| Citation: |
Xianyi Meng, Ke Jin, Zuo Xiao, Liming Ding. Side chain engineering on D18 polymers yields 18.74% power conversion efficiency[J]. Journal of Semiconductors, 2021, 42(10): 100501. doi: 10.1088/1674-4926/42/10/100501
X Y Meng, K Jin, Z Xiao, L M Ding, Side chain engineering on D18 polymers yields 18.74% power conversion efficiency[J]. J. Semicond., 2021, 42(10): 100501. doi: 10.1088/1674-4926/42/10/100501.
Export: BibTex EndNote
|
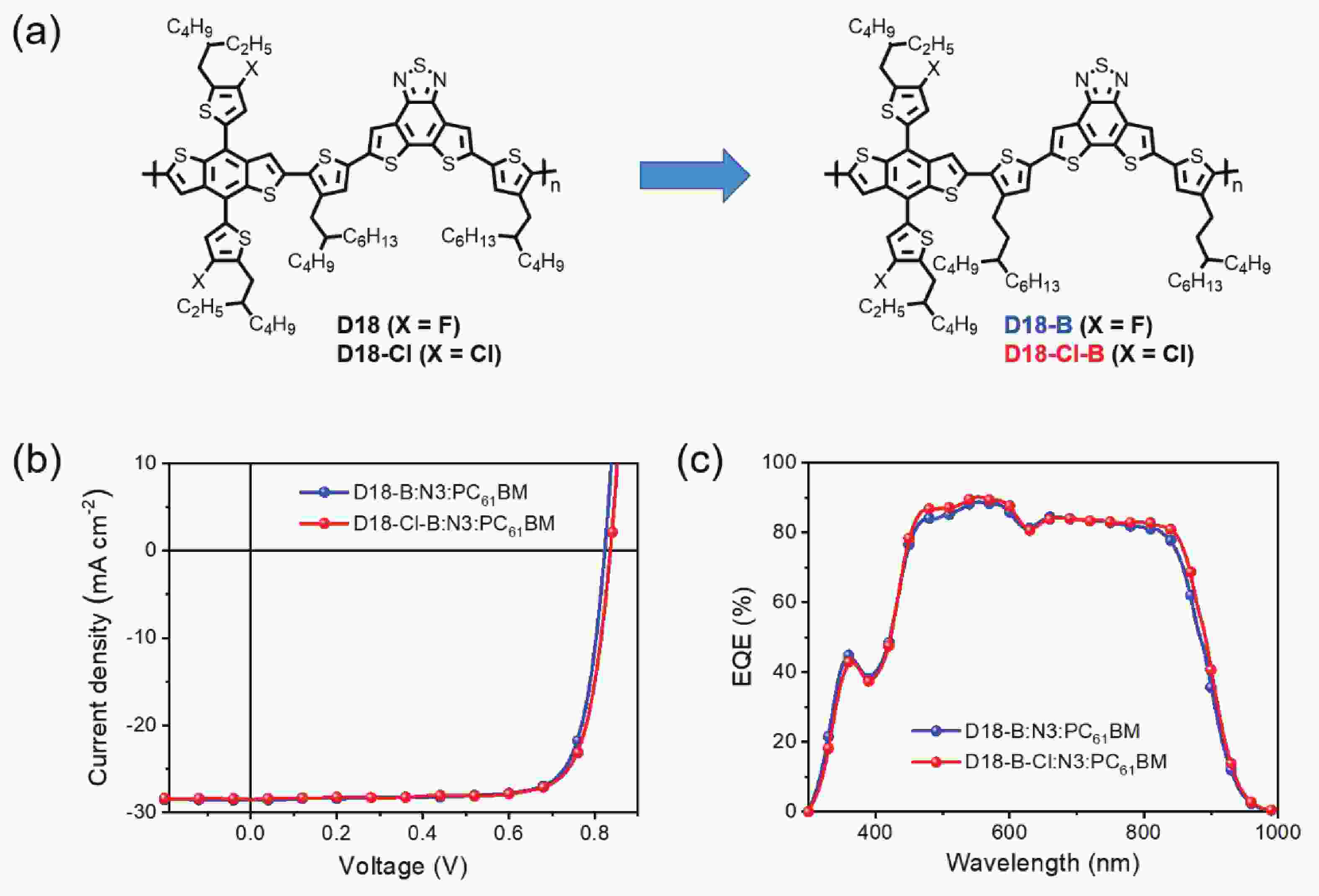
Side chain engineering on D18 polymers yields 18.74% power conversion efficiency
doi: 10.1088/1674-4926/42/10/100501
More Information-
References
[1] Liu Q, Jiang Y, Jin K, et al. 18% efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[2] Qin J, Zhang L, Zuo C, et al. A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency. J Semicond, 2021, 42, 010501 doi: 10.1088/1674-4926/42/1/010501[3] Jin K, Xiao Z, Ding L. 18.69% PCE from organic solar cells. J Semicond, 2021, 42, 060502 doi: 10.1088/1674-4926/42/6/060502[4] Jin K, Xiao Z, Ding L. D18, an eximious solar polymer!. J Semicond, 2021, 42, 010502 doi: 10.1088/1674-4926/42/1/010502[5] Xiong J, Jin K, Jiang Y, et al. Thiolactone copolymer donor gifts organic solar cells a 16.72% efficiency. Sci Bull, 2019, 64, 1573 doi: 10.1016/j.scib.2019.10.002[6] Liu J, Liu L, Zuo C, et al. 5H-dithieno[3,2-b:2',3'-d]pyran-5-one unit yields efficient wide-bandgap polymer donors. Sci Bull, 2019, 64, 1655 doi: 10.1016/j.scib.2019.09.001[7] Xu Y, Cui Y, Yao H, et al. A new conjugated polymer that enables the integration of photovoltaic and light-emitting functions in one device. Adv Mater, 2021, 33, 2101090 doi: 10.1002/adma.202101090[8] Jiang Y, Jin K, Chen X, et al. Post-sulphuration enhances the performance of a lactone polymer donor. J Semicond, 2021, 42, 070501 doi: 10.1088/1674-4926/42/7/070501[9] Tong Y, Xiao Z, Du X, et al. Progress of the key materials for organic solar cells. Sci China Chem, 2020, 63, 758 doi: 10.1007/s11426-020-9726-0[10] Sun C, Pan F, Bin H, et al. A low cost and high performance polymer donor material for polymer solar cells. Nat Commun, 2018, 9, 743 doi: 10.1038/s41467-018-03207-x[11] Zhang M, Guo X, Ma W, et al. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv Mater, 2015, 27, 4655 doi: 10.1002/adma.201502110[12] Lan L, Chen Z, Hu Q, et al. High-performance polymer solar cells based on a wide-bandgap polymer containing pyrrolo[3,4-f]benzotriazole-5,7-dione with a power conversion efficiency of 8.63%. Adv Sci, 2016, 3, 1600032 doi: 10.1002/advs.201600032[13] Zhu C, Meng L, Zhang J, et al. A quinoxaline-based D-A copolymer donor achieving 17.62% efficiency of organic solar cells. Adv Mater, 2021, 33, 2100474 doi: 10.1002/adma.202100474[14] Lei T, Dou J, Pei J. Influence of alkyl chain branching positions on the hole mobilities of polymer thin-film transistors. Adv Mater, 2012, 24, 6457 doi: 10.1002/adma.201202689[15] Fan B, Du X, Liu F, et al. Fine-tuning of the chemical structure of photoactive materials for highly efficient organic photovoltaics. Nat Energy, 2018, 3, 1051 doi: 10.1038/s41560-018-0263-4[16] Back J Y, Yu H, Song I, et al. Investigation of structure-property relationships in diketopyrrolopyrrole-based polymer semiconductors via side-chain engineering. Chem Mater, 2015, 27, 1732 doi: 10.1021/cm504545e[17] Barford W, Marcus M. Perspective: optical spectroscopy in π-conjugated polymers and how it can be used to determine multiscale polymer structures. J Chem Phys, 2017, 146, 130902 doi: 10.1063/1.4979495[18] Spano F C. Excitons in conjugated oligomer aggregates, films, and crystals. Annu Rev Phys Chem, 2006, 57, 217 doi: 10.1146/annurev.physchem.57.032905.104557[19] Jiang K, Wei Q, Lai J Y L, et al. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells. Joule, 2019, 3, 3020 doi: 10.1016/j.joule.2019.09.010[20] Xiao Z, Jia X, Li D, et al. 26 mA cm–2 Jsc from organic solar cells with a low-bandgap nonfullerene acceptor. Sci Bull, 2017, 62, 1494 doi: 10.1016/j.scib.2017.10.017[21] Xiao Z, Jia X, Ding L. Ternary organic solar cells offer 14% power conversion efficiency. Sci Bull, 2017, 62, 1562 doi: 10.1016/j.scib.2017.11.003 -
Supplements
 2021-100501suppl.pdf
2021-100501suppl.pdf
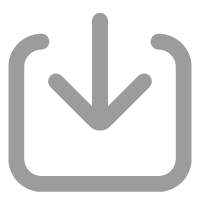
-
Proportional views




 DownLoad:
DownLoad: