| Citation: |
Hang Wang, Na Zhang, Shumin Li, Qinfei Ke, Zhengquan Li, Min Zhou. Metal-organic framework composites for energy conversion and storage[J]. Journal of Semiconductors, 2020, 41(9): 091707. doi: 10.1088/1674-4926/41/9/091707
H Wang, N Zhang, S M Li, Q F Ke, Z Q Li, M Zhou, Metal-organic framework composites for energy conversion and storage[J]. J. Semicond., 2020, 41(9): 091707. doi: 10.1088/1674-4926/41/9/091707.
Export: BibTex EndNote
|
Metal-organic framework composites for energy conversion and storage
doi: 10.1088/1674-4926/41/9/091707
More Information-
Abstract
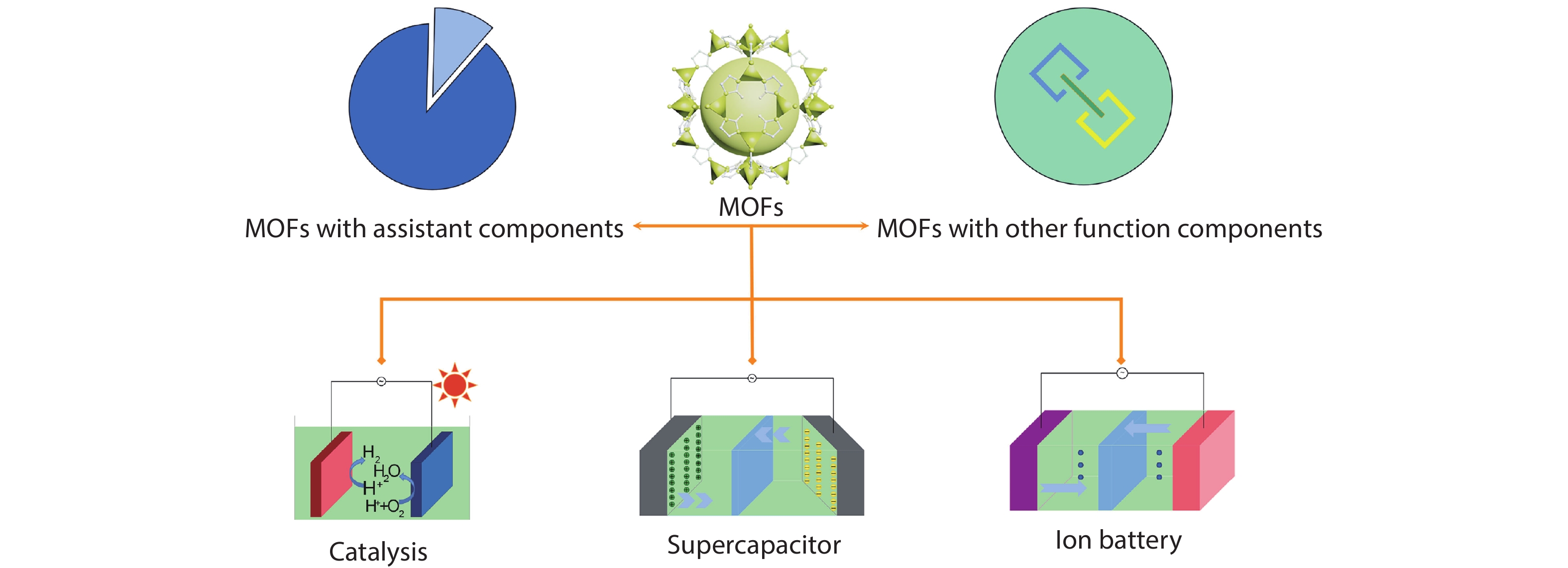
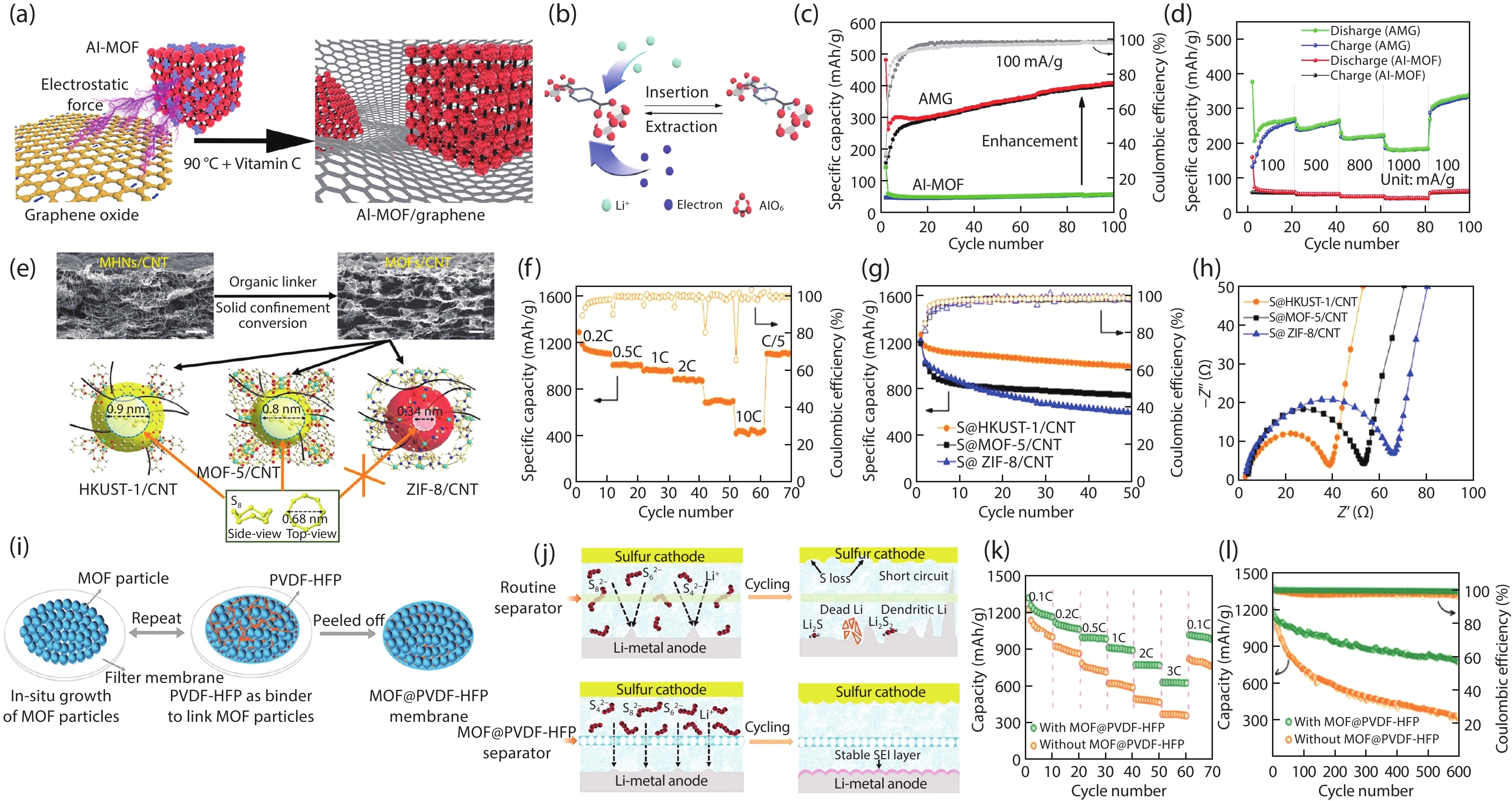
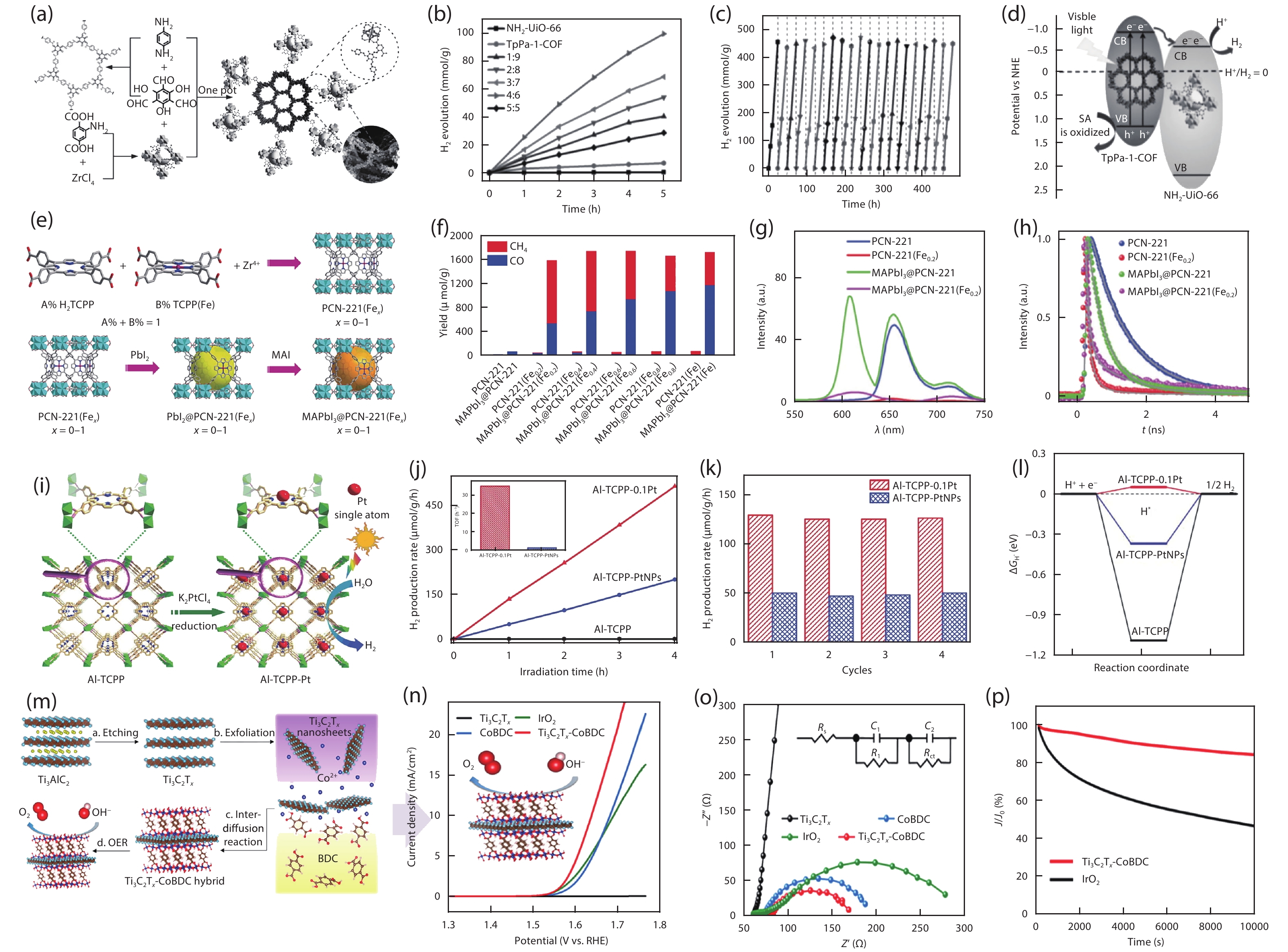
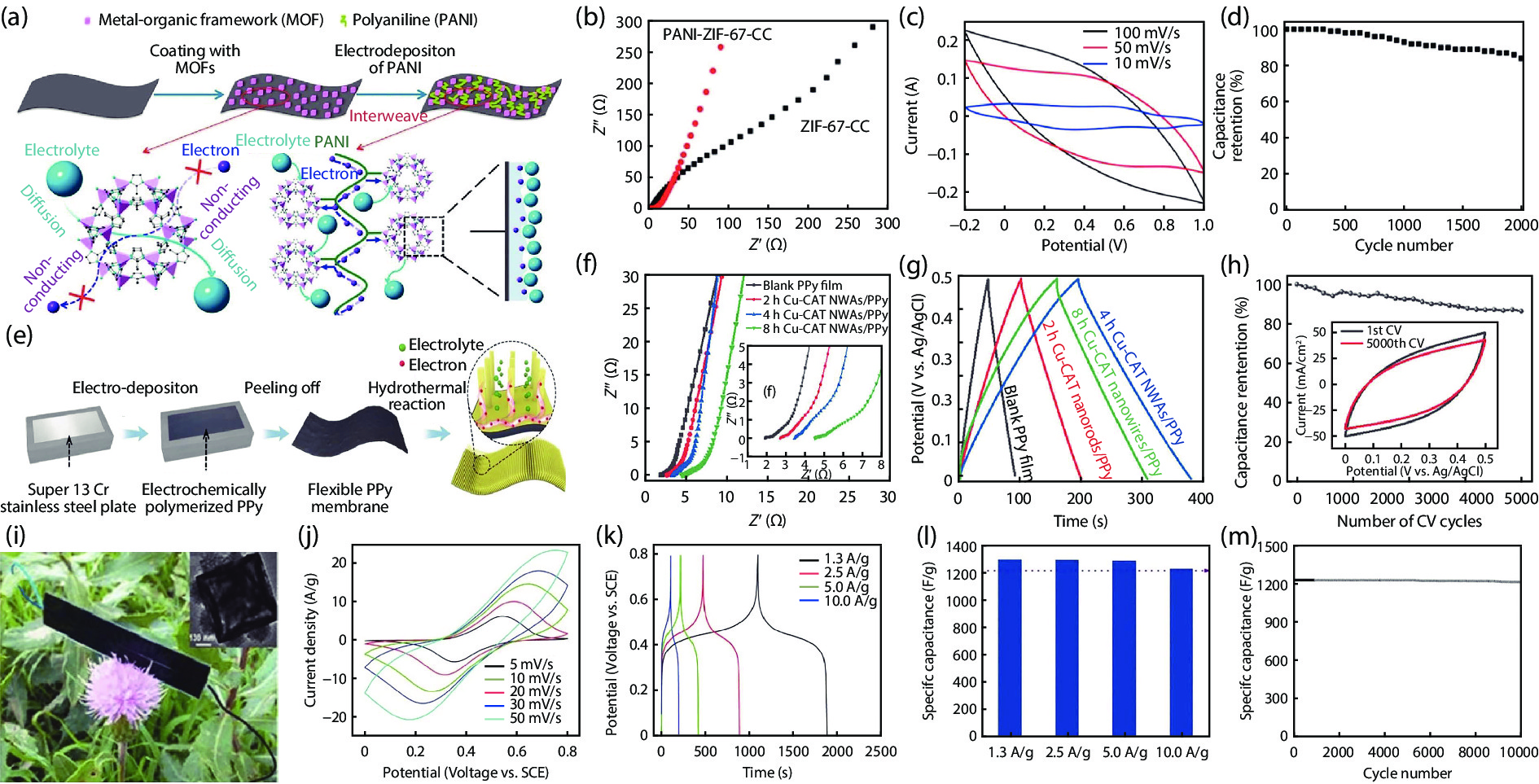
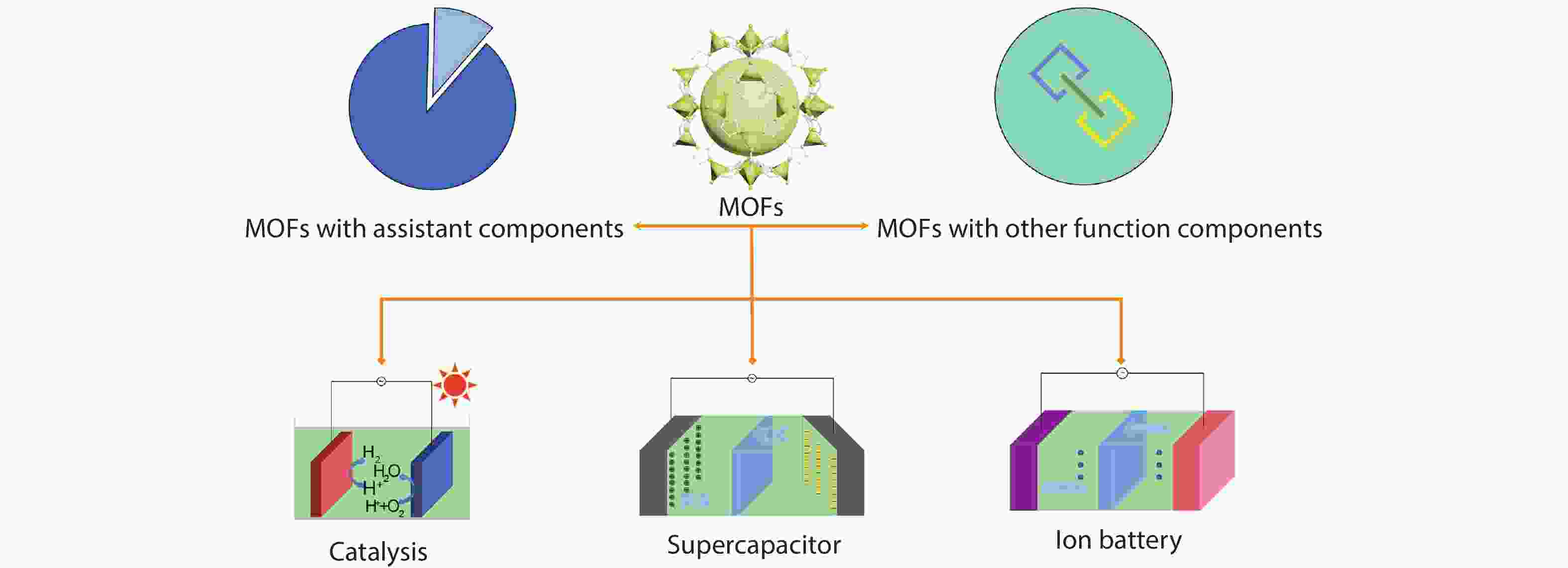
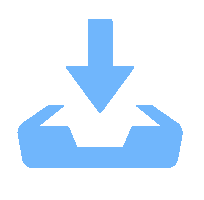
Metal-organic frameworks (MOFs) with orderly porous structure, large surface area, high electrochemical response and chemical tunability have been widely studied for energy conversion and storage. However, most reported MOFs still suffer from poor stability, insufficient conductivity, and low utilization of active sites. One strategy to circumvent these issues is to optimize MOFs via designing composites. Here, the design principle from the viewpoint of the intrinsic relationships among various components will be illuminated to acquire the synergistic effects, including two working modes: (1) MOFs with assistant components, (2) MOFs with other function components. This review introduces recent research progress of MOF-based composites with their typical applications in energy conversion (catalysis) and storage (supercapacitor and ion battery). Finally, the challenges and future prospects of MOF-based composites will be discussed in terms of maximizing composite properties. -
References
[1] Dhakshinamoorthy A, Asiri A M, García H. Metal-organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production. Angew Chem Int Ed, 2016, 55(18), 5414 doi: 10.1002/anie.201505581[2] Zheng S, Li X, Yan B, et al. Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage. Adv Energy Mater, 2017, 7(18), 1602733 doi: 10.1002/aenm.201602733[3] Liang Z, Qu C, Guo W, et al. Pristine metal-organic frameworks and their composites for energy storage and conversion. Adv Mater, 2018, 30(37), 1702891 doi: 10.1002/adma.201702891[4] Dong R, Han P, Arora H, et al. High-mobility band-like charge transport in a semiconducting two-dimensional metal-organic framework. Nat Mater, 2018, 17(11), 1027 doi: 10.1038/s41563-018-0189-z[5] Song Y, Li Z, Zhu Y, et al. Titanium hydroxide secondary building units in metal-organic frameworks catalyze hydrogen evolution under visible light. J Am Chem Soc, 2019, 141(31), 12219 doi: 10.1021/jacs.9b05964[6] Sheberla D, Bachman J C, Elias J S, et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat Mater, 2017, 16(2), 220 doi: 10.1038/nmat4766[7] Lee J H, Ali G, Kim D H, et al. Metal-organic framework cathodes based on a vanadium hexacyanoferrate prussian blue analogue for high-performance aqueous rechargeable batteries. Adv Energy Mater, 2017, 7(2), 1601491 doi: 10.1002/aenm.201601491[8] Wilmer C E, Leaf M, Lee C Y, et al. Large-scale screening of hypothetical metal-organic frameworks. Nat Chem, 2012, 4(2), 83 doi: 10.1038/nchem.1192[9] Falcaro P, Okada K, Hara T, et al. Centimetre-scale micropore alignment in oriented polycrystalline metal-organic framework films via heteroepitaxial growth. Nat Mater, 2017, 16(3), 342 doi: 10.1038/nmat4815[10] Zhu Y, Ciston J, Zheng B, et al. Unravelling surface and interfacial structures of a metal-organic framework by transmission electron microscopy. Nat Mater, 2017, 16(5), 532 doi: 10.1038/nmat4852[11] Liu G, Chernikova V, Liu Y, et al. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat Mater, 2018, 17(3), 283 doi: 10.1038/s41563-017-0013-1[12] Van Wyk A, Smith T, Park J, et al. Charge-transfer within Zr-based metal-organic framework: The role of polar node. J Am Chem Soc, 2018, 140(8), 2756 doi: 10.1021/jacs.7b13211[13] Feng D, Lei T, Lukatskaya M R, et al. Robust and conductive two-dimensional metal-organic frameworks with exceptionally high volumetric and areal capacitance. Nat Energy, 2018, 3(1), 30 doi: 10.1038/s41560-017-0044-5[14] Jiang Q, Xiong P, Liu J, et al. A redox-active 2D metal-organic framework for efficient lithium storage with extraordinary high capacity. Angew Chem Int Ed, 2020, 59(13), 5273 doi: 10.1002/anie.201914395[15] Yuan Y P, Yin L S, Cao S W, et al. Improving photocatalytic hydrogen production of metal-organic framework UiO-66 octahedrons by dye-sensitization. Appl Catal B, 2015, 168/169, 572 doi: 10.1016/j.apcatb.2014.11.007[16] Wu D, Guo Z, Yin X, et al. Metal-organic frameworks as cathode materials for Li-O2 batteries. Adv Mater, 2014, 26(20), 3258 doi: 10.1002/adma.201305492[17] Chen Y Z, Wang Z U, Wang H, et al. Singlet oxygen-engaged selective photo-oxidation over pt nanocrystals/porphyrinic MOF: The roles of photothermal effect and pt electronic state. J Am Chem Soc, 2017, 139(5), 2035 doi: 10.1021/jacs.6b12074[18] Asakura D, Li C H, Mizuno Y, et al. Bimetallic cyanide-bridged coordination polymers as lithium ion cathode materials: core@shell nanoparticles with enhanced cyclability. J Am Chem Soc, 2013, 135(7), 2793 doi: 10.1021/ja312160v[19] Zhong H, Ghorbani-Asl M, Ly K H, et al. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nat Commun, 2020, 11(1), 1409 doi: 10.1038/s41467-020-15141-y[20] Zhu Y P, Yin J, Abou-Hamad E, et al. Highly stable phosphonate-based MOFs with engineered bandgaps for efficient photocatalytic hydrogen production. Adv Mater, 2020, 32(16), 1906368 doi: 10.1002/adma.201906368[21] Li Q, Fan Z L, Xue D X, et al. A multi-dye@MOF composite boosts highly efficient photodegradation of an ultra-stubborn dye reactive blue 21 under visible-light irradiation. J Mater Chem A, 2018, 6(5), 2148 doi: 10.1039/C7TA10184H[22] Li M, Zheng Z, Zheng Y, et al. Controlled growth of metal-organic framework on upconversion nanocrystals for NIR-enhanced photocatalysis. ACS Appl Mater Interfaces, 2017, 9(3), 2899 doi: 10.1021/acsami.6b15792[23] Liu N, Huang W, Zhang X, et al. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB. Appl Catal B, 2018, 221, 119 doi: 10.1016/j.apcatb.2017.09.020[24] Li S, Ji K, Zhang M, et al. Boosting photocatalytic CO2 reduction of metal-organic frameworks by encapsulating carbon dots. Nanoscale, 2020, 12(17), 9533 doi: 10.1039/D0NR01696A[25] Jahan M, Bao Q, Loh K P. Electrocatalytically active graphene-porphyrin MOF composite for oxygen reduction reaction. J Am Chem Soc, 2012, 134(15), 6707 doi: 10.1021/ja211433h[26] Fang Y, Li X, Li F, et al. Self-assembly of cobalt-centered metal organic framework and multiwalled carbon nanotubes hybrids as a highly active and corrosion-resistant bifunctional oxygen catalyst. J Power Sources, 2016, 326, 50 doi: 10.1016/j.jpowsour.2016.06.114[27] Xiong W, Li H, You H, et al. Encapsulating metal organic framework into hollow mesoporous carbon sphere as efficient oxygen bifunctional electrocatalyst. Natl Sci Rev, 2019, 7(3), 609 doi: 10.1093/nsr/nwz166[28] Xu C, Pan Y, Wan G, et al. Turning on visible-light photocatalytic C-H oxidation over metal-organic frameworks by introducing metal-to-cluster charge transfer. J Am Chem Soc, 2019, 141(48), 19110 doi: 10.1021/jacs.9b09954[29] Zhang W, Wang Y, Zheng H, et al. Embedding ultrafine metal oxide nanoparticles in monolayered metal-organic framework nanosheets enables efficient electrocatalytic oxygen evolution. ACS Nano, 2020, 14(2), 1971 doi: 10.1021/acsnano.9b08458[30] Zhang H, Wei J, Dong J, et al. Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal-organic framework. Angew Chem Int Ed, 2016, 55(46), 14310 doi: 10.1002/anie.201608597[31] Bi S, Banda H, Chen M, et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat Mater, 2020, 19(5), 552 doi: 10.1038/s41563-019-0598-7[32] Skorupskii G, Trump B A, Kasel T W, et al. Efficient and tunable one-dimensional charge transport in layered lanthanide metal-organic frameworks. Nat Chem, 2020, 12(2), 131 doi: 10.1038/s41557-019-0372-0[33] Rajak R, Saraf M, Mobin S M. Robust heterostructures of a bimetallic sodium-zinc metal-organic framework and reduced graphene oxide for high-performance supercapacitors. J Mater Chem A, 2019, 7(4), 1725 doi: 10.1039/C8TA09528K[34] Zhou Y, Mao Z, Wang W, et al. In-situ fabrication of graphene oxide hybrid ni-based metal-organic framework (Ni-MOFs@GO) with ultrahigh capacitance as electrochemical pseudocapacitor materials. ACS Appl Mater Interfaces, 2016, 8(42), 28904 doi: 10.1021/acsami.6b10640[35] Wen P, Gong P, Sun J, et al. Design and synthesis of Ni-MOF/CNT composites and rGO/carbon nitride composites for an asymmetric supercapacitor with high energy and power density. J Mater Chem A, 2015, 3(26), 13874 doi: 10.1039/C5TA02461G[36] Deng T, Lu Y, Zhang W, et al. Inverted design for high-performance supercapacitor via Co(OH)2-derived highly oriented MOF electrodes. Adv Energy Mater, 2018, 8(7), 1702294 doi: 10.1002/aenm.201702294[37] Zhou S, Kong X, Zheng B, et al. Cellulose nanofiber @ conductive metal-organic frameworks for high-performance flexible supercapacitors. ACS Nano, 2019, 13(8), 9578 doi: 10.1021/acsnano.9b04670[38] Tian D, Song N, Zhong M, et al. Bimetallic MOF nanosheets decorated on electrospun nanofibers for high-performance asymmetric supercapacitors. ACS Appl Mater Interfaces, 2020, 12(1), 1280 doi: 10.1021/acsami.9b16420[39] Jiang H, Liu X C, Wu Y, et al. Metal-organic frameworks for high charge-discharge rates in lithium-sulfur batteries. Angew Chem Int Ed, 2018, 57(15), 3916 doi: 10.1002/anie.201712872[40] Gao C, Wang P, Wang Z, et al. The disordering-enhanced performances of the Al-MOF/graphene composite anodes for lithium ion batteries. Nano Energy, 2019, 65, 104032 doi: 10.1016/j.nanoen.2019.104032[41] Wei T, Zhang M, Wu P, et al. POM-based metal-organic framework/reduced graphene oxide nanocomposites with hybrid behavior of battery-supercapacitor for superior lithium storage. Nano Energy, 2017, 34, 205 doi: 10.1016/j.nanoen.2017.02.028[42] Hou Y, Mao H, Xu L. MIL-100(V) and MIL-100(V)/rGO with various valence states of vanadium ions as sulfur cathode hosts for lithium-sulfur batteries. Nano Res, 2017, 10(1), 344 doi: 10.1007/s12274-016-1326-0[43] Mao Y, Li G, Guo Y, et al. Foldable interpenetrated metal-organic frameworks/carbon nanotubes thin film for lithium-sulfur batteries. Nat Commun, 2017, 8(1), 14628 doi: 10.1038/ncomms14628[44] Zhang H, Zhao W, Zou M, et al. 3D, mutually embedded MOF@carbon nanotube hybrid networks for high-performance lithium-sulfur batteries. Adv Energy Mater, 2018, 8(19), 1800013 doi: 10.1002/aenm.201800013[45] Zang Y, Pei F, Huang J, et al. Large-area preparation of crack-free crystalline microporous conductive membrane to upgrade high energy lithium-sulfur batteries. Adv Energy Mater, 2018, 8(31), 1802052 doi: 10.1002/aenm.201802052[46] He Y, Chang Z, Wu S, et al. simultaneously inhibiting lithium dendrites growth and polysulfides shuttle by a flexible MOF-based membrane in Li –S batteries. Adv Energy Mater, 2018, 8(34), 1802130 doi: 10.1002/aenm.201802130[47] Zhang C, Shen L, Shen J, et al. Anion-sorbent composite separators for high-rate lithium-ion batteries. Adv Mater, 2019, 31(21), 1808338 doi: 10.1002/adma.201808338[48] Zhang F M, Sheng J L, Yang Z D, et al. Rational design of MOF/COF hybrid materials for photocatalytic H2 evolution in the presence of sacrificial electron donors. Angew Chem Int Ed, 2018, 57(37), 12106 doi: 10.1002/anie.201806862[49] Ran J, Qu J, Zhang H, et al. 2D metal organic framework nanosheet: A universal platform promoting highly efficient visible-light-induced hydrogen production. Adv Energy Mater, 2019, 9(11), 1803402 doi: 10.1002/aenm.201803402[50] Shi L, Wang T, Zhang H, et al. Electrostatic self-assembly of nanosized carbon nitride nanosheet onto a zirconium metal-organic framework for enhanced photocatalytic CO2 reduction. Adv Funct Mater, 2015, 25(33), 5360 doi: 10.1002/adfm.201502253[51] Kong Z C, Liao J F, Dong Y J, et al. Core@shell CsPbBr3@zeolitic imidazolate framework nanocomposite for efficient photocatalytic CO2 reduction. ACS Energy Lett, 2018, 3(11), 2656 doi: 10.1021/acsenergylett.8b01658[52] Wu L Y, Mu Y F, Guo X X, et al. encapsulating perovskite quantum dots in iron-based metal-organic frameworks (MOFs) for efficient photocatalytic CO2 reduction. Angew Chem Int Ed, 2019, 58(28), 9491 doi: 10.1002/anie.201904537[53] Fang X, Shang Q, Wang Y, et al. Single Pt atoms confined into a metal-organic framework for efficient photocatalysis. Adv Mater, 2018, 30(7), 1705112 doi: 10.1002/adma.201705112[54] Xia Z, Fang J, Zhang X, et al. Pt nanoparticles embedded metal-organic framework nanosheets: A synergistic strategy towards bifunctional oxygen electrocatalysis. Appl Catal B, 2019, 245, 389 doi: 10.1016/j.apcatb.2018.12.073[55] Rui K, Zhao G, Lao M, et al. Direct hybridization of noble metal nanostructures on 2D metal-organic framework nanosheets to catalyze hydrogen evolution. Nano Lett, 2019, 19(12), 8447 doi: 10.1021/acs.nanolett.9b02729[56] Zhao L, Dong B, Li S, et al. Interdiffusion reaction-assisted hybridization of two-dimensional metal-organic frameworks and Ti3C2Tx nanosheets for electrocatalytic oxygen evolution. ACS Nano, 2017, 11(6), 5800 doi: 10.1021/acsnano.7b01409[57] Liu T, Li P, Yao N, et al. CoP-doped MOF-based electrocatalyst for pH-universal hydrogen evolution reaction. Angew Chem Int Ed, 2019, 58(14), 4679 doi: 10.1002/anie.201901409[58] Wang L, Feng X, Ren L, et al. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited PANI. J Am Chem Soc, 2015, 137(15), 4920 doi: 10.1021/jacs.5b01613[59] Guo S, Zhu Y, Yan Y, et al. (Metal-organic framework)-polyaniline sandwich structure composites as novel hybrid electrode materials for high-performance supercapacitor. J Power Sources, 2016, 316, 176 doi: 10.1016/j.jpowsour.2016.03.040[60] Jiao Y, Chen G, Chen D, et al. Bimetal-organic framework assisted polymerization of pyrrole involving air oxidant to prepare composite electrodes for portable energy storage. J Mater Chem A, 2017, 5(45), 23744 doi: 10.1039/C7TA07464F[61] Xu X, Tang J, Qian H, et al. Three-dimensional networked metal-organic frameworks with conductive polypyrrole tubes for flexible supercapacitors. ACS Appl Mater Interfaces, 2017, 9(44), 38737 doi: 10.1021/acsami.7b09944[62] Wang H N, Zhang M, Zhang A M, et al. Polyoxometalate-based metal-organic frameworks with conductive polypyrrole for supercapacitors. ACS Appl Mater Interfaces, 2018, 10(38), 32265 doi: 10.1021/acsami.8b12194[63] Hou R, Miao M, Wang Q, et al. Integrated conductive hybrid architecture of metal-organic framework nanowire array on polypyrrole membrane for all-solid-state flexible supercapacitors. Adv Energy Mater, 2020, 10(1), 1901892 doi: 10.1002/aenm.201901892[64] Zhang Y Z, Cheng T, Wang Y, et al. A simple approach to boost capacitance: Flexible supercapacitors based on manganese oxides@MOFs via chemically induced in situ self-transformation. Adv Mater, 2016, 28(26), 5242 doi: 10.1002/adma.201600319[65] Yue L, Wang X, Sun T, et al. Ni-MOF coating MoS2 structures by hydrothermal intercalation as high-performance electrodes for asymmetric supercapacitors. Chem Eng J, 2019, 375, 121959 doi: 10.1016/j.cej.2019.121959[66] Zhang Z, Yoshikawa H, Awaga K. Monitoring the solid-state electrochemistry of Cu(2,7-AQDC) (AQDC = anthraquinone dicarboxylate) in a lithium battery: Coexistence of metal and ligand redox activities in a metal-organic framework. J Am Chem Soc, 2014, 136(46), 16112 doi: 10.1021/ja508197w[67] Wang P, Shen M, Zhou H, et al. MOF-derived CuS@Cu-BTC composites as high-performance anodes for lithium-ion batteries. Small, 2019, 15(47), 1903522 doi: 10.1002/smll.201903522[68] Jin J, Zheng Y, Huang S Z, et al. Directly anchoring 2D NiCo metal-organic frameworks on few-layer black phosphorus for advanced lithium-ion batteries. J Mater Chem A, 2019, 7(2), 783 doi: 10.1039/C8TA09327J[69] Baumann A E, Han X, Butala M M, et al. Lithium thiophosphate functionalized zirconium MOFs for Li–S batteries with enhanced rate capabilities. J Am Chem Soc, 2019, 141(44), 17891 doi: 10.1021/jacs.9b09538[70] Li Y, Lin S, Wang D, et al. Single atom array mimic on ultrathin MOF nanosheets boosts the safety and life of lithium-sulfur batteries. Adv Mater, 2020, 32(8), 1906722 doi: 10.1002/adma.201906722[71] Bai S, Liu X, Zhu K, et al. Metal-organic framework-based separator for lithium-sulfur batteries. Nat Energy, 2016, 1(7), 16094 doi: 10.1038/nenergy.2016.94[72] Hong X J, Song C L, Yang Y, et al. Cerium based metal-organic frameworks as an efficient separator coating catalyzing the conversion of polysulfides for high performance lithium-sulfur batteries. ACS Nano, 2019, 13(2), 1923 doi: 10.1021/acsnano.8b08155[73] Chen W, Pei J, He C T, et al. Single tungsten atoms supported on MOF-derived N-doped carbon for robust electrochemical hydrogen evolution. Adv Mater, 2018, 30(30), 1800396 doi: 10.1002/adma.201800396[74] Wang Q, Luo Y, Hou R, et al. Redox tuning in crystalline and electronic structure of bimetal-organic frameworks derived cobalt/nickel boride/sulfide for boosted faradaic capacitance. Adv Mater, 2019, 31(51), 1905744 doi: 10.1002/adma.201905744[75] Wang Z, Shen J, Liu J, et al. Self-supported and flexible sulfur cathode enabled via synergistic confinement for high-energy-density lithium-sulfur batteries. Adv Mater, 2019, 31(33), 1902228 doi: 10.1002/adma.201902228 -
Proportional views
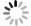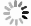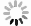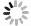




 DownLoad:
DownLoad: