| Citation: |
Fowziya Shaik Ali, Faisal Al Marzouqi, A. Afroos Banu, M. Ismail Fathima, A. R. Mohamed Jahangir, K. Mohamed Rafi, A. Ayeshamariam. Novel synthesis of cerium oxide nano photocatalyst by a hydrothermal method[J]. Journal of Semiconductors, 2021, 42(12): 122801. doi: 10.1088/1674-4926/42/12/122801
F S Ali, F Al Marzouqi, A A Banu, M I Fathima, A R M Jahangir, K M Rafi, A Ayeshamariam, Novel synthesis of cerium oxide nano photocatalyst by a hydrothermal method[J]. J. Semicond., 2021, 42(12): 122801. doi: 10.1088/1674-4926/42/12/122801.
Export: BibTex EndNote
|
Novel synthesis of cerium oxide nano photocatalyst by a hydrothermal method
doi: 10.1088/1674-4926/42/12/122801
More Information-
Abstract
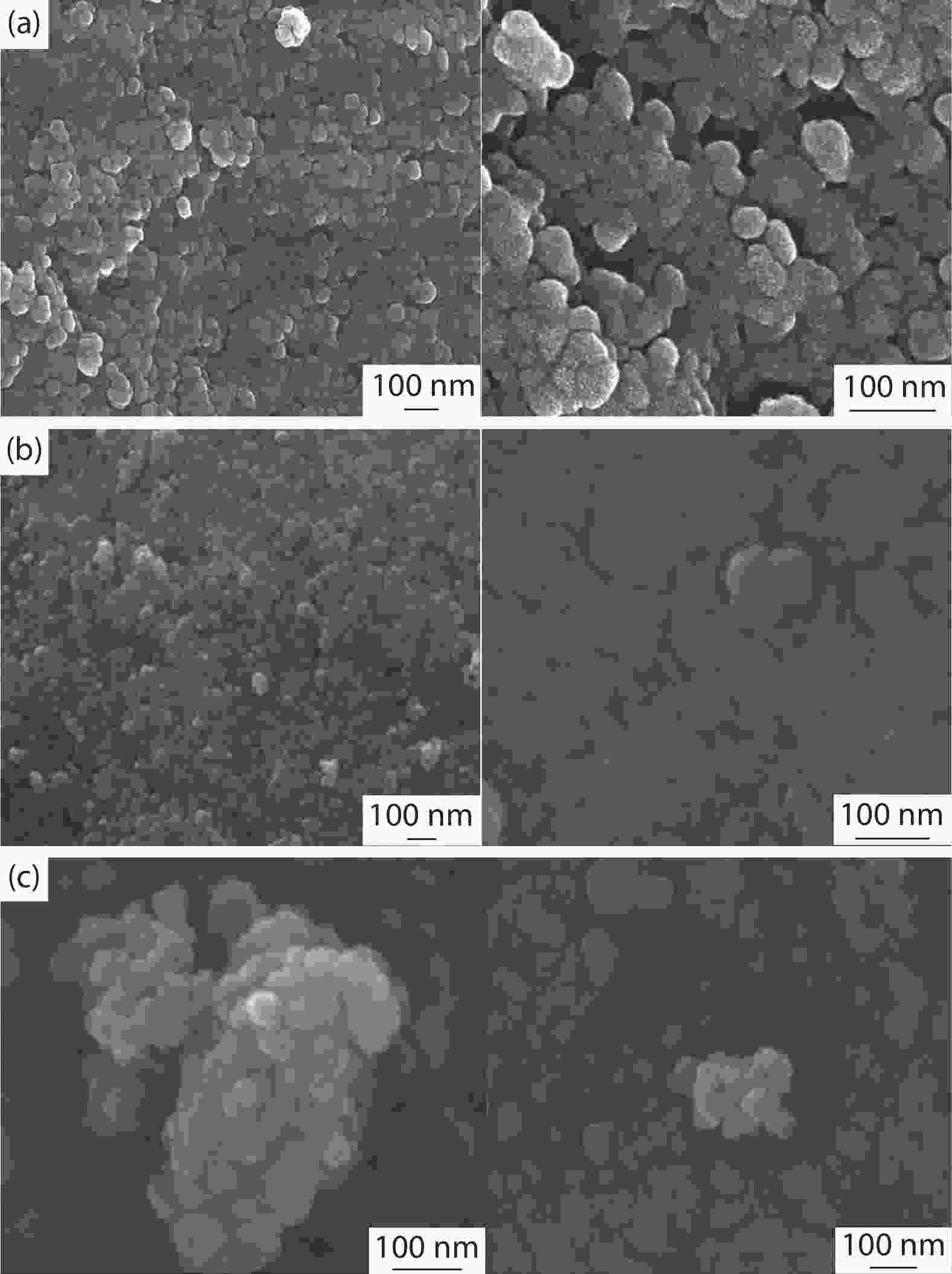
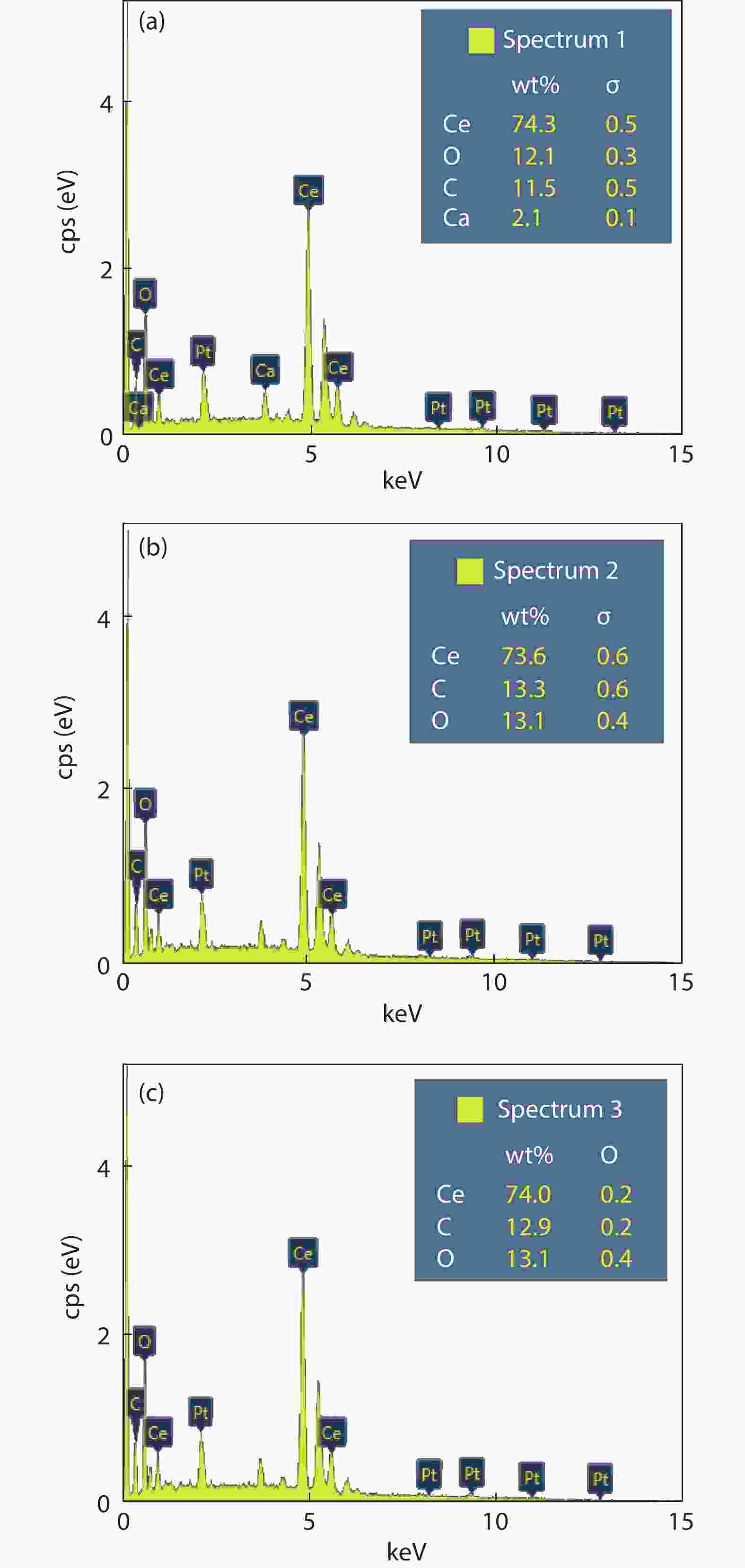
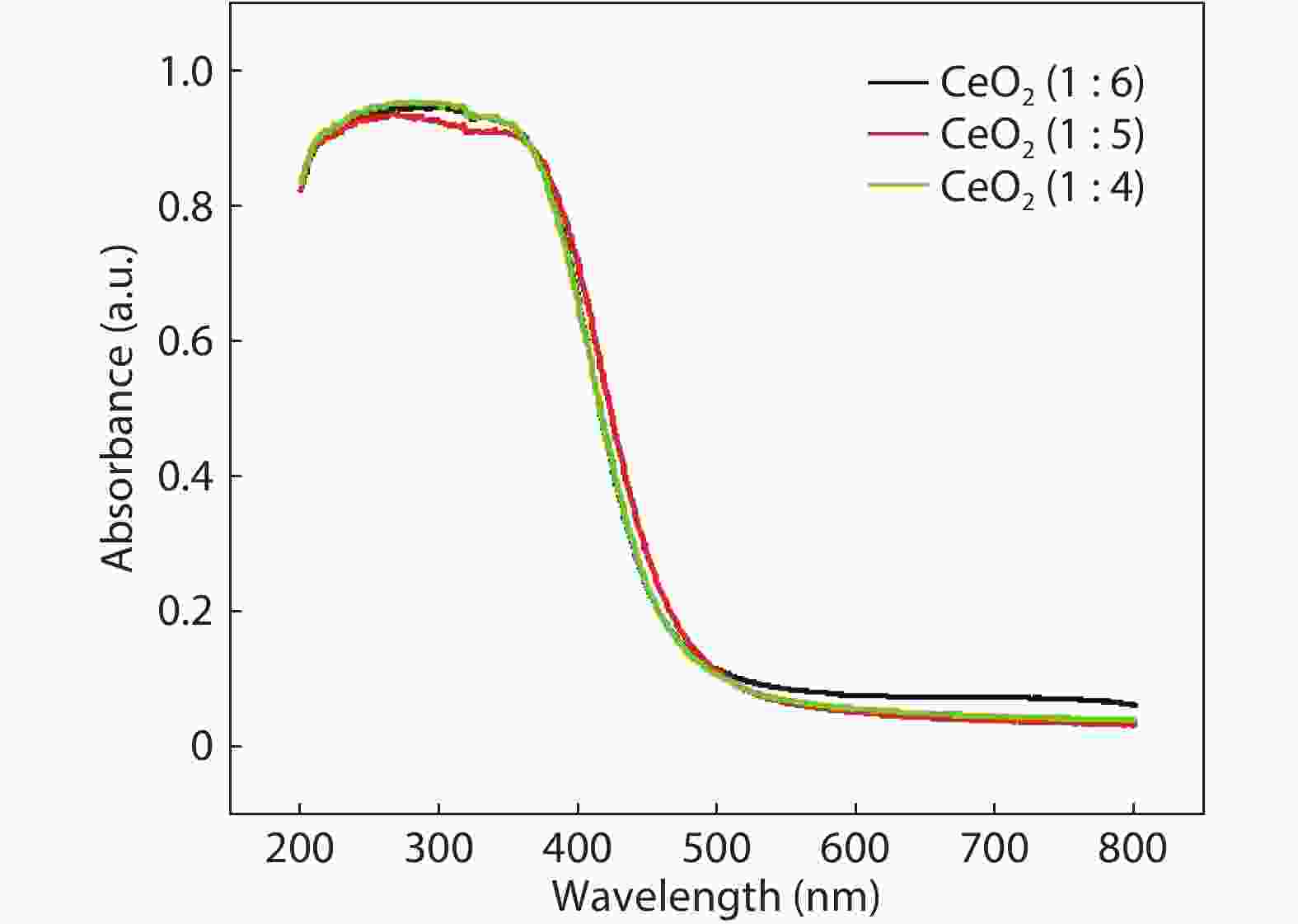
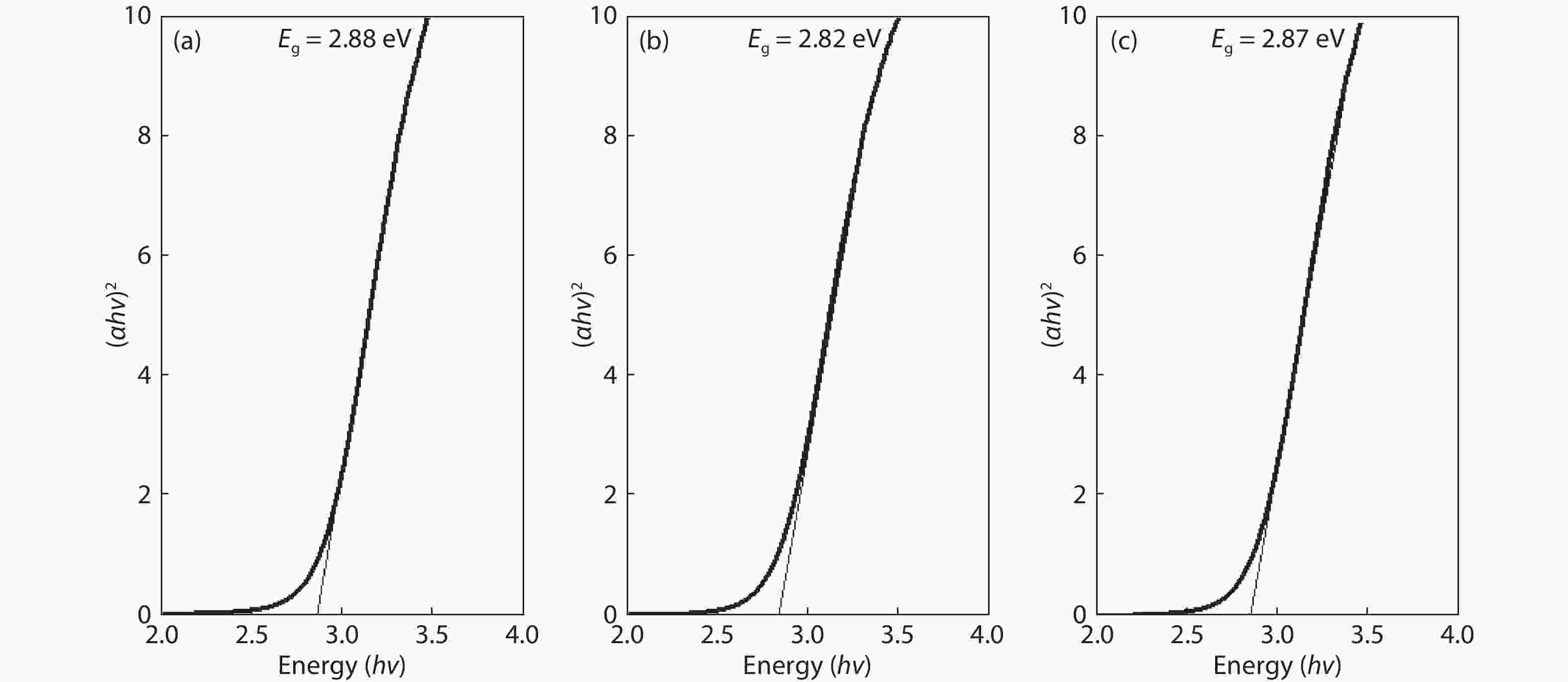
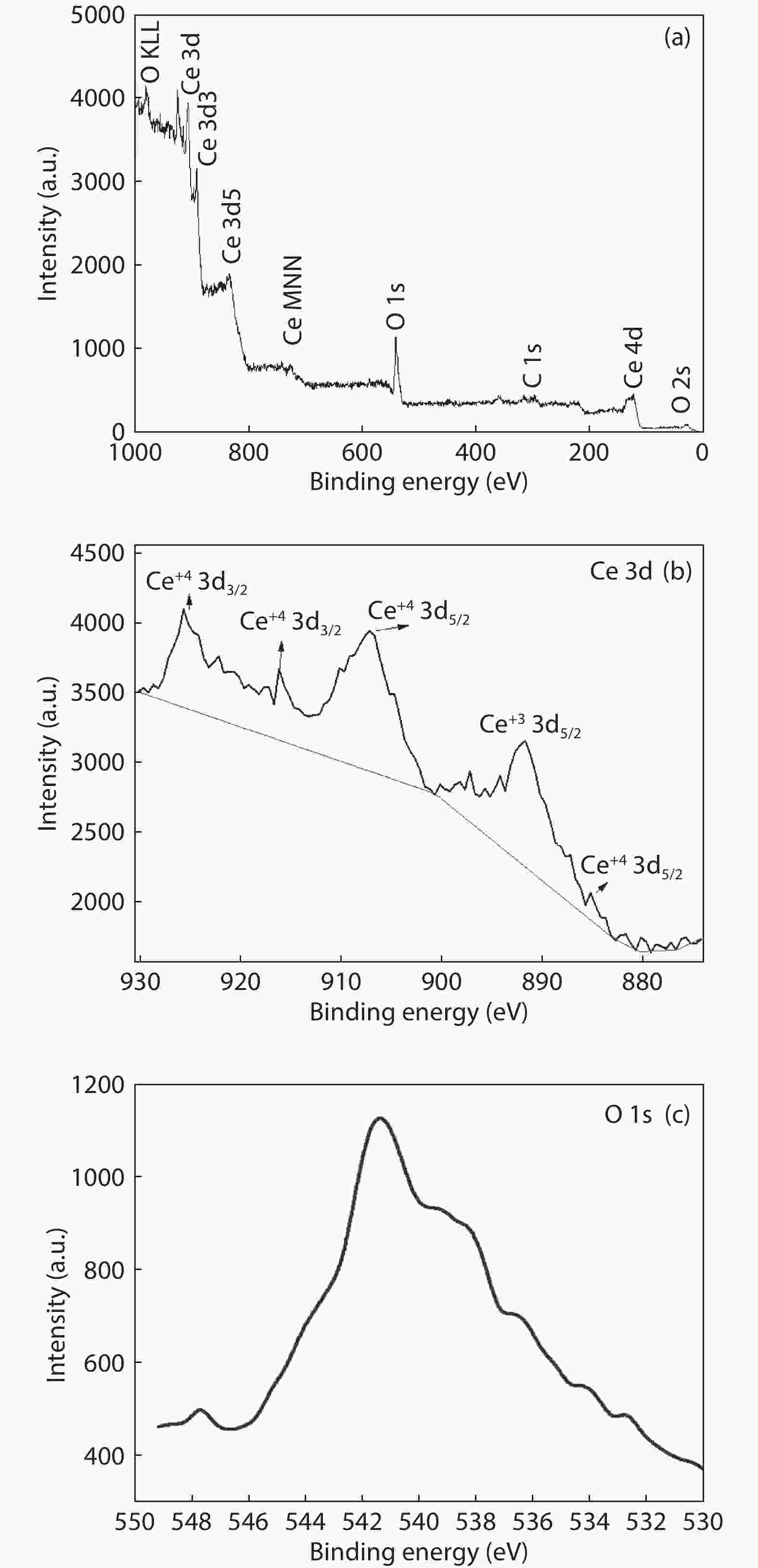
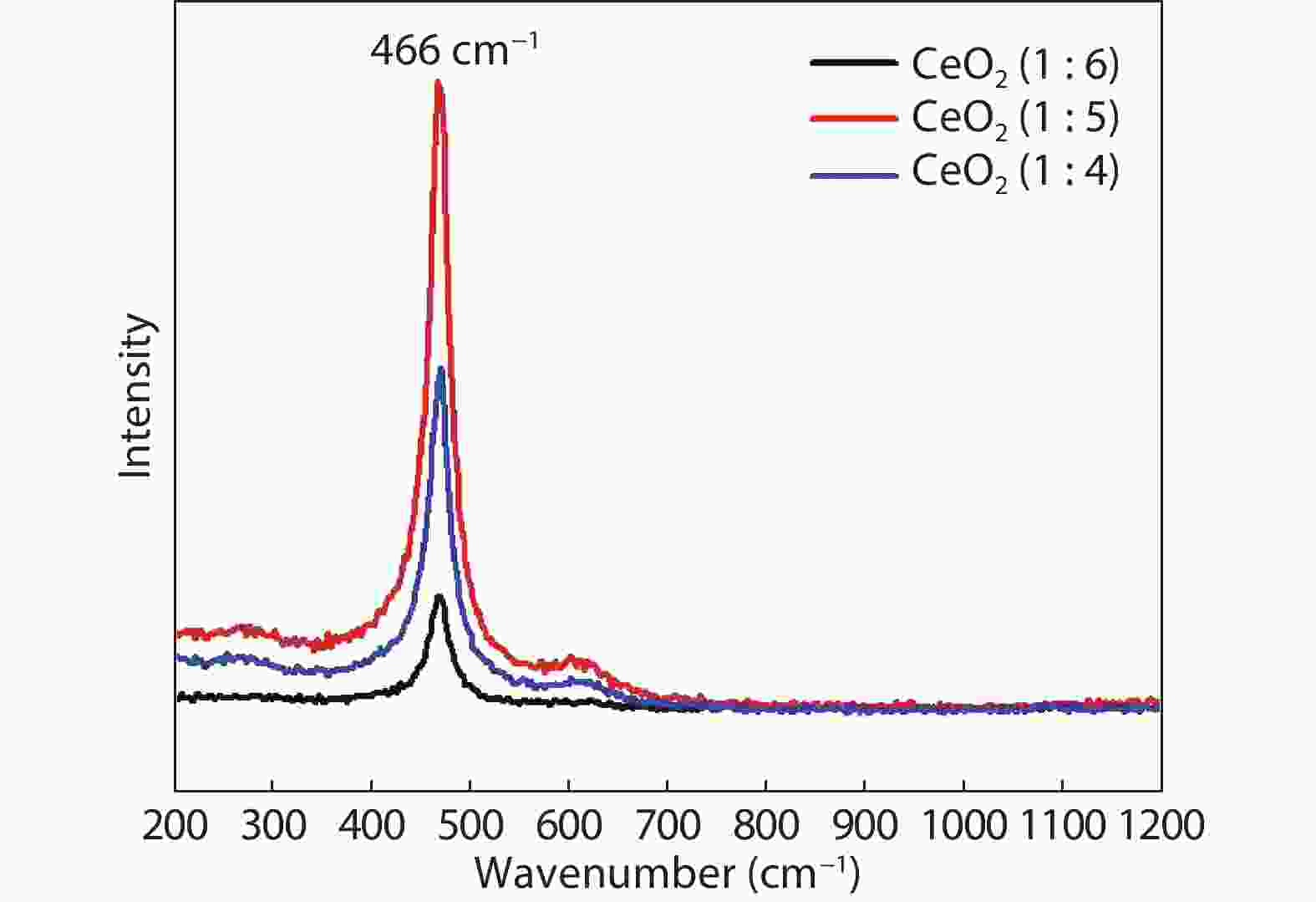
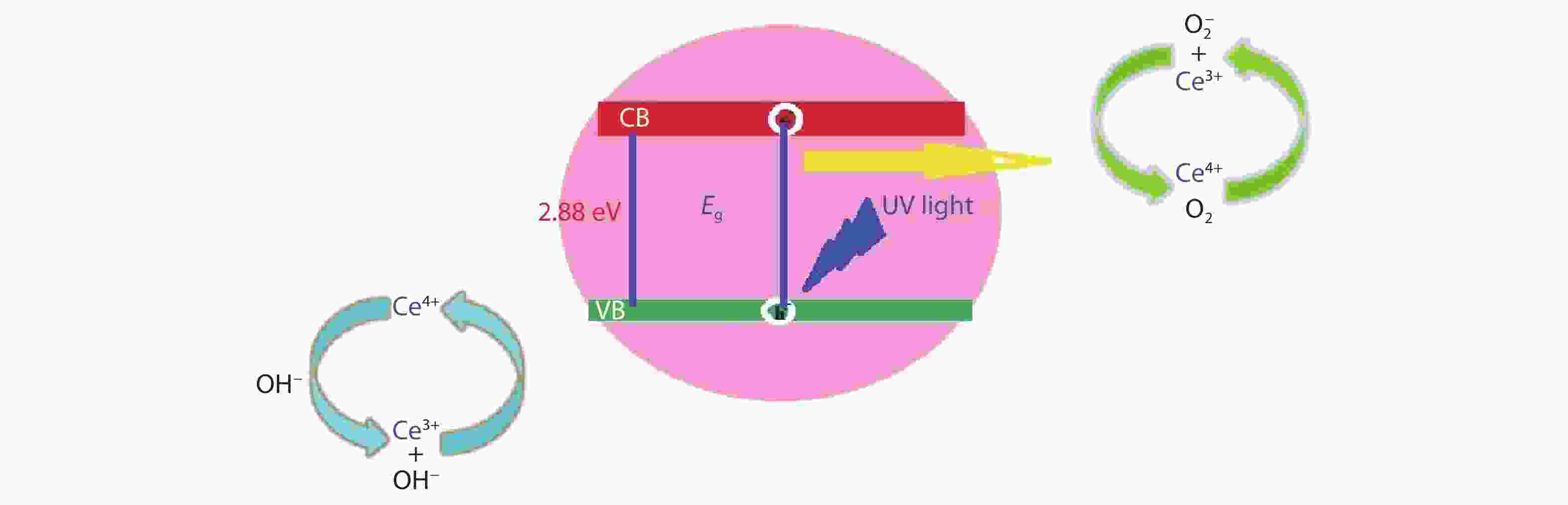
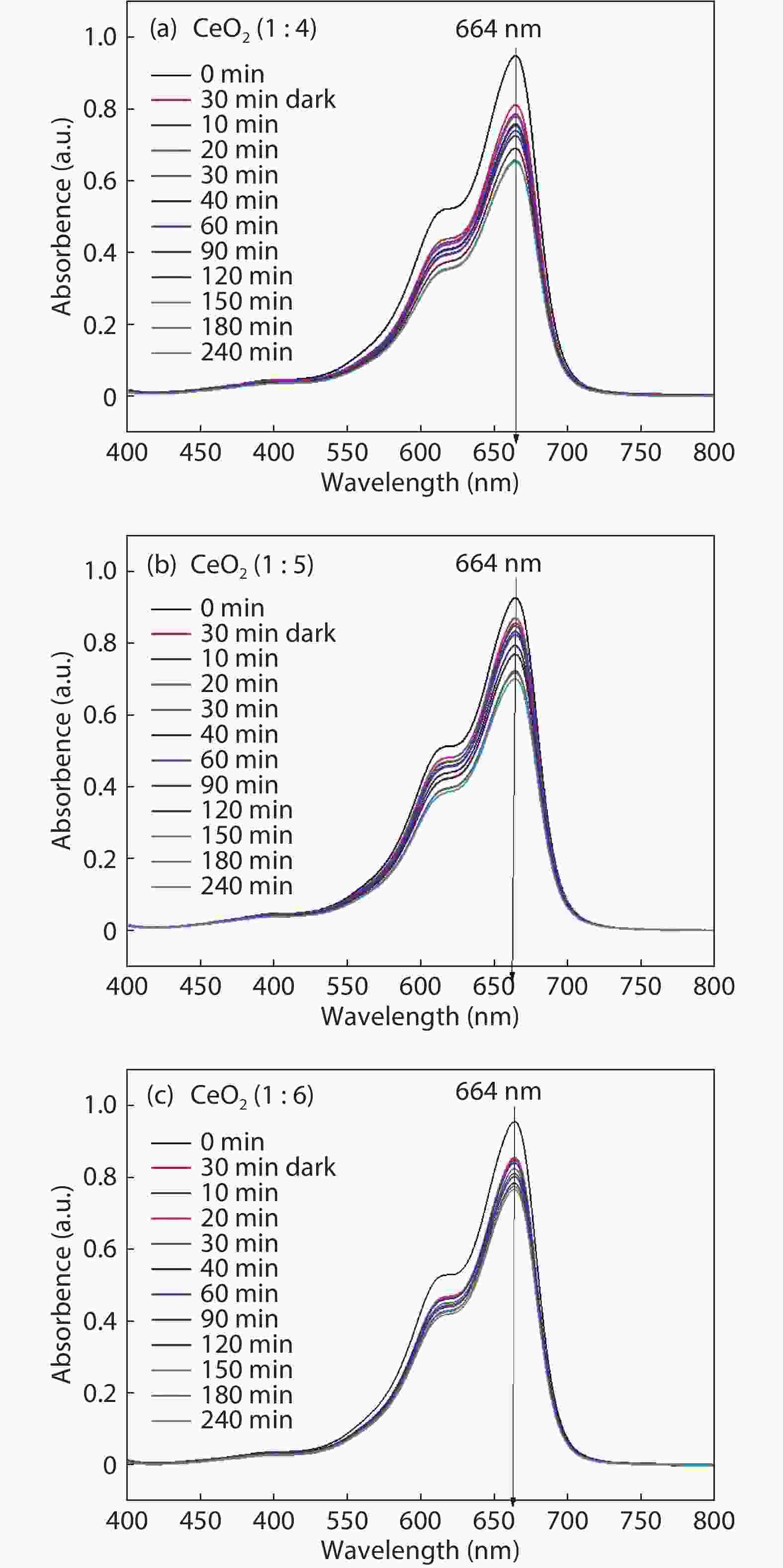
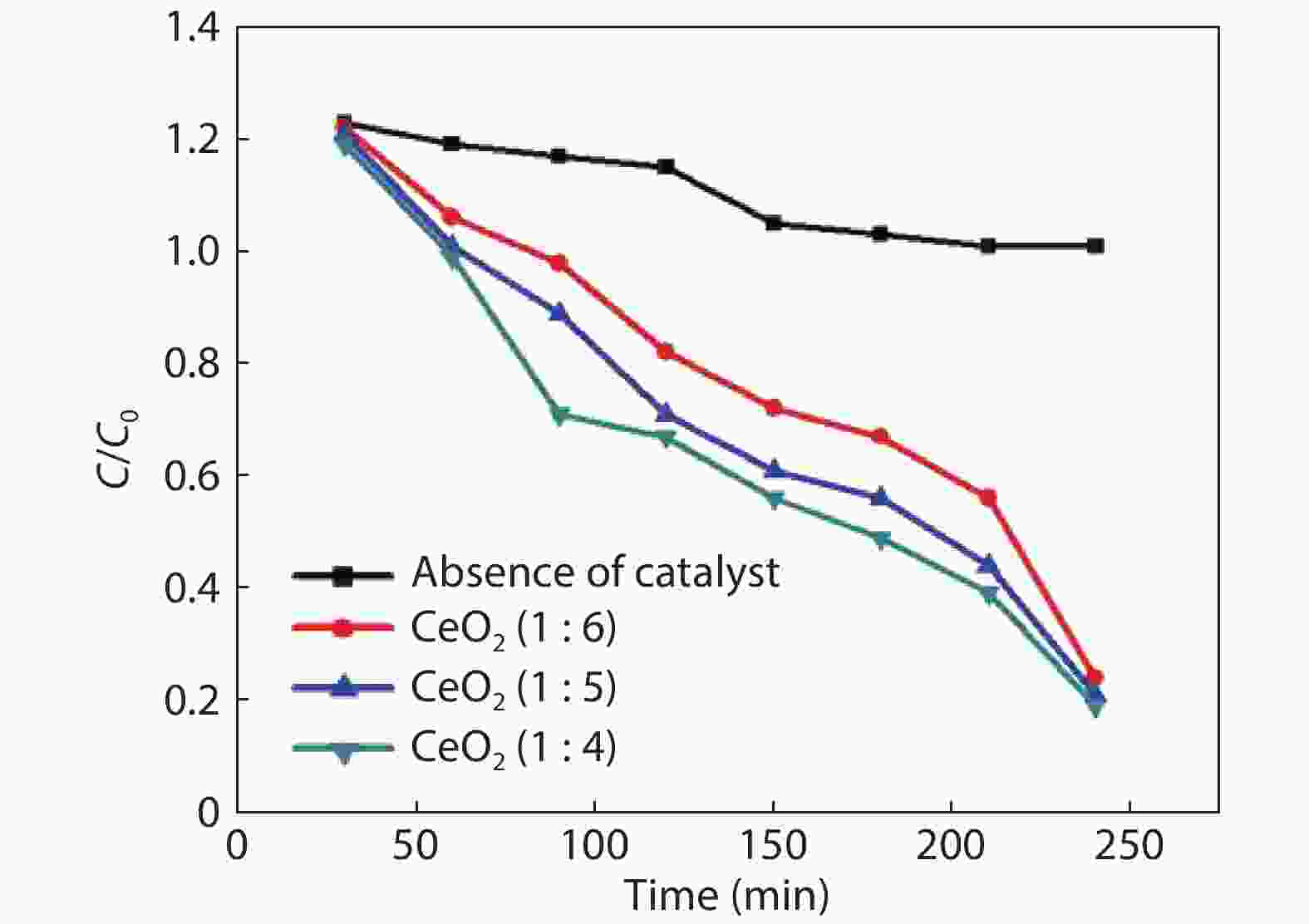
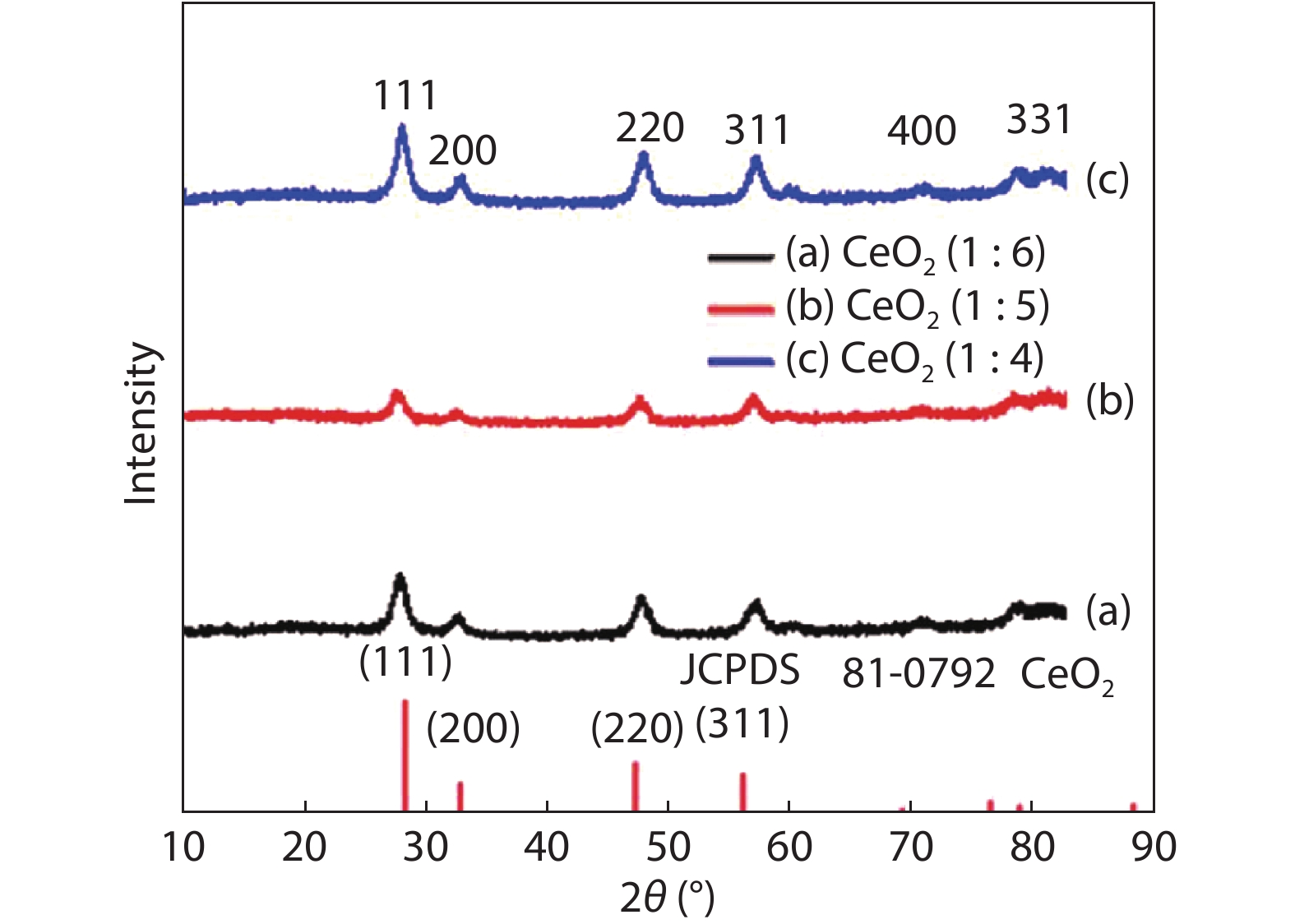
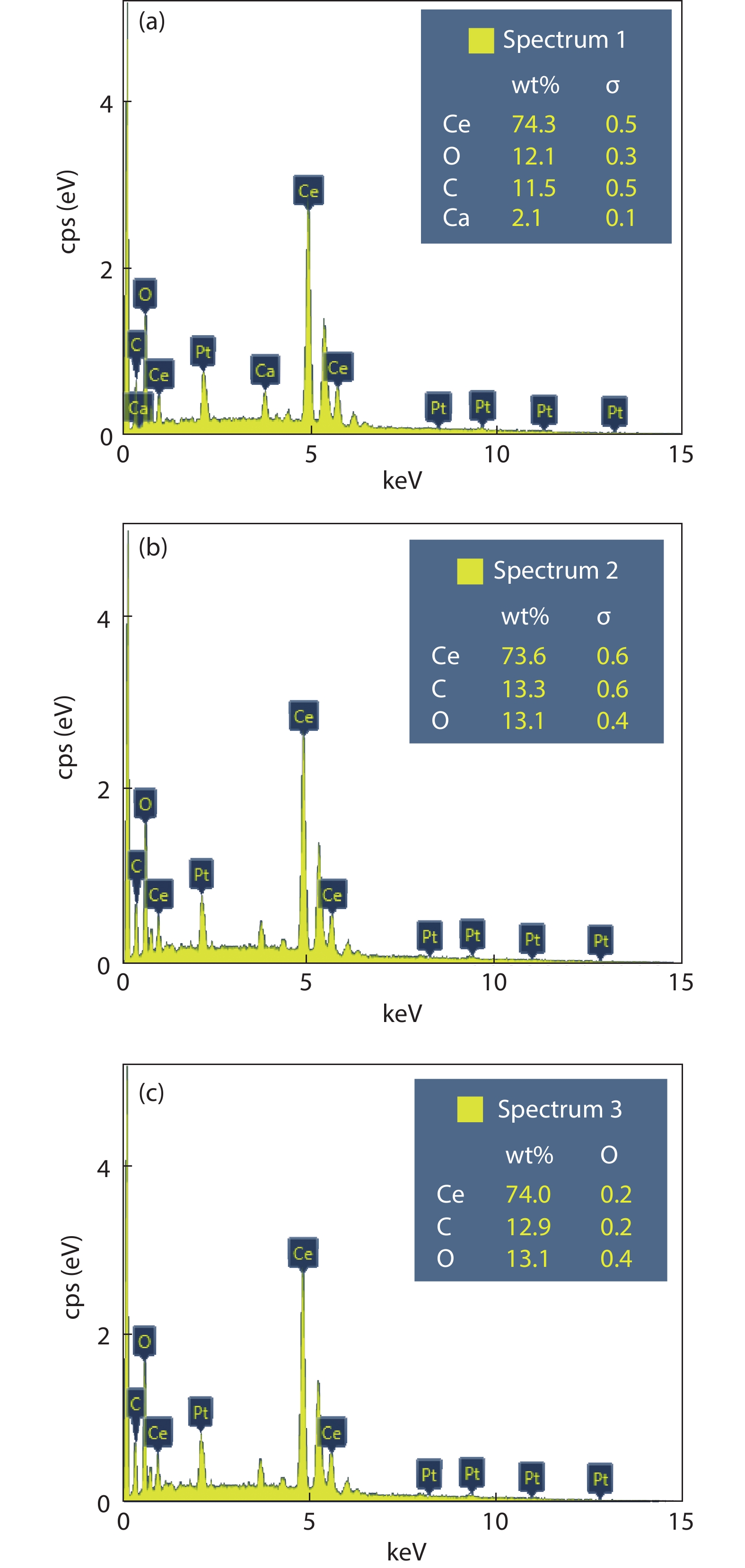
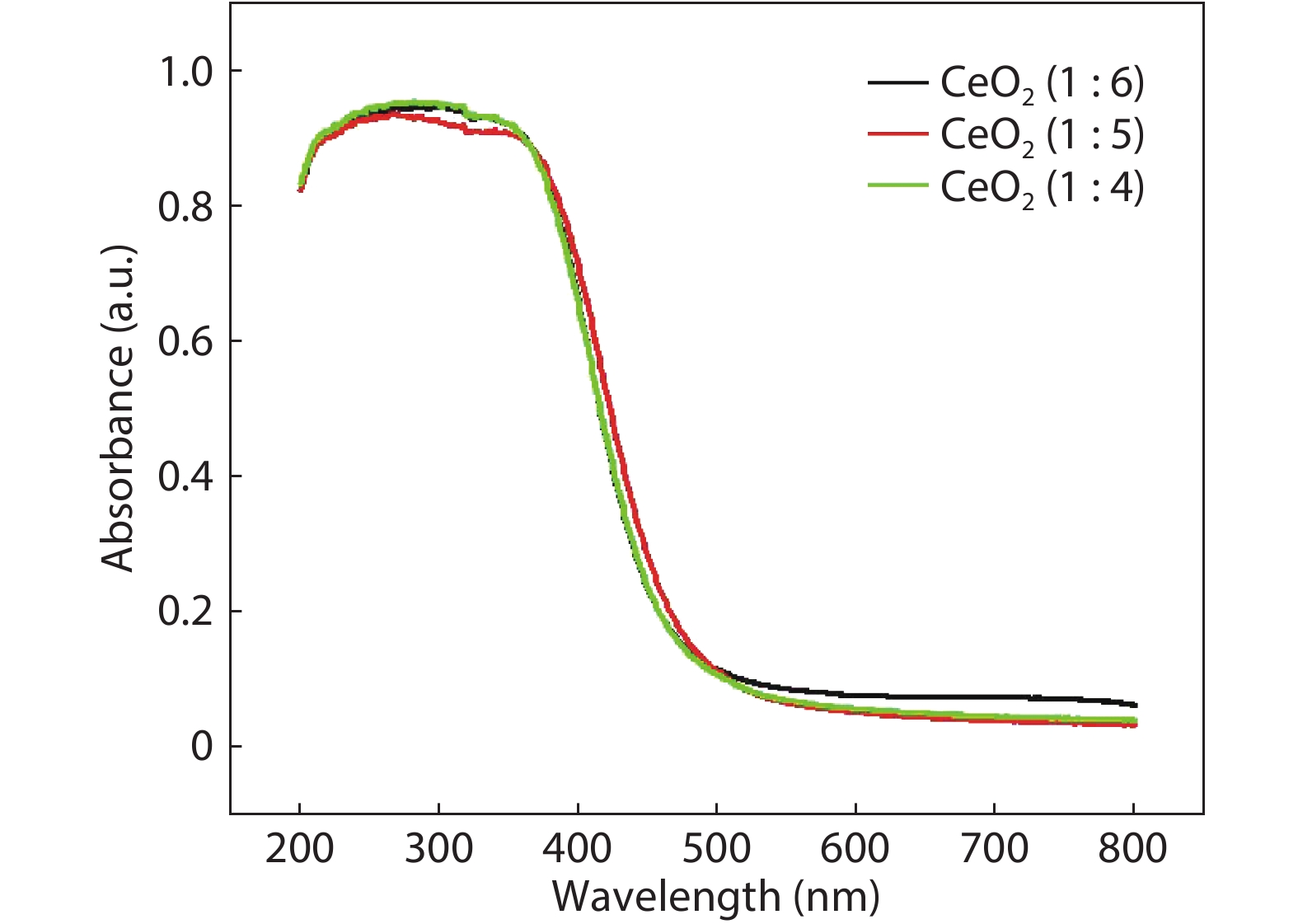
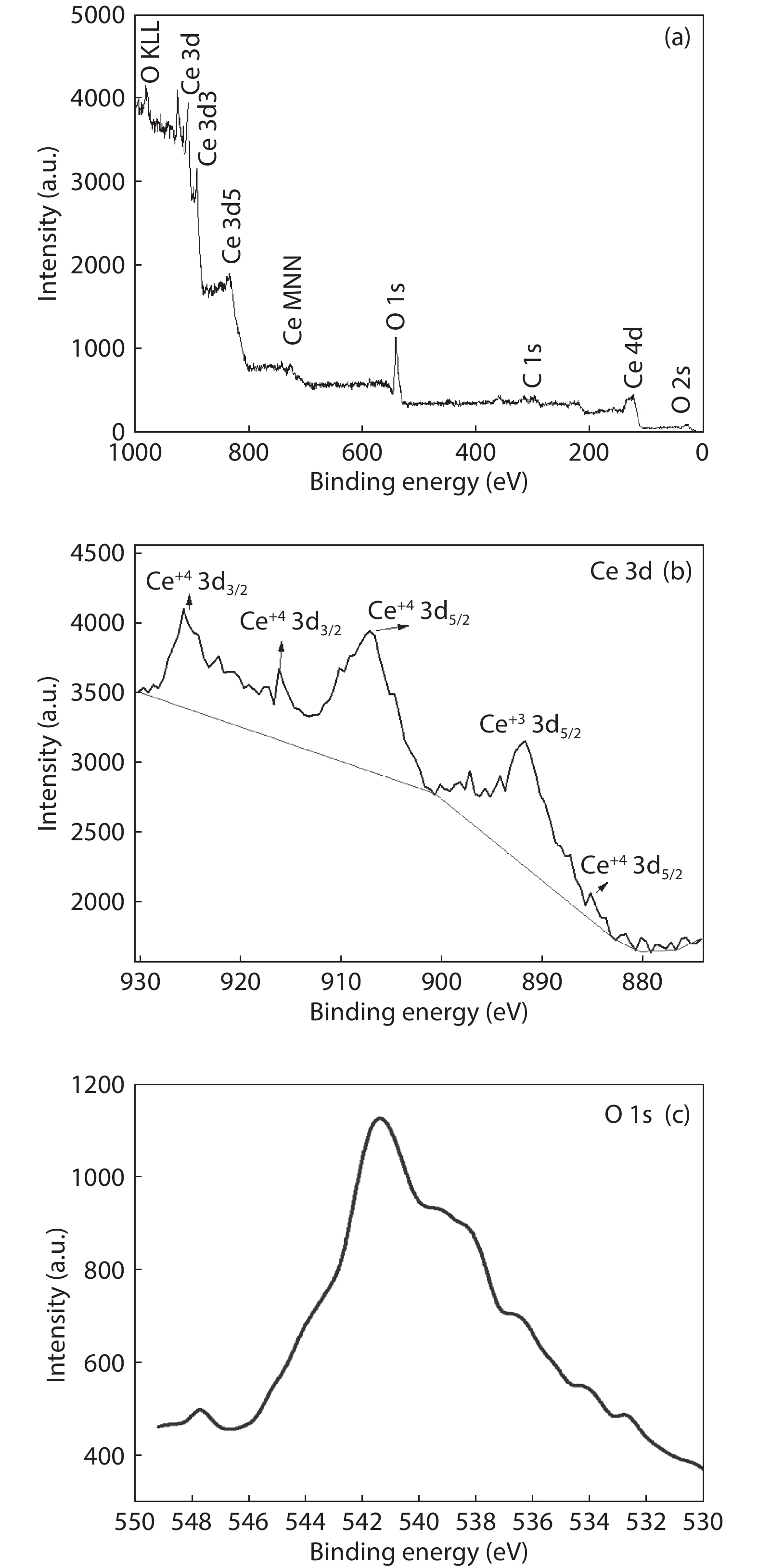
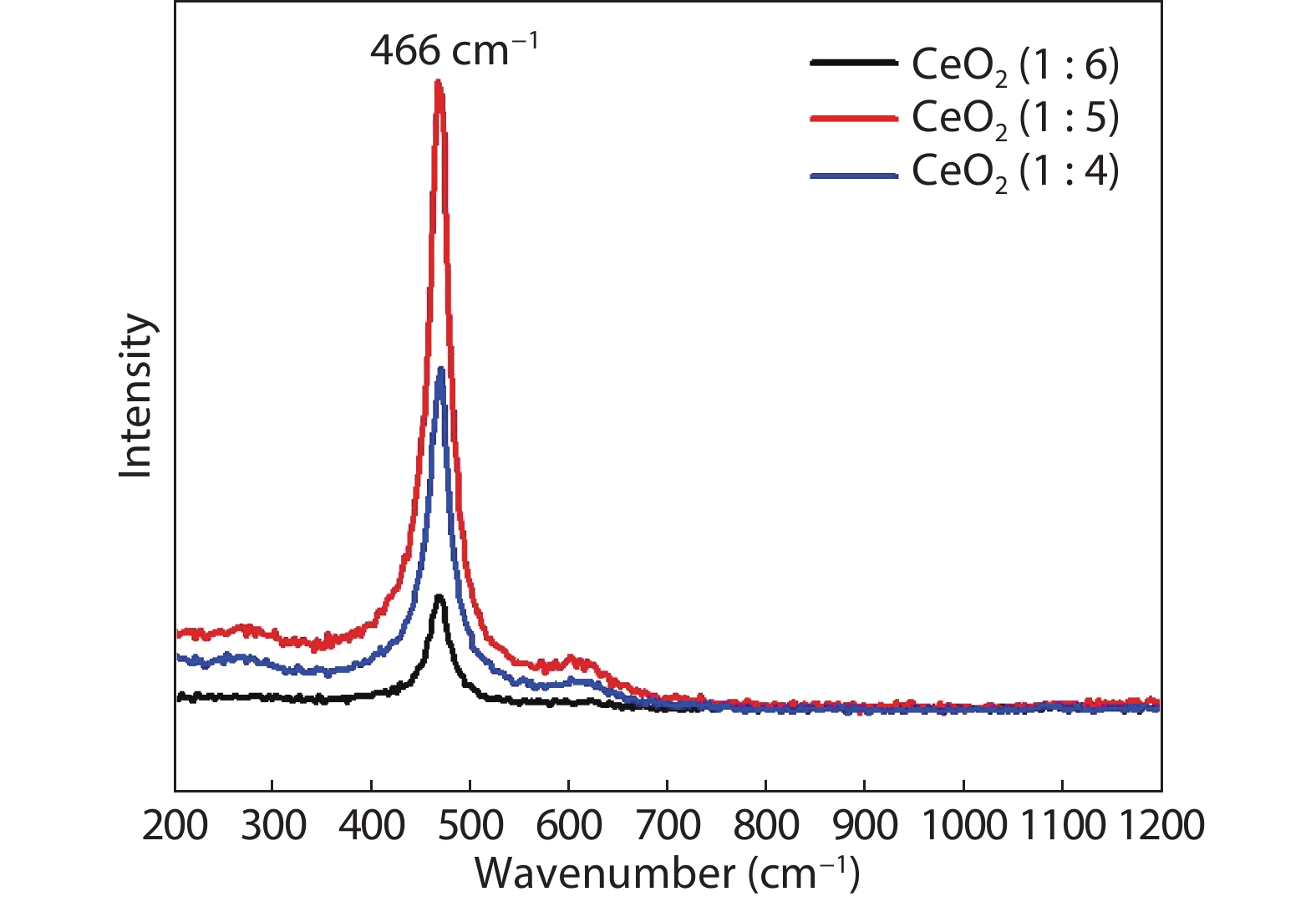
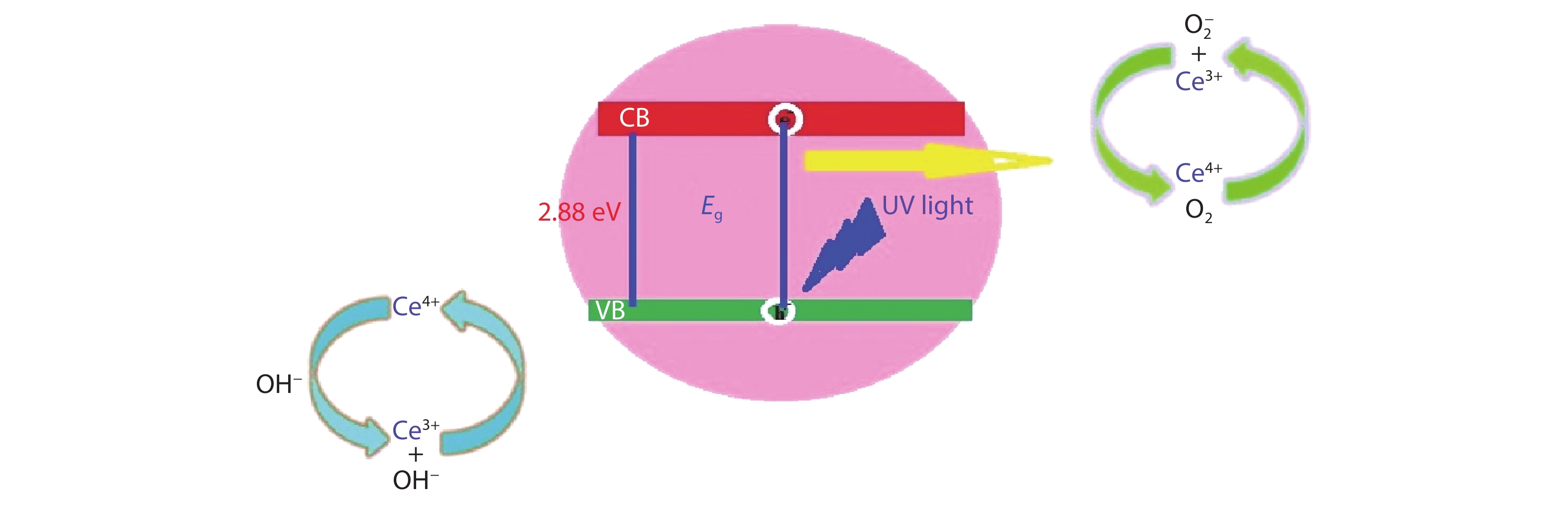
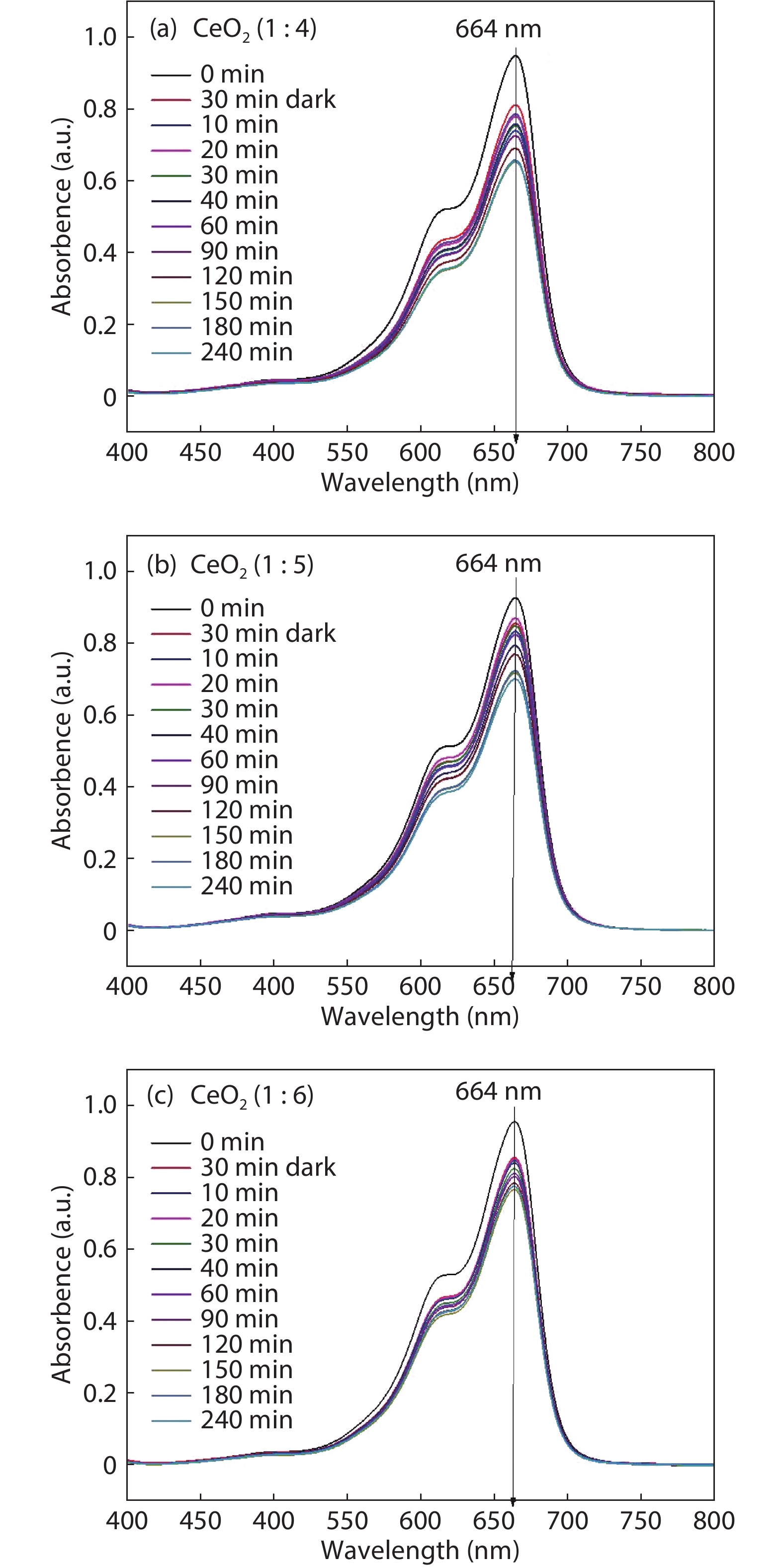
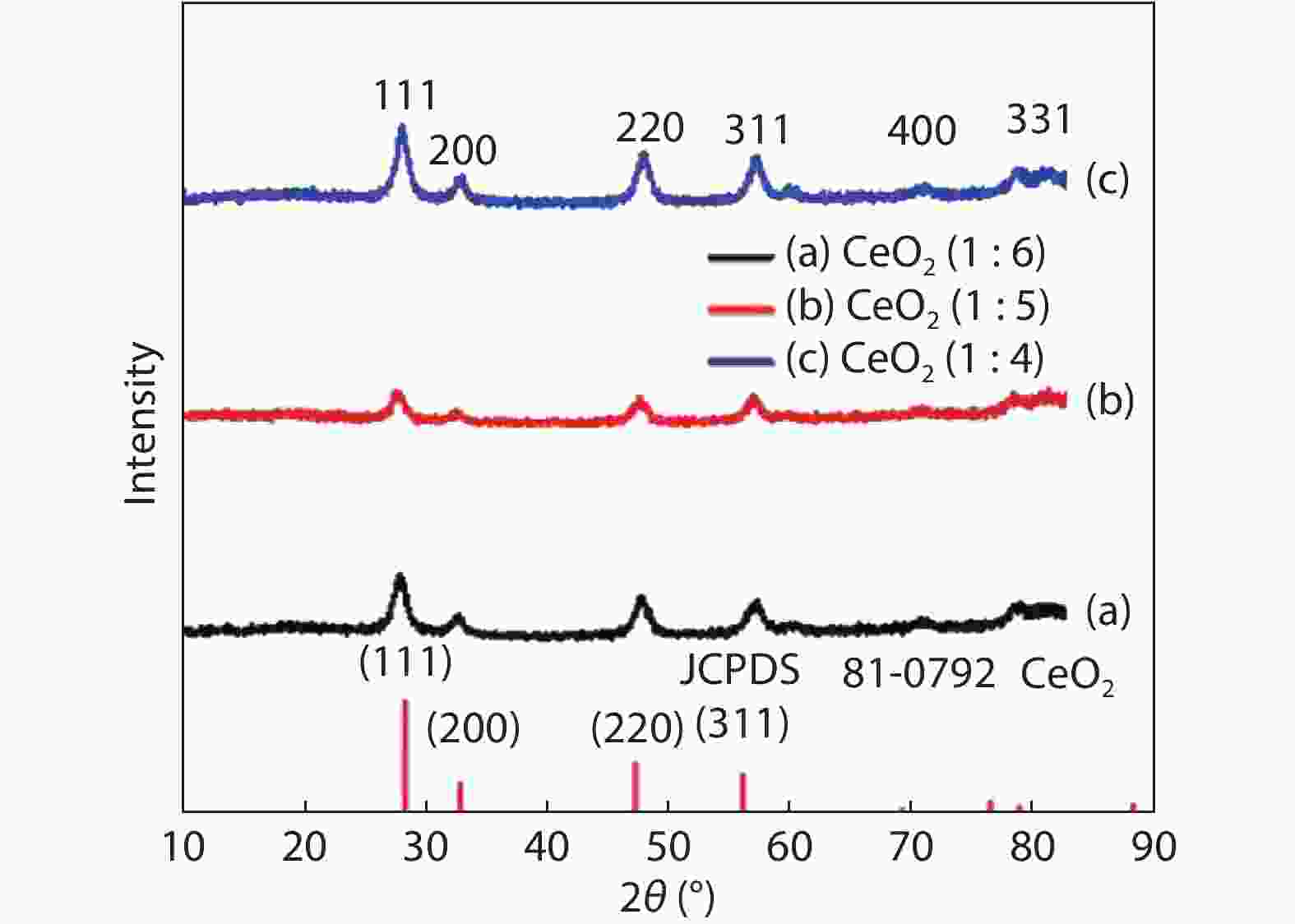
Crystalline cubic cerium oxide nano particles have been synthesized from cerium (III) nitrate (Ce (NO3)3.6H2O) and sodium hydroxide by a hydrothermal method. The effect of three different molar ratios of the NaOH precipitating agent on structural, optical, and photo catalytic activity was investigated. The synthesized cerium oxide nano particles were characterized by X-ray diffraction (XRD), a UV–vis spectrometer, scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDAX), Raman spectroscopy and X-ray photo electron spectroscopy (XPS). According to the findings, hydrothermally synthesized cerium oxide NPs have a high efficiency for photocatalytic degradation of methylene blue when exposed to UV light. Environmental water pollution is the major issue of the atmosphere. To get fresh water, humans could search the resources to purify the water in simple way and degradation is the one of the methods to purify salt water.-
Keywords:
- cerium oxide,
- nano particles,
- photo catalysis,
- hydrothermal process
-
References
[1] Mogensen M, Sammes N M, Tompsett G A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion, 2000, 129, 63 doi: 10.1016/S0167-2738(99)00318-5[2] Yashima M, Sasaki S, Yamaguchi Y, et al. Internal distortion in ZrO2-CeO2 solid solutions: Neutron and high-resolution synchrotron X-ray diffraction study. Appl Phys Lett, 1998, 72, 182 doi: 10.1063/1.120678[3] Feng X, Sayle D C, Wang Z L, et al. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science, 2006, 312, 1504 doi: 10.1126/science.1125767[4] Yamashita K. Hydrothermal synthesis and low temperature conduction properties of substituted ceria ceramics. Solid State Ion, 1995, 81, 53 doi: 10.1016/0167-2738(95)99031-H[5] Imanaka N, Masui T, Hirai H, et al. Amorphous cerium−titanium solid solution phosphate as a novel family of band gap tunable sunscreen materials. Chem Mater, 2003, 15, 2289 doi: 10.1021/cm034200w[6] Kubacka A, Fernández-García M, Colón G. Advanced nanoarchitectures for solar photocatalytic applications. Chem Rev, 2012, 112, 1555 doi: 10.1021/cr100454n[7] Yu J, Wang S, Low J, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys Chem Chem Phys, 2013, 15, 16883 doi: 10.1039/c3cp53131g[8] Miranda C, Mansilla H, Yáñez J, et al. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J Photochem Photobiol A, 2013, 253, 16 doi: 10.1016/j.jphotochem.2012.12.014[9] Muñoz-Batista M J, Kubacka A, Fernández-García M. Effect of g-C3N4 loading on TiO2-based photocatalysts: UV and visible degradation of toluene. Catal Sci Technol, 2014, 4, 2006 doi: 10.1039/c4cy00226a[10] Murrell L L, Tauster S J, Anderson D R. Laser Raman characterization of surface phase precious metal oxides formed on CeO2. Stud Surf Sci Catal, 1991, 71, 275 doi: 10.1016/S0167-2991(08)62984-5[11] Hussain K, Hussain T. Gold nanoparticles: A boon to drug delivery system. South Ind J Biol Sci, 2015, 1, 128 doi: 10.22205/sijbs/2015/v1/i3/100407[12] Ali F S, Qi K Z, Al Wahaibi B, et al. Photocatalytic degradation of bisphenol A in the presence of TiO2 nanoparticle: Effect of solvent on size control. Desal Water Treat, 2017, 79, 301 doi: 10.5004/dwt.2017.20808[13] Qi K Z, Selvaraj R, Al Fahdi T, et al. Enhanced photocatalytic activity of anatase-TiO2 nanoparticles by fullerene modification: A theoretical and experimental study. Appl Surf Sci, 2016, 387, 750 doi: 10.1016/j.apsusc.2016.06.134[14] Zhang Y F, Selvaraj R, Sillanpää M, et al. Enhanced solar photocatalytic activity of Er3+:YAlO3-loaded BiPO4 composite. J Ind Eng Chem, 2015, 24, 161 doi: 10.1016/j.jiec.2014.09.024[15] Sim J H, Umh H N, Shin H H, et al. Comparison of adsorptive features between silver ion and silver nanoparticles on nanoporous materials. J Ind Eng Chem, 2014, 20, 2864 doi: 10.1016/j.jiec.2013.11.019[16] Mokkelbost T, Kaus I, Grande T, et al. Combustion synthesis and characterization of nanocrystalline CeO2-based powders. Chem Mater, 2004, 16, 5489 doi: 10.1021/cm048583p[17] Maric R, Oljaca M, Vukasinovic B, et al. Synthesis of oxide nanopowders in nano spray diffusion flames. Mater Manuf Process, 2004, 19, 1143 doi: 10.1081/AMP-200035278[18] Kockrick E, Schrage C, Grigas A, et al. Synthesis and catalytic properties of microemulsion-derived cerium oxide nanoparticles. J Solid State Chem, 2008, 181, 1614 doi: 10.1016/j.jssc.2008.04.036[19] Mädler L, Stark W J, Pratsinis S E. Flame-made ceria nanoparticles. J Mater Res, 2002, 17, 1356 doi: 10.1557/JMR.2002.0202[20] Laberty-Robert C, Long J W, Lucas E M, et al. Sol–gel-derived ceria nanoarchitectures: Synthesis, characterization, and electrical properties. Chem Mater, 2006, 18, 50 doi: 10.1021/cm051385t[21] Cho M Y, Roh K C, Park S M, et al. Control of particle size and shape of precursors for ceria using ammonium carbonate as a precipitant. Mater Lett, 2010, 64, 323 doi: 10.1016/j.matlet.2009.11.004[22] Gulicovski J J, Milonjić S K, Szécsényi K M. Synthesis and characterization of stable aqueous ceria sols. Mater Manuf Process, 2009, 24, 1080 doi: 10.1080/10426910903032162[23] Hirano M, Inagaki M. Preparation of mono disperse cerium (IV) oxide particles by thermal hydrolysis. J Mater Chem, 2000, 10, 473 doi: 10.1039/a907510k[24] Cui R R, Lu W C, Zhang L M, et al. Template-free synthesis and self-assembly of CeO2 nanospheres fabricated with foursquare nanoflakes. J Phys Chem C, 2009, 113, 21520 doi: 10.1021/jp9065168[25] Athawale A A, Bapat M S, Desai P A. Hydroxide directed routes to synthesize nanosized cubic ceria (CeO2). J Alloys Compd, 2009, 484, 211 doi: 10.1016/j.jallcom.2009.03.125[26] Sahoo S K, Mohapatra M, Singh A K, et al. Hydrothermal synthesis of single crystalline nano CeO2 and its structural, optical, and electronic characterization. Mater Manuf Process, 2010, 25, 982 doi: 10.1080/10426914.2010.480995[27] Zhai Y Q, Zhang S Y, Pang H. Preparation, characterization and photocatalytic activity of CeO2 nanocrystalline using ammonium bicarbonate as precipitant. Mater Lett, 2007, 61, 1863 doi: 10.1016/j.matlet.2006.07.146[28] Zhou Y C, Rahaman M N. Hydrothermal synthesis and sintering of ultrafine CeO2 powders. J Mater Res, 1993, 8, 1680 doi: 10.1557/JMR.1993.1680[29] Nikolaou K. Emissions reduction of high and low polluting new technology vehicles equipped with a CeO2 catalytic system. Sci Total Environ, 1999, 235, 71 doi: 10.1016/S0048-9697(99)00191-6[30] Goharshadi E K, Samiee S, Nancarrow P. Fabrication of cerium oxide nanoparticles: Characterization and optical properties. J Colloid Interface Sci, 2011, 356, 473 doi: 10.1016/j.jcis.2011.01.063[31] Khan M M, Ansari S A, Pradhan D, et al. Defect-induced band gap narrowed CeO2 nanostructures for visible light activities. Ind Eng Chem Res, 2014, 53, 9754 doi: 10.1021/ie500986n[32] Zheng X G, Huang S, Yang D M, et al. Synthesis of X-architecture CeO2 for the photodegradation of methylene blue under UV-light irradiation. J Alloys Compd, 2017, 705, 131 doi: 10.1016/j.jallcom.2017.02.110[33] Li S J, Wang C C, Liu Y P, et al. Photocatalytic degradation of antibiotics using a novel Ag/Ag2S/Bi2MoO6 plasmonic p-n heterojunction photocatalyst: Mineralization activity, degradation pathways and boosted charge separation mechanism. Chem Eng J, 2021, 415, 128991 doi: 10.1016/j.cej.2021.128991[34] Li S J, Chen J L, Hu S W, et al. Facile construction of novel Bi2WO6/Ta3N5 Z-scheme heterojunction nanofibers for efficient degradation of harmful pharmaceutical pollutants. Chem Eng J, 2020, 402, 126165 doi: 10.1016/j.cej.2020.126165[35] Li S J, Xue B, Chen J L, et al. Constructing a plasmonic p-n heterojunction photocatalyst of 3D Ag/Ag6Si2O7/Bi2MoO6 for efficiently removing broad-spectrum antibiotics. Sep Purif Technol, 2021, 254, 117579 doi: 10.1016/j.seppur.2020.117579[36] Li S J, Hu S W, Jiang W, et al. In situ construction of WO3 nanoparticles decorated Bi2MoO6 microspheres for boosting photocatalytic degradation of refractory pollutants. J Colloid Interface Sci, 2019, 556, 335 doi: 10.1016/j.jcis.2019.08.077[37] Dong B, Li L Y, Dong Z F, et al. Fabrication of CeO2 nanorods for enhanced solar photocatalysts. Int J Hydrog Energy, 2018, 43, 5275 doi: 10.1016/j.ijhydene.2017.10.061[38] Wang L N, Meng F M, Li K K, et al. Characterization and optical properties of pole-like nano-CeO2 synthesized by a facile hydrothermal method. Appl Surf Sci, 2013, 286, 269 doi: 10.1016/j.apsusc.2013.09.067[39] Klug H P, Alexandef L E. X-ray diffraction procedures. New York: Wiley, 1974, 689.[40] Lin M, Fu Z Y, Tan H R, et al. Hydrothermal synthesis of CeO2 nanocrystals: Ostwald ripening or oriented attachment. Cryst Growth Des, 2012, 12, 3296 doi: 10.1021/cg300421x[41] Jawor-Baczynska A, Moore B D, Sefcik J. Effect of mixing, concentration and temperature on the formation of mesostructured solutions and their role in the nucleation of dl-valine crystals. Faraday Discuss, 2015, 179, 141 doi: 10.1039/C4FD00262H[42] Vekilov P G. The two-step mechanism of nucleation of crystals in solution. Nanoscale, 2010, 2, 2346 doi: 10.1039/c0nr00628a[43] Tauc J C. Amorphous and liquid semiconductors. London: Plenum Press 1974[44] Wang Z L, Quan Z W, Lin J. Remarkable changes in the optical properties of CeO2 nanocrystals induced by lanthanide ions doping. Inorg Chem, 2007, 46, 5237 doi: 10.1021/ic0701256[45] Wang X Y, Tian J J, Fei C B, et al. Rapid construction of TiO2 aggregates using microwave assisted synthesis and its application for dye-sensitized solar cells. RSC Adv, 2015, 5, 8622 doi: 10.1039/C4RA11266K[46] Lu F, Meng F M, Wang L N, et al. Morphology-selective synthesis method of nanopolyhedra and square-like CeO2 nanoparticles. Mater Lett, 2012, 73, 154 doi: 10.1016/j.matlet.2012.01.041[47] Li C R, Zhang X Q, Dong W J, et al. High photocatalytic activity material based on high-porosity ZnO/CeO2 nanofibers. Mater Lett, 2012, 80, 145 doi: 10.1016/j.matlet.2012.04.105[48] Li S N, Zhu H Q, Qin Z F, et al. Catalytic performance of gold supported on mn, Fe and Ni doped ceria in the preferential oxidation of CO in H2-rich stream. Catalysts, 2018, 8, 469 doi: 10.3390/catal8100469[49] Palmqvist A E C, Wirde M, Gelius U, et al. Surfaces of doped nanophase cerium oxide catalysts. Nanostruct Mater, 1999, 11, 995 doi: 10.1016/S0965-9773(00)00431-1[50] Weber W H, Hass K C, McBride J R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys Rev B, 1993, 48, 178 doi: 10.1103/PhysRevB.48.178[51] Lu X H, Huang X, Xie S L, et al. Facile electrochemical synthesis of single crystalline CeO2 octahedrons and their optical properties. Langmuir, 2010, 26, 7569 doi: 10.1021/la904882t[52] Wang S L, Xu M, Peng T Y, et al. Porous hypercrosslinked polymer-TiO2-graphene composite photocatalysts for visible-light-driven CO2 conversion. Nat Commun, 2019, 10, 676 doi: 10.1038/s41467-019-08651-x[53] Yang X F, Cui H Y, Li Y, et al. Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance. ACS Catal, 2013, 3, 363 doi: 10.1021/cs3008126[54] Chai R J, Li Y K, Zhang Q F, et al. Monolithic Ni-MOx/Ni-foam (M = Al, Zr or Y) catalysts with enhanced heat/mass transfer for energy-efficient catalytic oxy-methane reforming. Catal Commun, 2015, 70, 1 doi: 10.1016/j.catcom.2015.07.007[55] Solsona B, Concepción P, Hernández S, et al. Oxidative dehydrogenation of ethane over NiO-CeO2 mixed oxides catalysts. Catal Today, 2012, 180, 51 doi: 10.1016/j.cattod.2011.03.056[56] Zhang Z Q, Han L P, Chai R J, et al. Microstructured CeO2-NiO-Al2O3/Ni-foam catalyst for oxidative dehydrogenation of ethane to ethylene. Catal Commun, 2017, 88, 90 doi: 10.1016/j.catcom.2016.10.004[57] Kohantorabi M, Gholami M R. Kinetic analysis of the reduction of 4-nitrophenol catalyzed by CeO2 nanorods-supported CuNi nanoparticles. Ind Eng Chem Res, 2017, 56, 1159 doi: 10.1021/acs.iecr.6b04208[58] Liang Y, Chen Z, Yao W, et al. Decorating of Ag and CuO on Cu nanoparticles for enhanced high catalytic activity to the degradation of organic pollutants. Langmuir, 2017, 33, 7606 doi: 10.1021/acs.langmuir.7b01540[59] Park S, Park J, Selvaraj R, et al. Facile microwave-assisted synthesis of SnS2 nanoparticles for visible-light responsive photocatalyst. J Ind Eng Chem, 2015, 31, 269 doi: 10.1016/j.jiec.2015.06.036[60] Capodaglio A. Contaminants of emerging concern removal by high-energy oxidation-reduction processes: State of the art. Appl Sci, 2019, 9, 4562 doi: 10.3390/app9214562 -
Proportional views




 DownLoad:
DownLoad: