| Citation: |
Weiqin Wei, Shuxin Ouyang, Tierui Zhang. Perylene diimide self-assembly: From electronic structural modulation to photocatalytic applications[J]. Journal of Semiconductors, 2020, 41(9): 091708. doi: 10.1088/1674-4926/41/9/091708
****
W Q Wei, S X O yang, T R Zhang, Perylene diimide self-assembly: From electronic structural modulation to photocatalytic applications[J]. J. Semicond., 2020, 41(9): 091708. doi: 10.1088/1674-4926/41/9/091708.
|
Perylene diimide self-assembly: From electronic structural modulation to photocatalytic applications
DOI: 10.1088/1674-4926/41/9/091708
More Information
-
Abstract
As an emerging organic semiconductor, perylene diimide (PDI) self-assembly has attracted tremendous attention in the aspects of solar cells, sensors, fluorescence probes and n-transistors, etc. In term of photocatalysis, various photocatalysts based on PDI self-assembly exhibit some unique properties, such as intrinsic Π-Π stacking structure, fast internal charge transfer, band-like electronic structure, flexible structural modifiability, well-defined morphological adjustability and excellent light absorption. This paper mainly presents recent progress on PDI self-assembly regarding how to regulate the electronic structure of PDI self-assembly. In addition, the photocatalytic applications of PDI self-assembly and its complexes were reviewed, such as environmental remedy, energy productions, organic synthesis and photodynamic/photothermal therapy, further highlighting related photocatalytic mechanisms. Finally, the review contents and some perspectives on photocatalytic research of PDI self-assembly were summarized, and some key scientific problems were put forward to direct related photocatalytic research in future.-
Keywords:
- perylene diimide,
- self-assembly,
- photocatalysis,
- Π-Π stacking,
- electron transfer
-
References
[1] Bard A J. Photoelectrochemistry. Science, 1980, 207, 139 doi: 10.1126/science.207.4427.139[2] Hoffmann M R, Martin S T, Choi W, et al. Environmental applications of semiconductor photocatalysis. Chem Rev, 1995, 95, 69 doi: 10.1021/cr00033a004[3] Quay P D, Tilbrook B, Wong C S. Oceanic uptake of fossil fuel CO2: Carbon-13 evidence. Science, 1992, 256, 74 doi: 10.1126/science.256.5053.74[4] Gustafsson O, Krusa M, Zencak Z, et al. Brown clouds over south asia: biomass or fossil fuel combustion. Science, 2009, 323, 495 doi: 10.1126/science.1164857[5] Wigley T M L. Could reducing fossil-fuel emissions cause global warming. Nature, 1991, 349, 503 doi: 10.1038/349503a0[6] Tong H, Ouyang S, Bi Y P, et al. Nano-photocatalytic materials: Possibilities and challenges. Adv Mater, 2012, 24, 229 doi: 10.1002/adma.201102752[7] Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev, 2009, 38, 253 doi: 10.1039/B800489G[8] Wei W Q, Liu D, Wei Z, et al. Short-range π–π stacking assembly on P25 TiO2 nanoparticles for enhanced visible-light photocatalysis. ACS Catal, 2017, 7, 652 doi: 10.1021/acscatal.6b03064[9] Wei W Q, Wei Z, Liu D, et al. Enhanced visible-light photocatalysis via back-electron transfer from palladium quantum dots to perylene diimide. Appl Catal B, 2018, 230, 49 doi: 10.1016/j.apcatb.2018.02.032[10] Wei W Q, Zhu Y F. TiO2@perylene diimide full-spectrum photocatalysts via semi-core–shell structure. Small, 2019, 15, 1903933 doi: 10.1002/smll.201903933[11] Hu W, Lin L, Zhang R Q, et al. Highly efficient photocatalytic water splitting over edge-modified phosphorene nanoribbons. J Am Chem Soc, 2017, 139, 15429 doi: 10.1021/jacs.7b08474[12] Fujito H, Kunioku H, Kato D, et al. Layered perovskite oxychloride Bi4NbO8Cl: A stable visible light responsive photocatalyst for water splitting. ChemInform, 2016, 138, 2082 doi: 10.1002/chin.201626012[13] Wei Z, Liu M L, Zhang Z J, et al. Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energy Environ Sci, 2018, 11, 2581 doi: 10.1039/C8EE01316K[14] Zeng G T, Qiu J, Li Z, et al. CO2 reduction to methanol on TiO2-passivated GaP photocatalysts. ACS Catal, 2014, 4, 3512 doi: 10.1021/cs500697w[15] Li X, Yu J G, Jaroniec M, et al. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem Rev, 2019, 119, 3962 doi: 10.1021/acs.chemrev.8b00400[16] Zeng L, Liu T, He C, et al. Organized aggregation makes insoluble perylene diimide efficient for the reduction of aryl halides via consecutive visible light-induced electron-transfer processes. J Am Chem Soc, 2016, 138, 3958 doi: 10.1021/jacs.5b12931[17] Ghosh I, Ghosh T, Bardagi J I, et al. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science, 2014, 346, 725 doi: 10.1126/science.1258232[18] Robertson P K J, Robertson J M C, Bahnemann D W. Removal of microorganisms and their chemical metabolites from water using semiconductor photocatalysis. J Hazard Mater, 2012, 211/212, 161 doi: 10.1016/j.jhazmat.2011.11.058[19] Wang J, Liu D, Zhu Y F, et al. Supramolecular packing dominant photocatalytic oxidation and anticancer performance of PDI. Appl Catal B, 2018, 231, 251 doi: 10.1016/j.apcatb.2018.03.026[20] Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature, 1972, 238, 37 doi: 10.1038/238037a0[21] Chai Z G, Zeng T T, Li Q, et al. Efficient visible light-driven splitting of alcohols into hydrogen and corresponding carbonyl compounds over a Ni-modified CdS photocatalyst. J Am Chem Soc, 2016, 138, 10128 doi: 10.1021/jacs.6b06860[22] Hu J Q, Liu A L, Jin H L, et al. A versatile strategy for shish-kebab-like multi-heterostructured chalcogenides and enhanced photocatalytic hydrogen evolution. J Am Chem Soc, 2015, 137, 11004 doi: 10.1021/jacs.5b04784[23] Song H, Meng X G, Wang S Y, et al. Direct and selective photocatalytic oxidation of CH4 to oxygenates with O2 on cocatalysts/ZnO at room temperature in water. J Am Chem Soc, 2019, 141, 20507 doi: 10.1021/jacs.9b11440[24] He W W, Kim H K, Wamer W G, et al. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J Am Chem Soc, 2014, 136, 750 doi: 10.1021/ja410800y[25] Zhang K, Liu J L, Wang L Y, et al. Near-complete suppression of oxygen evolution for photoelectrochemical H2O oxidative H2O2 synthesis. J Am Chem Soc, 2020, 142, 8641 doi: 10.1021/jacs.9b13410[26] Yu Y Y, Ma K, Zhuang R, et al. Hydroxyl-mediated formation of highly dispersed SnO2/TiO2 heterojunction via pulsed chemical vapor deposition to enhance photocatalytic activity. Ind Eng Chem Res, 2019, 58, 14655 doi: 10.1021/acs.iecr.9b02360[27] Wang Y Y, Jiang W J, Luo W J, et al. Ultrathin nanosheets g-C3N4@Bi2WO6 core-shell structure via low temperature reassembled strategy to promote photocatalytic activity. Appl Catal B, 2018, 237, 633 doi: 10.1016/j.apcatb.2018.06.013[28] Yang J J, Chen D M, Zhu Y, et al. 3D–3D porous Bi2WO6/ graphene hydrogel composite with excellent synergistic effect of adsorption-enrichment and photocatalytic degradation. Appl Catal B, 2017, 205, 228 doi: 10.1016/j.apcatb.2016.12.035[29] Iwase A, Yoshino S, Takayama T, et al. Water splitting and CO2 reduction under visible light irradiation using Z-scheme systems consisting of metal sulfides, CoO x-loaded BiVO4, and a reduced graphene oxide electron mediator. J Am Chem Soc, 2016, 138, 10260 doi: 10.1021/jacs.6b05304[30] Zou Z G, Ye J H, Sayama K, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature, 2001, 414, 625 doi: 10.1038/414625a[31] Weng B, Qi M Y, Han C, et al. Photocorrosion inhibition of semiconductor-based photocatalysts: Basic principle, current development, and future perspective. ACS Catal, 2019, 9, 4642 doi: 10.1021/acscatal.9b00313[32] Ghosh S, Kouame N A, Ramos L, et al. Conducting polymer nanostructures for photocatalysis under visible light. Nat Mater, 2015, 14, 505 doi: 10.1038/nmat4220[33] Yang F X, Cheng S S, Zhang X T, et al. 2D organic materials for optoelectronic applications. Adv Mater, 2018, 30, 1702415 doi: 10.1002/adma.201702415[34] Cao S W, Low J, Yu J G, et al. Polymeric photocatalysts based on graphitic carbon nitride. Adv Mater, 2015, 27, 2150 doi: 10.1002/adma.201500033[35] Zhao N N, Yan L M, Zhao X Y, et al. Versatile types of organic/inorganic nanohybrids: From strategic design to biomedical applications. Chem Rev, 2019, 119, 1666 doi: 10.1021/acs.chemrev.8b00401[36] Li L L, Chen Y, Zhu J J. Recent advances in electrochemiluminescence analysis. Anal Chem, 2017, 89, 358 doi: 10.1021/acs.analchem.6b04675[37] Choudhuri I, Bhauriyal P, Pathak B. Recent advances in graphene-like 2D materials for spintronics applications. Chem Mater, 2019, 31, 8260 doi: 10.1021/acs.chemmater.9b02243[38] Niu W H, Yang Y. Graphitic carbon nitride for electrochemical energy conversion and storage. ACS Energy Lett, 2018, 3, 2796 doi: 10.1021/acsenergylett.8b01594[39] Meng Z, Stolz R M, Mendecki L, et al. Electrically-transduced chemical sensors based on two-dimensional nanomaterials. Chem Rev, 2019, 119, 478 doi: 10.1021/acs.chemrev.8b00311[40] Ong W J, Tan L L, Ng Y H, et al. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability. Chem Rev, 2016, 116, 7159 doi: 10.1021/acs.chemrev.6b00075[41] Wang Z H, Hu X, Liu Z Z, et al. Recent developments in polymeric carbon nitride-derived photocatalysts and electrocatalysts for nitrogen fixation. ACS Catal, 2019, 9, 10260 doi: 10.1021/acscatal.9b03015[42] Wang X C, Maeda K, Thomas A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater, 2009, 8, 76 doi: 10.1038/nmat2317[43] Takeda H, Kamiyama H, Okamoto K, et al. Highly efficient and robust photocatalytic systems for CO2 reduction consisting of a Cu(I) photosensitizer and Mn(I) catalysts. J Am Chem Soc, 2018, 140, 17241 doi: 10.1021/jacs.8b10619[44] Higgins R F, Fatur S M, Shepard S G, et al. Uncovering the roles of oxygen in Cr(III) photoredox catalysis. J Am Chem Soc, 2016, 138, 5451 doi: 10.1021/jacs.6b02723[45] Hong D C, Kawanishi T, Tsukakoshi Y, et al. Efficient photocatalytic CO2 reduction by a Ni(II) complex having pyridine pendants through capturing a Mg2+ ion as a lewis-acid cocatalyst. J Am Chem Soc, 2019, 141, 20309 doi: 10.1021/jacs.9b10597[46] Zhang D, Wu L Z, Zhou L, et al. Photocatalytic hydrogen production from hantzsch 1, 4-dihydropyridines by platinum(II) terpyridyl complexes in homogeneous solution. J Am Chem Soc, 2004, 126, 3440 doi: 10.1021/ja037631o[47] Fernández S, Franco F, Casadevall C, et al. A unified electro- and photocatalytic CO2 to CO reduction mechanism with aminopyridine cobalt complexes. J Am Chem Soc, 2020, 142, 120 doi: 10.1021/jacs.9b06633[48] Xu B, Troian-Gautier L, Dykstra R, et al. Photocatalyzed diastereoselective isomerization of cinnamyl chlorides to cyclopropanes. J Am Chem Soc, 2020, 142, 6206 doi: 10.1021/jacs.0c00147[49] Elvington M, Brown J, Arachchige S M, et al. Photocatalytic hydrogen production from water employing a Ru, Rh, Ru molecular device for photoinitiated electron collection. J Am Chem Soc, 2007, 129, 10644 doi: 10.1021/ja073123t[50] Cheung P L, Kapper S C, Zeng T, et al. Improving photocatalysis for the reduction of CO2 through non-covalent supramolecular assembly. J Am Chem Soc, 2019, 141, 14961 doi: 10.1021/jacs.9b07067[51] Rabe E J, Corp K L, Sobolewski A L, et al. Proton-coupled electron transfer from water to a model heptazine-based molecular photocatalyst. J Phys Chem Lett, 2018, 9, 6257 doi: 10.1021/acs.jpclett.8b02519[52] Wang C, Xie Z G, de Krafft K E, et al. Doping metal–organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J Am Chem Soc, 2011, 133, 13445 doi: 10.1021/ja203564w[53] Yang X J, Liang T, Sun J X, et al. Template-directed synthesis of photocatalyst-encapsulating metal-organic frameworks with boosted photocatalytic activity. ACS Catal, 2019, 9, 7486 doi: 10.1021/acscatal.9b01783[54] Chambers M B, Wang X, Ellezam L, et al. Maximizing the photocatalytic activity of metal–organic frameworks with aminated-functionalized linkers: Substoichiometric effects in MIL-125-NH2. J Am Chem Soc, 2017, 139, 8222 doi: 10.1021/jacs.7b02186[55] Wei P F, Qi M Z, Wang Z P, et al. Benzoxazole-linked ultrastable covalent organic frameworks for photocatalysis. J Am Chem Soc, 2018, 140, 4623 doi: 10.1021/jacs.8b00571[56] Wan Y, Wang L, Xu H, et al. A simple molecular design strategy for two-dimensional covalent organic framework capable of visible-light-driven water splitting. J Am Chem Soc, 2020, 149(9), 4508 doi: 10.1021/jacs.0c00564[57] Luo Q Z, Bao L L, Wang D S, et al. Preparation and strongly enhanced visible light photocatalytic activity of TiO2 nanoparticles modified by conjugated derivatives of polyisoprene. J Phys Chem C, 2012, 116, 25806 doi: 10.1021/jp308150j[58] Floresyona D, Goubard F, Aubert P H, et al. Highly active poly(3-hexylthiophene) nanostructures for photocatalysis under solar light. Appl Catal B, 2017, 209, 23 doi: 10.1016/j.apcatb.2017.02.069[59] Zhang M, Rouch W D, McCulla R D. Conjugated polymers as photoredox catalysts: Visible-light-driven reduction of aryl aldehydes by poly(p-phenylene). Eur J Org Chem, 2012, 2012, 6187 doi: 10.1002/ejoc.201200437[60] Muktha B, Madras G, Guru Row T N, et al. Conjugated polymers for photocatalysis. J Phys Chem B, 2007, 111, 7994 doi: 10.1021/jp071096n[61] Ghosh S, Mallik A K, Basu R N. Enhanced photocatalytic activity and photoresponse of poly(3, 4-ethylenedioxythiophene) nanofibers decorated with gold nanoparticle under visible light. Sol Energy, 2018, 159, 548 doi: 10.1016/j.solener.2017.11.036[62] Li L W, Cai Z X, Wu Q H, et al. Rational design of porous conjugated polymers and roles of residual palladium for photocatalytic hydrogen production. J Am Chem Soc, 2016, 138, 7681 doi: 10.1021/jacs.6b03472[63] Zhang Z J, Zhu Y F, Chen X J, et al. A full-spectrum metal-free porphyrin supramolecular photocatalyst for dual functions of highly efficient hydrogen and oxygen evolution. Adv Mater, 2019, 31, 1806626 doi: 10.1002/adma.201806626[64] Liu D, Wang J, Bai X J, et al. Self-assembled PDINH supramolecular system for photocatalysis under visible light. Adv Mater, 2016, 28, 7284 doi: 10.1002/adma.201601168[65] Wei Z, Hu J S, Zhu K J, et al. Self-assembled polymer phenylethnylcopper nanowires for photoelectrochemical and photocatalytic performance under visible light. Appl Catal B, 2018, 226, 616 doi: 10.1016/j.apcatb.2017.12.070[66] Würthner F, Saha-Möller C R, Fimmel B, et al. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem Rev, 2016, 116, 962 doi: 10.1021/acs.chemrev.5b00188[67] Zollinger H. Color chemistry. 3rd ed. Wiley-VCH: Weinheim, 2003[68] Wuerthner F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. ChemInform, 2004, 35, 1564 doi: 10.1002/chin.200430145[69] Saito G, Yoshida Y. Development of conductive organic molecular assemblies: Organic metals, superconductors, and exotic functional materials. ChemInform, 2007, 38, 1 doi: 10.1002/chin.200714259[70] Weingarten A S, Kazantsev R V, Palmer L C, et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat Chem, 2014, 6, 964 doi: 10.1038/nchem.2075[71] Ke D M, Zhan C L, Xu S P, et al. Self-assembled hollow nanospheres strongly enhance photoluminescence. J Am Chem Soc, 2011, 133, 11022 doi: 10.1021/ja202179t[72] Balakrishnan K, Datar A, Naddo T, et al. Effect of side-chain substituents on self-assembly of perylene diimide molecules: Morphology control. J Am Chem Soc, 2006, 128, 7390 doi: 10.1021/ja061810z[73] Bai S, Debnath S, Javid N, et al. Differential self-assembly and tunable emission of aromatic peptide bola-amphiphiles containing perylene bisimide in polar solvents including water. Langmuir, 2014, 30, 7576 doi: 10.1021/la501335e[74] Wang J, Shi W, Liu D, et al. Supramolecular organic nanofibers with highly efficient and stable visible light photooxidation performance. Appl Catal B, 2017, 202, 289 doi: 10.1016/j.apcatb.2016.09.037[75] Balakrishnan K, Datar A, Oitker R, et al. Nanobelt self-assembly from an organic n-type semiconductor: Propoxyethyl-PTCDI. J Am Chem Soc, 2005, 127, 10496 doi: 10.1021/ja052940v[76] Jonkheijm P, van der Schoot P, Schenning A P H J, et al. Probing the solvent-assisted nucleation pathway in chemical self-assembly. Science, 2006, 313, 80 doi: 10.1126/science.1127884[77] Gong Q Y, Xing J, Huang Y J, et al. Perylene diimide oligomer nanoparticles with ultrahigh photothermal conversion efficiency for cancer theranostics. ACS Appl Bio Mater, 2020, 3, 1607 doi: 10.1021/acsabm.9b01187[78] Zang L. Interfacial donor–acceptor engineering of nanofiber materials to achieve photoconductivity and applications. Acc Chem Res, 2015, 48, 2705 doi: 10.1021/acs.accounts.5b00176[79] Che Y K, Datar A, Yang X M, et al. Enhancing one-dimensional charge transport through intermolecular π-electron delocalization: Conductivity improvement for organic nanobelts. J Am Chem Soc, 2007, 129, 6354 doi: 10.1021/ja070164g[80] Zang L, Che Y K, Moore J S. One-dimensional self-assembly of planar π-conjugated molecules: Adaptable building blocks for organic nanodevices. Acc Chem Res, 2008, 41, 1596 doi: 10.1021/ar800030w[81] Miao H, Yang J, Peng G L, et al. Enhancement of the degradation ability for organic pollutants via the synergistic effect of photoelectrocatalysis on a self-assembled perylene diimide (SA-PDI) thin film. Sci Bull, 2019, 64, 896 doi: 10.1016/j.scib.2019.05.006[82] Yang J, Miao H, Wei Y X, et al. Π-Π Interaction between self-assembled perylene diimide and 3D graphene for excellent visible-light photocatalytic activity. Appl Catal B, 2019, 240, 225 doi: 10.1016/j.apcatb.2018.09.003[83] Yang J, Miao H, Li W L, et al. Designed synthesis of a p-Ag2S/n-PDI self-assembled supramolecular heterojunction for enhanced full-spectrum photocatalytic activity. J Mater Chem A, 2019, 7, 6482 doi: 10.1039/C9TA00580C[84] Miao H, Yang J, Wei Y X, et al. Visible-light photocatalysis of PDI nanowires enhanced by plasmonic effect of the gold nanoparticles. Appl Catal B, 2018, 239, 61 doi: 10.1016/j.apcatb.2018.08.009[85] Wei Y X, Ma M G, Li W L, et al. Enhanced photocatalytic activity of PTCDI-C60 via π-π interaction. Appl Catal B, 2018, 238, 302 doi: 10.1016/j.apcatb.2018.07.043[86] Zhang Z J, Wang J, Liu D, et al. Highly efficient organic photocatalyst with full visible light spectrum through π–π stacking of TCNQ–PTCDI. ACS Appl Mater Interfaces, 2016, 8, 30225 doi: 10.1021/acsami.6b10186[87] Chen S, Wang C, Bunes B R, et al. Enhancement of visible-light-driven photocatalytic H2 evolution from water over g-C3N4 through combination with perylene diimide aggregates. Appl Catal A, 2015, 498, 63 doi: 10.1016/j.apcata.2015.03.026[88] Chen S, Jacobs D L, Xu J K, et al. 1D nanofiber composites of perylene diimides for visible-light-driven hydrogen evolution from water. RSC Adv, 2014, 4, 48486 doi: 10.1039/C4RA09258A[89] Yang Z, Fan W P, Zou J H, et al. Precision cancer theranostic platform by in situ polymerization in perylene diimide-hybridized hollow mesoporous organosilica nanoparticles. J Am Chem Soc, 2019, 141, 14687 doi: 10.1021/jacs.9b06086[90] Stergiou A, Tagmatarchis N. Fluorene–perylene diimide arrays onto graphene sheets for photocatalysis. ACS Appl Mater Interfaces, 2016, 8, 21576 doi: 10.1021/acsami.6b06797[91] Chen S, Slattum P, Wang C Y, et al. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem Rev, 2015, 115, 11967 doi: 10.1021/acs.chemrev.5b00312[92] Huang C, Barlow S, Marder S R. Perylene-3, 4, 9, 10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J Org Chem, 2011, 76, 2386 doi: 10.1021/jo2001963[93] Peng H Q, Niu L Y, Chen Y Z, et al. Biological applications of supramolecular assemblies designed for excitation energy transfer. Chem Rev, 2015, 115, 7502 doi: 10.1021/cr5007057[94] Teo Y N, Kool E T. DNA-multichromophore systems. Chem Rev, 2012, 112, 4221 doi: 10.1021/cr100351g[95] Chen Z J, Debije M G, Debaerdemaeker T, et al. Tetrachloro-substituted perylene bisimide dyes as promising n-type organic semiconductors: Studies on structural, electrochemical and charge transport properties. ChemPhysChem, 2004, 5, 137 doi: 10.1002/cphc.200300882[96] Würthner F, Sautter A, Schilling J. Synthesis of diazadibenzoperylenes and characterization of their structural, optical, redox, and coordination properties. J Org Chem, 2002, 67, 3037 doi: 10.1021/jo011133l[97] Yan P, Chowdhury A, Holman M W, et al. Self-organized perylene diimide nanofibers. J Phys Chem B, 2005, 109, 724 doi: 10.1021/jp046133e[98] Che Y K, Datar A, Balakrishnan K, et al. Ultralong nanobelts self-assembled from an asymmetric perylene tetracarboxylic diimide. J Am Chem Soc, 2007, 129, 7234 doi: 10.1021/ja071903w[99] Che Y K, Huang H L, Xu M, et al. Interfacial engineering of organic nanofibril heterojunctions into highly photoconductive materials. J Am Chem Soc, 2011, 133, 1087 doi: 10.1021/ja109396g[100] Graser F, Hädicke E. Kristallstruktur und Farbe Bei Perylen-3, 4: 9, 10-bis(dicarboximid)-Pigmenten. Liebigs Ann Chem, 1980, 1980, 1994 doi: 10.1002/jlac.198019801210[101] Graser F, Hädike E. Kristallstruktur und Farbe Bei Perylen-3, 4: 9, 10-bis(dicarboximid)-Pigmenten, 2. Liebigs Ann Chem, 1984, 1984, 483 doi: 10.1002/jlac.198419840308[102] Struijk C W, Sieval A B, Dakhorst J E J, et al. Liquid crystalline perylene diimides: architecture and charge carrier mobilities. J Am Chem Soc, 2000, 122, 11057 doi: 10.1021/ja000991g[103] Datar A, Balakrishnan K, Yang X M, et al. Linearly polarized emission of an organic semiconductor nanobelt. J Phys Chem B, 2006, 110, 12327 doi: 10.1021/jp061739j[104] Yamagata H, Maxwell D S, Fan J, et al. HJ-aggregate behavior of crystalline 7, 8, 15, 16-tetraazaterrylene: Introducing a new design paradigm for organic materials. J Phys Chem C, 2014, 118, 28842 doi: 10.1021/jp509011u[105] Chen Y C, Lam J W Y, Kwok R T K, et al. Aggregation-induced emission: Fundamental understanding and future developments. Mater Horiz, 2019, 6, 428 doi: 10.1039/C8MH01331D[106] Che Y K, Yang X M, Liu G L, et al. Ultrathin n-type organic nanoribbons with high photoconductivity and application in optoelectronic vapor sensing of explosives. J Am Chem Soc, 2010, 132, 5743 doi: 10.1021/ja909797q[107] Rodler F, Schade B, Jäger C M, et al. Amphiphilic perylene–calix. J Am Chem Soc, 2015, 137, 3308 doi: 10.1021/ja512048t[108] Wang J L, Yu Y, Zhang L Z. Highly efficient photocatalytic removal of sodium pentachlorophenate with Bi3O4Br under visible light. Appl Catal B, 2013, 136/137, 112 doi: 10.1016/j.apcatb.2013.02.009[109] Liang Y M, Lan S Q, Deng P, et al. Regioregular and regioirregular poly(selenophene-perylene diimide) acceptors for polymer–polymer solar cells. ACS Appl Mater Interfaces, 2018, 10, 32397 doi: 10.1021/acsami.8b09061[110] Li X, Wang H, Nakayama H, et al. Multi-sulfur-annulated fused perylene diimides for organic solar cells with low open-circuit voltage loss. ACS Appl Energy Mater, 2019, 2, 3805 doi: 10.1021/acsaem.9b00492[111] Samanta S K, Song I, Yoo J H, et al. Organic n-channel transistors based on. ACS Appl Mater Interfaces, 2018, 10, 32444 doi: 10.1021/acsami.8b10831[112] Yang J, Yin Y, Chen F, et al. Comparison of three n-type copolymers based on benzodithiophene and naphthalene diimide/perylene diimide/fused perylene diimides for all-polymer solar cells application. ACS Appl Mater Interfaces, 2018, 10, 23263 doi: 10.1021/acsami.8b06306[113] Woodhouse M, Perkins C L, Rawls M T, et al. Non-conjugated polymers for organic photovoltaics: Physical and optoelectronic properties of poly(perylene diimides). J Phys Chem C, 2010, 114, 6784 doi: 10.1021/jp910738a[114] Zhang J, Li Y, Huang J, et al. Ring-fusion of perylene diimide acceptor enabling efficient nonfullerene organic solar cells with a small voltage loss. J Am Chem Soc, 2017, 139, 16092 doi: 10.1021/jacs.7b09998[115] An T C, An J B, Gao Y P, et al. Photocatalytic degradation and mineralization mechanism and toxicity assessment of antivirus drug acyclovir: Experimental and theoretical studies. Appl Catal B, 2015, 164, 279 doi: 10.1016/j.apcatb.2014.09.009[116] Iwase M, Yamada K, Kurisaki T, et al. Visible-light photocatalysis with phosphorus-doped titanium(IV) oxide particles prepared using a phosphide compound. Appl Catal B, 2013, 132/133, 39 doi: 10.1016/j.apcatb.2012.11.014[117] Kitano S, Hashimoto K, Kominami H. Photocatalytic degradation of 2-propanol over metal-ion-loaded titanium(IV) oxide under visible light irradiation: Effect of physical properties of nano-crystalline titanium(IV) oxide. Appl Catal B, 2011, 101, 206 doi: 10.1016/j.apcatb.2010.09.021[118] Li Q, Shang J K. Composite photocatalyst of nitrogen and fluorine codoped titanium oxide nanotube arrays with dispersed palladium oxide nanoparticles for enhanced visible light photocatalytic performance. Environ Sci Technol, 2010, 44, 3493 doi: 10.1021/es903928n[119] Shi Q, Murcia-López S, Tang P Y, et al. Role of tungsten doping on the surface states in BiVO4 photoanodes for water oxidation: Tuning the electron trapping process. ACS Catal, 2018, 8, 3331 doi: 10.1021/acscatal.7b04277[120] An L J, Onishi H. Electron–hole recombination controlled by metal doping sites in NaTaO3 photocatalysts. ACS Catal, 2015, 5, 3196 doi: 10.1021/acscatal.5b00484[121] Liu X, Gao S, Xu H, et al. Stable blue TiO2– x nanoparticles for efficient visible light photocatalysts. Nanoscale, 2013, 5, 1870 doi: 10.1039/c2nr33563h[122] Zhu Q, Peng Y, Lin L, et al. Green synthetic approach for Ti3+ self-doped TiO2– x nanoparticles with efficient visible light photocatalytic activity. J Mater Chem A, 2014, 2, 4429 doi: 10.1039/C3TA14484D[123] Huang H W, Zhou C, Jiao X C, et al. Subsurface defect engineering in single-unit-cell Bi2WO6 monolayers boosts solar-driven photocatalytic performance. ACS Catal, 2020, 10, 1439 doi: 10.1021/acscatal.9b04789[124] Jiang D, Wang W Z, Zhang L, et al. Insights into the surface-defect dependence of photoreactivity over CeO2 nanocrystals with well-defined crystal facets. ACS Catal, 2015, 5, 4851 doi: 10.1021/acscatal.5b01128[125] Cushing S K, Meng F K, Zhang J Y, et al. Effects of defects on photocatalytic activity of hydrogen-treated titanium oxide nanobelts. ACS Catal, 2017, 7, 1742 doi: 10.1021/acscatal.6b02177[126] Seybold G, Wagenblast G. New perylene and violanthrone dyestuffs for fluorescent collectors. Dye Pigment, 1989, 11, 303 doi: 10.1016/0143-7208(89)85048-X[127] Sadrai M, Hadel L, Sauers R R, et al. Lasing action in a family of perylene derivatives: Singlet absorption and emission spectra, triplet absorption and oxygen quenching constants, and molecular mechanics and semiempirical molecular orbital calculations. J Phys Chem, 1992, 96, 7988 doi: 10.1021/j100199a032[128] Ahrens M J, Fuller M J, Wasielewski M R. Cyanated perylene-3, 4-dicarboximides and perylene-3, 4: 9, 10-bis(dicarboximide): Facile chromophoric oxidants for organic photonics and electronics. Chem Mater, 2003, 15, 2684 doi: 10.1021/cm034140u[129] Zhao Y Y, Wasielewski M R. 3, 4: 9, 10-Perylenebis(dicarboximide) chromophores that function as both electron donors and acceptors. Tetrahedron Lett, 1999, 40, 7047 doi: 10.1016/S0040-4039(99)01468-9[130] Lukas A S, Zhao Y Y, Miller S E, et al. Biomimetic electron transfer using low energy excited states: A green perylene-based analogue of chlorophylla. J Phys Chem B, 2002, 106, 1299 doi: 10.1021/jp014073w[131] Yoshida J I, Kataoka K, Horcajada R, et al. Modern strategies in electroorganic synthesis. Chem Rev, 2008, 108, 2265 doi: 10.1021/cr0680843[132] Kingston C, Palkowitz M D, Takahira Y, et al. A survival guide for the "electro-curious". Acc Chem Res, 2020, 53, 72 doi: 10.1021/acs.accounts.9b00539[133] Ruffoni A, Juliá F, Svejstrup T D, et al. Practical and regioselective amination of arenes using alkyl amines. Nat Chem, 2019, 11, 426 doi: 10.1038/s41557-019-0254-5[134] Bariwal J, van der Eycken E. C–N bond forming cross-coupling reactions: An overview. Chem Soc Rev, 2013, 42, 9283 doi: 10.1039/c3cs60228a[135] Moeller K D. Synthetic applications of anodic electrochemistry. Tetrahedron, 2000, 56, 9527 doi: 10.1016/S0040-4020(00)00840-1[136] Yang Q L, Wang X Y, Lu J Y, et al. Copper-catalyzed electrochemical C–H amination of arenes with secondary amines. J Am Chem Soc, 2018, 140, 11487 doi: 10.1021/jacs.8b07380[137] Morofuji T, Shimizu A, Yoshida J I. Electrochemical C–H amination: Synthesis of aromatic primary amines viaN-arylpyridinium ions. J Am Chem Soc, 2013, 135, 5000 doi: 10.1021/ja402083e[138] Ham W S, Hillenbrand J, Jacq J, et al. Divergent late-stage (hetero)aryl C–H amination by the pyridinium radical cation. Angew Chem Int Ed, 2019, 58, 532 doi: 10.1002/anie.201810262[139] Hayashi R, Shimizu A, Yoshida J I. The stabilized cation pool method: Metal- and oxidant-free benzylic C–H/aromatic C–H cross-coupling. J Am Chem Soc, 2016, 138, 8400 doi: 10.1021/jacs.6b05273[140] Hou Z W, Mao Z Y, Melcamu Y Y, et al. Back cover: Electrochemical synthesis of imidazo-fused N-heteroaromatic compounds through a C–N bond-forming radical. Angew Chem Int Ed, 2018, 57, 1722 doi: 10.1002/anie.201800266[141] Hou Z W, Mao Z Y, Zhao H B, et al. Frontispiece: electrochemical C–H/N–H functionalization for the synthesis of highly functionalized (aza)indoles. Angew Chem Int Ed, 2016, 55, 9168 doi: 10.1002/anie.201683261[142] Waldvogel S R, Selt M. Electrochemical allylic oxidation of olefins: Sustainable and safe. Angew Chem Int Ed, 2016, 55, 12578 doi: 10.1002/anie.201606727[143] Jiang Y Y, Xu K, Zeng C C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem Rev, 2018, 118, 4485 doi: 10.1021/acs.chemrev.7b00271[144] Yan M, Kawamata Y, Baran P S. Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem Rev, 2017, 117, 13230 doi: 10.1021/acs.chemrev.7b00397[145] Jutand A. Contribution of electrochemistry to organometallic catalysis. Chem Rev, 2008, 108, 2300 doi: 10.1021/cr068072h[146] Feng R Z, Smith J A, Moeller K D. Anodic cyclization reactions and the mechanistic strategies that enable optimization. Acc Chem Res, 2017, 50, 2346 doi: 10.1021/acs.accounts.7b00287[147] Krieg E, Weissman H, Shimoni E, et al. Understanding the effect of fluorocarbons in aqueous supramolecular polymerization: Ultrastrong noncovalent binding and cooperativity. J Am Chem Soc, 2014, 136, 9443 doi: 10.1021/ja503906p[148] Zhao Q L, Zhang S, Liu Y, et al. Tetraphenylethenyl-modified perylene bisimide: Aggregation-induced red emission, electrochemical properties and ordered microstructures. J Mater Chem, 2012, 22, 7387 doi: 10.1039/c2jm16613e[149] Hendsbee A D, Sun J P, Law W K, et al. Synthesis, self-assembly, and solar cell performance of N-annulated perylene diimide non-fullerene acceptors. Chem Mater, 2016, 28, 7098 doi: 10.1021/acs.chemmater.6b03292[150] Li G, Zhao Y B, Li J B, et al. Synthesis, characterization, physical properties, and OLED application of single BN-fused perylene diimide. J Org Chem, 2015, 80, 196 doi: 10.1021/jo502296z[151] Seifert S, Schmidt D, Würthner F. An ambient stable core-substituted perylene bisimide dianion: Isolation and single crystal structure analysis. Chem Sci, 2015, 6, 1663 doi: 10.1039/C4SC03671A[152] Schuster N J, Joyce L A, Paley D W, et al. The structural origins of intense circular dichroism in a waggling helicene nanoribbon. J Am Chem Soc, 2020, 142, 7066 doi: 10.1021/jacs.0c00646[153] Lee S K, Zu Y B, Herrmann A, et al. Electrochemistry, spectroscopy and electrogenerated chemiluminescence of perylene, terrylene, and quaterrylene diimides in aprotic solution. J Am Chem Soc, 1999, 121, 3513 doi: 10.1021/ja984188m[154] Zhang A D, Jiang W, Wang Z H. Fulvalene-embedded perylene diimide and its stable radical anion. Angew Chem, 2020, 132, 762 doi: 10.1002/ange.201912536[155] Jones B A, Facchetti A, Wasielewski M R, et al. Tuning orbital energetics in arylene diimide semiconductors. materials design for ambient stability of n-type charge transport. J Am Chem Soc, 2007, 129, 15259 doi: 10.1021/ja075242e[156] Peurifoy S R, Castro E, Liu F, et al. Three-dimensional graphene nanostructures. J Am Chem Soc, 2018, 140, 9341 doi: 10.1021/jacs.8b04119[157] Gao G P, Liang N N, Geng H, et al. Spiro-fused perylene diimide arrays. J Am Chem Soc, 2017, 139, 15914 doi: 10.1021/jacs.7b09140[158] Liu B, Böckmann M, Jiang W, et al. Perylene diimide-embedded double. J Am Chem Soc, 2020, 142, 7092 doi: 10.1021/jacs.0c00954[159] Langhals H. Cyclic carboxylic imide structures as structure elements of high stability. Novel developments in perylene dye chemistry. Heterocycles, 1995, 1, 477 doi: 10.3987/REV-94-SR2[160] Wang W, Wang L Q, Palmer B J, et al. Cyclization and catenation directed by molecular self-assembly. J Am Chem Soc, 2006, 128, 11150 doi: 10.1021/ja061826p[161] Barendt T A, Ferreira L, Marques I, et al. Anion- and solvent-induced rotary dynamics and sensing in a perylene diimide. J Am Chem Soc, 2017, 139, 9026 doi: 10.1021/jacs.7b04295[162] Pochas C M, Kistler K A, Yamagata H, et al. Contrasting photophysical properties of star-shaped vs linear perylene diimide complexes. J Am Chem Soc, 2013, 135, 3056 doi: 10.1021/ja3087449[163] Wang J, Yang Z, Gao X X, et al. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl Catal B, 2017, 217, 169 doi: 10.1016/j.apcatb.2017.05.034[164] You C C, Würthner F. Self-assembly of ferrocene-functionalized perylene bisimide bridging ligands with Pt(II) corner to electrochemically active molecular squares. J Am Chem Soc, 2003, 125, 9716 doi: 10.1021/ja029648x[165] Delgado M C R, Kim E G, Filho D A D S, et al. Tuning the charge-transport parameters of perylene diimide single crystals via end and/or core functionalization: A density functional theory investigation. J Am Chem Soc, 2010, 132, 3375 doi: 10.1021/ja908173x[166] Kim Y J, Lee Y, Park K, et al. Hierarchical self-assembly of perylene diimide (PDI) crystals. J Phys Chem Lett, 2020, 11, 3934 doi: 10.1021/acs.jpclett.0c01226[167] Zhou E J, Cong J Z, Wei Q S, et al. Berichtigung: all-polymer solar cells from perylene diimide based copolymers: Material design and phase separation control. Angew Chem, 2011, 123, 8120 doi: 10.1002/ange.201104815[168] Luo Z H, Wu F, Zhang T, et al. Designing a perylene diimide/fullerene hybrid as effective electron transporting material in inverted perovskite solar cells with enhanced efficiency and stability. Angew Chem Int Ed, 2019, 58, 8520 doi: 10.1002/anie.201904195[169] Dössel L F, Kamm V, Howard I A, et al. Synthesis and controlled self-assembly of covalently linked hexa-peri-hexabenzocoronene/perylene diimide dyads as models to study fundamental energy and electron transfer processes. J Am Chem Soc, 2012, 134, 5876 doi: 10.1021/ja211504a[170] Jin S B, Supur M, Addicoat M, et al. Creation of superheterojunction polymers via direct polycondensation: Segregated and bicontinuous donor–acceptor π-columnar arrays in covalent organic frameworks for long-lived charge separation. J Am Chem Soc, 2015, 137, 7817 doi: 10.1021/jacs.5b03553[171] Prathapan S, Yang S I, Seth J, et al. Synthesis and excited-state photodynamics of perylene–porphyrin dyads. 1. parallel energy and charge transfer via a diphenylethyne linker. J Phys Chem B, 2001, 105, 8237 doi: 10.1021/jp010335i[172] O'Neil M P, Niemczyk M P, Svec W A, et al. Picosecond optical switching based on biphotonic excitation of an electron donor-acceptor-donor molecule. Science, 1992, 257, 63 doi: 10.1126/science.257.5066.63[173] van der Boom T, Hayes R T, Zhao Y Y, et al. Charge transport in photofunctional nanoparticles self-assembled from zinc 5, 10, 15, 20-tetrakis(perylenediimide)porphyrin building blocks. J Am Chem Soc, 2002, 124, 9582 doi: 10.1021/ja026286k[174] Baram J, Shirman E, Ben-Shitrit N, et al. Control over self-assembly through reversible charging of the aromatic building blocks in photofunctional supramolecular fibers. J Am Chem Soc, 2008, 130, 14966 doi: 10.1021/ja807027w[175] Jung C, Müller B K, Lamb D C, et al. A new photostable terrylene diimide dye for applications in single molecule studies and membrane labeling. J Am Chem Soc, 2006, 128, 5283 doi: 10.1021/ja0588104[176] Marcon V, Breiby D W, Pisula W, et al. Understanding structure–mobility relations for perylene tetracarboxydiimide derivatives. J Am Chem Soc, 2009, 131, 11426 doi: 10.1021/ja900963v[177] Dsouza R N, Pischel U, Nau W M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem Rev, 2011, 111, 7941 doi: 10.1021/cr200213s[178] Yin X M, Wang Q X, Zheng Y J, et al. Molecular alignment and electronic structure of N, N'-dibutyl-3, 4, 9, 10-perylene-tetracarboxylic-diimide molecules on MoS2 surfaces. ACS Appl Mater Interfaces, 2017, 9, 5566 doi: 10.1021/acsami.6b14000[179] Gigli L, Arletti R, Tabacchi G, et al. Structure and host-guest interactions of perylene-diimide dyes in zeolite L nanochannels. J Phys Chem C, 2018, 122, 3401 doi: 10.1021/acs.jpcc.7b10607[180] Liu N, Shi M M, Pan X W, et al. Photoinduced electron transfer and enhancement of photoconductivity in silicon nanoparticles/perylene diimide composites in a polymer matrix. J Phys Chem C, 2008, 112, 15865 doi: 10.1021/jp802385g[181] Xie A F, Liu B, Hall J E, et al. Self-assembled photoactive polyelectrolyte/perylene-diimide composites. Langmuir, 2005, 21, 4149 doi: 10.1021/la0471700[182] Gosztola D, Niemczyk M P, Svec W, et al. Excited doublet states of electrochemically generated aromatic imide and diimide radical anions. J Phys Chem A, 2000, 104, 6545 doi: 10.1021/jp000706f[183] Adegoke O O, Jung I H, Orr M, et al. Effect of acceptor strength on optical and electronic properties in conjugated polymers for solar applications. J Am Chem Soc, 2015, 137, 5759 doi: 10.1021/ja513002h[184] Shoaee S, Clarke T M, Huang C, et al. Acceptor energy level control of charge photogeneration in organic donor/acceptor blends. J Am Chem Soc, 2010, 132, 12919 doi: 10.1021/ja1042726[185] Dubey R K, Niemi M, Kaunisto K, et al. Direct evidence of significantly different chemical behavior and excited-state dynamics of 1, 7- and 1, 6-regioisomers of pyrrolidinyl-substituted perylene diimide. Chem Eur J, 2013, 19, 6791 doi: 10.1002/chem.201203387[186] Ryan S T, Young R M, Henkelis J J , et al. Energy and electron transfer dynamics within a series of perylene diimide/cyclophane systems. J Am Chem Soc, 2015, 137, 15299 doi: 10.1021/jacs.5b10329[187] Ramos A M, Beckers E H A, Offermans T, et al. Photoinduced multistep electron transfer in an oligoaniline–oligo(p-phenylene vinylene)–perylene diimide molecular array. J Phys Chem A, 2004, 108, 8201 doi: 10.1021/jp048971e[188] Ryan S T J, del Barrio J, Ghosh I, et al. Efficient host–guest energy transfer in polycationic cyclophane–perylene diimide complexes in water. J Am Chem Soc, 2014, 136, 9053 doi: 10.1021/ja5032437[189] Santos E R D, Pina J, Venâncio T, et al. Photoinduced energy and electron-transfer reactions by polypyridine ruthenium(II) complexes containing a derivatized perylene diimide. J Phys Chem C, 2016, 120, 22831 doi: 10.1021/acs.jpcc.6b06693[190] Song Y, Zhang W, He S J, et al. Perylene diimide and luminol as potential-resolved electrochemiluminescence nanoprobes for dual targets immunoassay at low potential. ACS Appl Mater Interfaces, 2019, 11, 33676 doi: 10.1021/acsami.9b11416[191] Huang Y W, Fu L N, Zou W J, et al. Ammonia sensory properties based on single-crystalline micro/nanostructures of perylenediimide derivatives: Core-substituted effect. J Phys Chem C, 2011, 115, 10399 doi: 10.1021/jp200735m[192] Che Y K, Yang X M, Loser S, et al. Expedient vapor probing of organic amines using fluorescent nanofibers fabricated from an n-type organic semiconductor. Nano Lett, 2008, 8, 2219 doi: 10.1021/nl080761g[193] Schuster N J, Paley D W, Jockusch S, et al. Electron delocalization in perylene diimide helicenes. Angew Chem Int Ed, 2016, 55, 13519 doi: 10.1002/anie.201607878[194] Ball M L, Zhang B Y, Xu Q Z, et al. Influence of molecular conformation on electron transport in giant, conjugated macrocycles. J Am Chem Soc, 2018, 140, 10135 doi: 10.1021/jacs.8b06565[195] Malenfant P R L, Dimitrakopoulos C D, Gelorme J D, et al. N-type organic thin-film transistor with high field-effect mobility based on a N, N'-dialkyl-3, 4, 9, 10-perylene tetracarboxylic diimide derivative. Appl Phys Lett, 2002, 80, 2517 doi: 10.1063/1.1467706[196] Wang X Y, Meng J Q, Yang X, et al. Fabrication of a perylene tetracarboxylic diimide–graphitic carbon nitride heterojunction photocatalyst for efficient degradation of aqueous organic pollutants. ACS Appl Mater Interfaces, 2019, 11, 588 doi: 10.1021/acsami.8b15122[197] Liu W Q, Bobbala S, Stern C L, et al. XCage: A tricyclic octacationic receptor for perylene diimide with picomolar affinity in water. J Am Chem Soc, 2020, 142, 3165 doi: 10.1021/jacs.9b12982[198] Zhang Q C, Jiang L, Wang J, et al. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl Catal B, 2020, 277, 119122 doi: 10.1016/j.apcatb.2020.119122[199] Chen P, Blaney L, Cagnetta G, et al. Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis. Environ Sci Technol, 2019, 53, 1564 doi: 10.1021/acs.est.8b05827[200] Guldi D M. Fullerene–porphyrin architectures; photosynthetic antenna and reaction center models. Chem Soc Rev, 2002, 31, 22 doi: 10.1039/b106962b[201] Liu L, Yue M T, Lu J R, et al. The enrichment of photo-catalysis via self-assembly perylenetetracarboxylic acid diimide polymer nanostructures incorporating TiO2 nano-particles. Appl Surf Sci, 2018, 456, 645 doi: 10.1016/j.apsusc.2018.06.183[202] Araújo R F, Silva C J R, Paiva M C, et al. Efficient dispersion of multi-walled carbon nanotubes in aqueous solution by non-covalent interaction with perylene bisimides. RSC Adv, 2013, 3, 24535 doi: 10.1039/c3ra42422g[203] Liu Y, Zhu E W, Bian L Y, et al. Robust graphene dispersion with amphiphlic perylene-polyglycidol. Mater Lett, 2014, 118, 188 doi: 10.1016/j.matlet.2013.12.073[204] Oelsner C, Schmidt C, Hauke F, et al. Interfacing strong electron acceptors with single wall carbon nanotubes. J Am Chem Soc, 2011, 133, 4580 doi: 10.1021/ja1108744[205] Tsarfati Y, Strauss V, Kuhri S, et al. Dispersing perylene diimide/SWCNT hybrids: Structural insights at the molecular level and fabricating advanced materials. J Am Chem Soc, 2015, 137, 7429 doi: 10.1021/jacs.5b03167[206] Zhang K, Wang J, Jiang W J, et al. Self-assembled perylene diimide based supramolecular heterojunction with Bi2WO6 for efficient visible-light-driven photocatalysis. Appl Catal B, 2018, 232, 175 doi: 10.1016/j.apcatb.2018.03.059[207] Ji Q Y, Xu Z, Xiang W M, et al. Enhancing the performance of pollution degradation through secondary self-assembled composite supramolecular heterojunction photocatalyst BiOCl/PDI under visible light irradiation. Chemosphere, 2020, 253, 126751 doi: 10.1016/j.chemosphere.2020.126751[208] Gao Q Z, Xu J, Wang Z P, et al. Enhanced visible photocatalytic oxidation activity of perylene diimide/g-C3N4 n–n heterojunction via π–π interaction and interfacial charge separation. Appl Catal B, 2020, 271, 118933 doi: 10.1016/j.apcatb.2020.118933[209] Wang H L, Zhao L L, Liu X Q, et al. Novel hydrogen bonding composite based on copper phthalocyanine/perylene diimide derivatives p–n heterojunction with improved photocatalytic activity. Dye Pigment, 2017, 137, 322 doi: 10.1016/j.dyepig.2016.11.014[210] Zeng W G, Cai T, Liu Y T, et al. An artificial organic-inorganic Z-scheme photocatalyst WO3@Cu@PDI supramolecular with excellent visible light absorption and photocatalytic activity. Chem Eng J, 2020, 381, 122691 doi: 10.1016/j.cej.2019.122691[211] Cheng Y, Song R Q, Wu K, et al. The enhanced visible-light-driven antibacterial performances of PTCDI-PANI(Fe(III)-doped) heterostructure. J Hazard Mater, 2020, 383, 121166 doi: 10.1016/j.jhazmat.2019.121166[212] Gao X M, Gao K L, Li X B, et al. Hybrid PDI/BiOCl heterojunction with enhanced interfacial charge transfer for a full-spectrum photocatalytic degradation of pollutants. Catal Sci Technol, 2020, 10, 372 doi: 10.1039/C9CY01722D[213] Jeon T H, Koo M S, Kim H, et al. Dual-functional photocatalytic and photoelectrocatalytic systems for energy- and resource-recovering water treatment. ACS Catal, 2018, 8, 11542 doi: 10.1021/acscatal.8b03521[214] Ding C M, Shi J Y, Wang Z L, et al. Photoelectrocatalytic water splitting: Significance of cocatalysts, electrolyte, and interfaces. ACS Catal, 2017, 7, 675 doi: 10.1021/acscatal.6b03107[215] Brereton K R, Bonn A G, Miller A J M. Molecular photoelectrocatalysts for light-driven hydrogen production. ACS Energy Lett, 2018, 3, 1128 doi: 10.1021/acsenergylett.8b00255[216] Sheng Y Q, Miao H, Jing J F, et al. Perylene diimide anchored graphene 3D structure via π–π interaction for enhanced photoelectrochemical degradation performances. Appl Catal B, 2020, 272, 118897 doi: 10.1016/j.apcatb.2020.118897[217] Kirner J T, Stracke J J, Gregg B A, et al. Visible-light-assisted photoelectrochemical water oxidation by thin films of a phosphonate-functionalized perylene diimide plus CoO x cocatalyst. ACS Appl Mater Interfaces, 2014, 6, 13367 doi: 10.1021/am405598w[218] Kirner J T, Finke R G. Sensitization of nanocrystalline metal oxides with a phosphonate-functionalized perylene diimide for photoelectrochemical water oxidation with a CoO x catalyst. ACS Appl Mater Interfaces, 2017, 9, 27625 doi: 10.1021/acsami.7b05874[219] Linkous C A, Slattery D K. Solar photocatalytic hydrogen production from water using a dual bed photosystem-phase I final report and phase II proposal. Office of Scientific and Technical Information (OSTI), 2000[220] Kunz V, Stepanenko V, Würthner F. Embedding of a ruthenium(ii) water oxidation catalyst into nanofibers via self-assembly. Chem Commun, 2015, 51, 290 doi: 10.1039/C4CC08314H[221] Li J X, Li Z J, Ye C, et al. Visible light-induced photochemical oxygen evolution from water by 3, 4, 9, 10-perylenetetracarboxylic dianhydride nanorods as an n-type organic semiconductor. Catal Sci Technol, 2016, 6, 672 doi: 10.1039/C5CY01570G[222] Zhong Z, Li R F, Lin W L, et al. One-dimensional nanocrystals of cobalt perylene diimide polymer with in situ generated FeOOH for efficient photocatalytic water oxidation. Appl Catal B, 2020, 260, 118135 doi: 10.1016/j.apcatb.2019.118135[223] Zheng R J, Zhang M, Sun X, et al. Perylene-3, 4, 9, 10-tetracarboxylic acid accelerated light-driven water oxidation on ultrathin indium oxide porous sheets. Appl Catal B, 2019, 254, 667 doi: 10.1016/j.apcatb.2019.05.003[224] Vagnini M T, Smeigh A L, Blakemore J D, et al. Ultrafast photodriven intramolecular electron transfer from an iridium-based water-oxidation catalyst to perylene diimide derivatives. PNAS, 2012, 109, 15651 doi: 10.1073/pnas.1202075109[225] Chen S, Li Y X, Wang C Y. Visible-light-driven photocatalytic H2 evolution from aqueous suspensions of perylene diimide dye-sensitized Pt/TiO2 catalysts. RSC Adv, 2015, 5, 15880 doi: 10.1039/C4RA16245E[226] Sun T, Song J G, Jia J, et al. Real roles of perylenetetracarboxylic diimide for enhancing photocatalytic H2-production. Nano Energy, 2016, 26, 83 doi: 10.1016/j.nanoen.2016.04.058[227] Chen Y Z, Li A X, Yue X Q, et al. Facile fabrication of organic/inorganic nanotube heterojunction arrays for enhanced photoelectrochemical water splitting. Nanoscale, 2016, 8, 13228 doi: 10.1039/C5NR07893H[228] Li L W, Cai Z X. Structure control and photocatalytic performance of porous conjugated polymers based on perylene diimide. Polym Chem, 2016, 7, 4937 doi: 10.1039/C6PY00972G[229] Nolan M C, Walsh J J, Mears L L E, et al. pH dependent photocatalytic hydrogen evolution by self-assembled perylene bisimides. J Mater Chem A, 2017, 5, 7555 doi: 10.1039/C7TA01845B[230] Wang R, Li G, Zhang A D, et al. Efficient energy-level modification of novel pyran-annulated perylene diimides for photocatalytic water splitting. Chem Commun, 2017, 53, 6918 doi: 10.1039/C7CC03682E[231] Kong K Y, Zhang S C, Chu Y M, et al. A self-assembled perylene diimide nanobelt for efficient visible-light-driven photocatalytic H2 evolution. Chem Commun, 2019, 55, 8090 doi: 10.1039/C9CC03465J[232] Concepcion J J, House R L, Papanikolas J M, et al. Chemical approaches to artificial photosynthesis. PANS, 2012, 109, 15560 doi: 10.1073/pnas.1212254109[233] Xu Y C, Zheng J X, Lindner J O, et al. Consecutive charging of a perylene bisimide dye by multistep low-energy solar-light-induced electron transfer towards H2 evolution. Angew Chem Int Ed, 2020, 59, 10363 doi: 10.1002/anie.202001231[234] Li X, Lv X, Zhang Q Q, et al. Self-assembled supramolecular system PDINH on TiO2 surface enhances hydrogen production. J Colloid Interface Sci, 2018, 525, 136 doi: 10.1016/j.jcis.2018.04.041[235] Yao L, Guijarro N, Boudoire F, et al. Establishing stability in organic semiconductor photocathodes for solar hydrogen production. J Am Chem Soc, 2020, 142, 7795 doi: 10.1021/jacs.0c00126[236] Weingarten A S, Kazantsev R V, Palmer L C, et al. Supramolecular packing controls H2 photocatalysis in chromophore amphiphile hydrogels. J Am Chem Soc, 2015, 137, 15241 doi: 10.1021/jacs.5b10027[237] Kazantsev R V, Dannenhoffer A J, Weingarten A S, et al. Crystal-phase transitions and photocatalysis in supramolecular scaffolds. J Am Chem Soc, 2017, 139, 6120 doi: 10.1021/jacs.6b13156[238] Kazantsev R V, Dannenhoffer A J, Aytun T, et al. Molecular control of internal crystallization and photocatalytic function in supramolecular nanostructures. Chem, 2018, 4, 1596 doi: 10.1016/j.chempr.2018.04.002[239] Weingarten A S, Dannenhoffer A J, Kazantsev R V, et al. Chromophore dipole directs morphology and photocatalytic hydrogen generation. J Am Chem Soc, 2018, 140, 4965 doi: 10.1021/jacs.7b12641[240] Sai H, Erbas A, Dannenhoffer A, et al. Chromophore amphiphile-polyelectrolyte hybrid hydrogels for photocatalytic hydrogen production. J Mater Chem A, 2020, 8, 158 doi: 10.1039/C9TA08974H[241] Dumele O, Chen J H, Passarelli J V, et al. Supramolecular energy materials. Adv Mater, 2020, 32, 1907247 doi: 10.1002/adma.201907247[242] Prier C K, Rankic D A, MacMillan D W C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem Rev, 2013, 113, 5322 doi: 10.1021/cr300503r[243] Anastas P T, Warner J. Green chemistry: Theory and practice. Oxford Univ Press, 1998[244] Zeman C J IV, Kim S, Zhang F, et al. Direct observation of the reduction of aryl halides by a photoexcited perylene diimide radical anion. J Am Chem Soc, 2020, 142, 2204 doi: 10.1021/jacs.9b13027[245] Ghosh I. Excited radical anions and excited anions in visible light photoredox catalysis. Phys Sci Rev, 2019, 4, 20170185 doi: 10.1515/psr-2017-0185[246] Shang J T, Tang H Y, Ji H W, et al. Synthesis, characterization, and activity of a covalently anchored heterogeneous perylene diimide photocatalyst. Chin J Catal, 2017, 38, 2094 doi: 10.1016/S1872-2067(17)62960-7[247] Wang L W, Zhang X, Yu X, et al. An all-organic semiconductor C3N4/PDINH heterostructure with advanced antibacterial photocatalytic therapy activity. Adv Mater, 2019, 31, 1901965 doi: 10.1002/adma.201901965[248] Yang Z, Chen X Y. Semiconducting perylene diimide nanostructure: Multifunctional phototheranostic nanoplatform. Acc Chem Res, 2019, 52, 1245 doi: 10.1021/acs.accounts.9b00064[249] Hu X M, Lu F, Chen L, et al. Perylene diimide-grafted polymeric nanoparticles chelated with Gd3+ for photoacoustic/T1-weighted magnetic resonance imaging-guided photothermal therapy. ACS Appl Mater Interfaces, 2017, 9, 30458 doi: 10.1021/acsami.7b09633[250] Tang W, Yang Z, Wang S, et al. Organic semiconducting photoacoustic nanodroplets for laser-activatable ultrasound imaging and combinational cancer therapy. ACS Nano, 2018, 12, 2610 doi: 10.1021/acsnano.7b08628[251] Yang Z, Tian R, Wu J J, et al. Impact of semiconducting perylene diimide nanoparticle size on lymph node mapping and cancer imaging. ACS Nano, 2017, 11, 4247 doi: 10.1021/acsnano.7b01261[252] Fan Q L, Cheng K, Yang Z, et al. Photoacoustic imaging: Perylene-diimide-based nanoparticles as highly efficient photoacoustic agents for deep brain tumor imaging in living mice. Adv Mater, 2015, 27, 774 doi: 10.1002/adma.201570028[253] Lü B, Chen Y F, Li P Y, et al. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat Commun, 2019, 10, 767 doi: 10.1038/s41467-019-08434-4[254] Englman R, Jortner J. The energy gap law for radiationless transitions in large molecules. Mol Phys, 1970, 18, 145 doi: 10.1080/00268977000100171 -
Proportional views





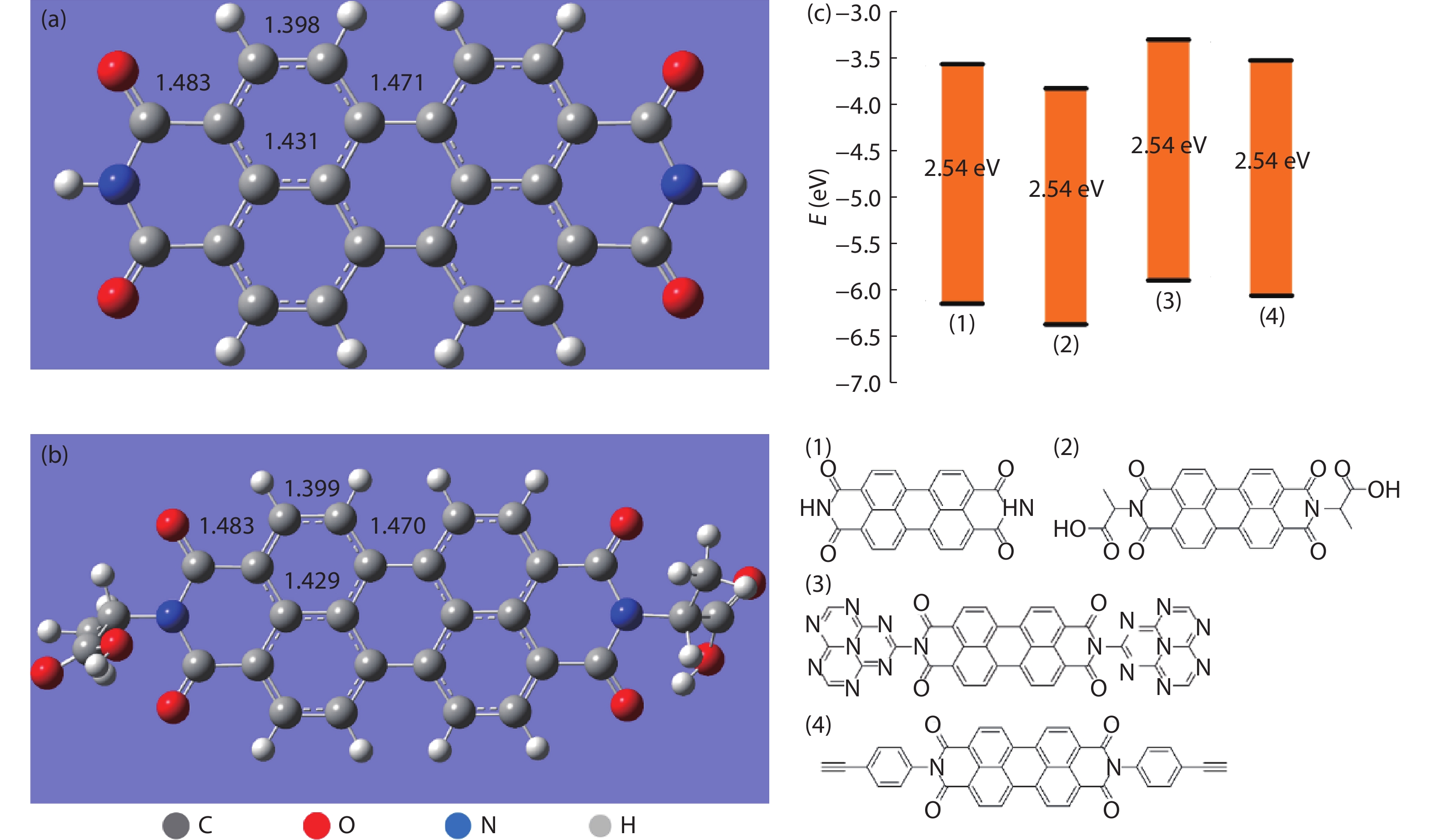
 DownLoad:
DownLoad: