| Citation: |
Haobo Yuan, Yuling Liu, Mengting Jiang, Guodong Chen, Weijuan Liu, Shengli Wang. Effect of H2O2 and nonionic surfactant in alkaline copper slurry[J]. Journal of Semiconductors, 2015, 36(1): 016001. doi: 10.1088/1674-4926/36/1/016001
H B Yuan, Y L Liu, M T Jiang, G D Chen, W J Liu, S L Wang. Effect of H2O2 and nonionic surfactant in alkaline copper slurry[J]. J. Semicond., 2015, 36(1): 016001. doi: 10.1088/1674-4926/36/1/016001.
Export: BibTex EndNote
|
Effect of H2O2 and nonionic surfactant in alkaline copper slurry
doi: 10.1088/1674-4926/36/1/016001
More Information-
Abstract
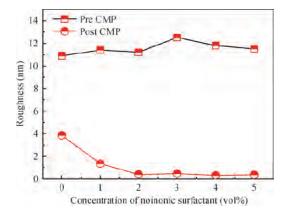
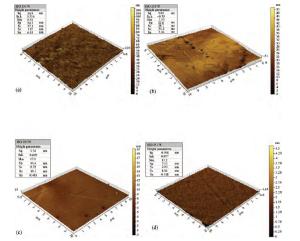
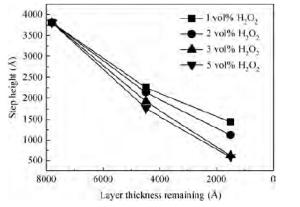
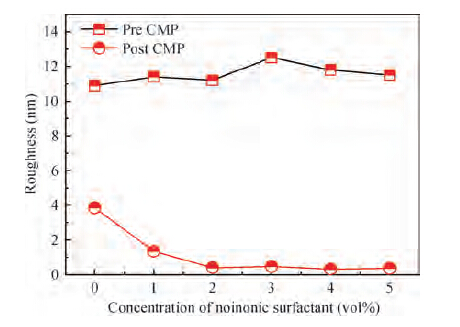
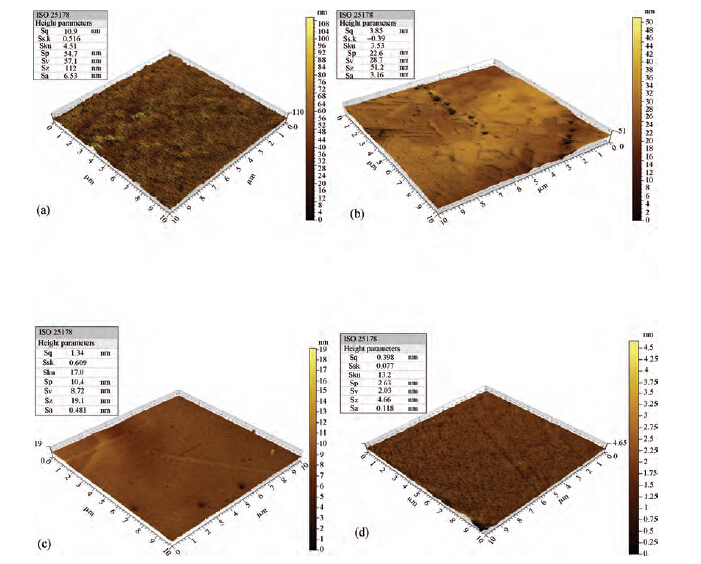
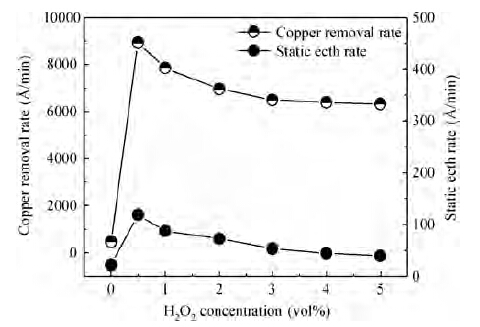
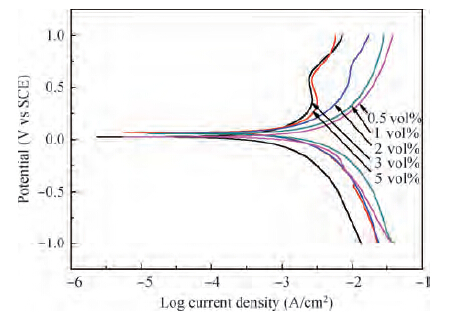
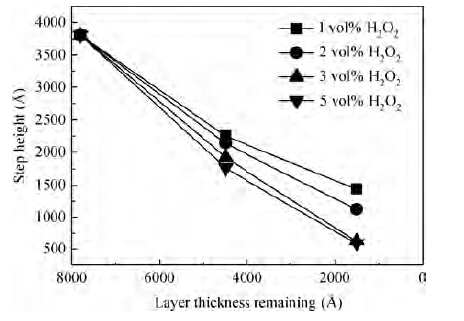
For improving the polishing performance, in this article, the roles of a nonionic surfactant (Fatty alcohol polyoxyethylene ether) and H2O2 were investigated in the chemical mechanical planarization process, respectively. Firstly, the effects of the nonionic surfactant on the within-wafer non-uniformity (WIWNU) and the surface roughness were mainly analyzed. In addition, the passivation ability of the slurry, which had no addition of BTA, was also discussed from the viewpoint of the static etch rate, electrochemical curve and residual step height under different concentrations of H2O2. The experimental results distinctly revealed that the nonionic surfactant introduced in the slurry improved the WIWNU and surface roughness, and that a 2 vol% was considered as an appropriate concentration relatively. When the concentration of H2O2 surpasses 3 vol%, the slurry will possess a relatively preferable passivation ability, which can effectively decrease the step height and contribute to acquiring a flat and smooth surface. Hence, based on the result of these experiments, the influences of the nonionic surfactant and H2O2 are further understood, which means the properties of slurry can be improved. -
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] -
Proportional views





 DownLoad:
DownLoad: