| Citation: |
Fengjun Ye, Wenqiang Yang, Deying Luo, Rui Zhu, Qihuang Gong. Applications of cesium in the perovskite solar cells[J]. Journal of Semiconductors, 2017, 38(1): 011003. doi: 10.1088/1674-4926/38/1/011003
****
F J Ye, W Q Yang, D Y Luo, R Zhu, Q H Gong. Applications of cesium in the perovskite solar cells[J]. J. Semicond., 2017, 38(1): 011003. doi: 10.1088/1674-4926/38/1/011003.
|
Applications of cesium in the perovskite solar cells
DOI: 10.1088/1674-4926/38/1/011003
More Information
-
Abstract
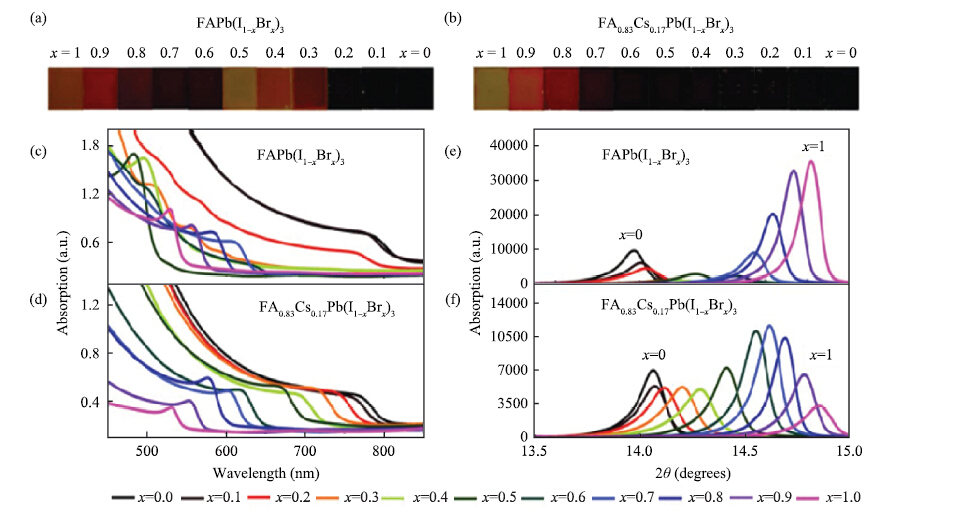
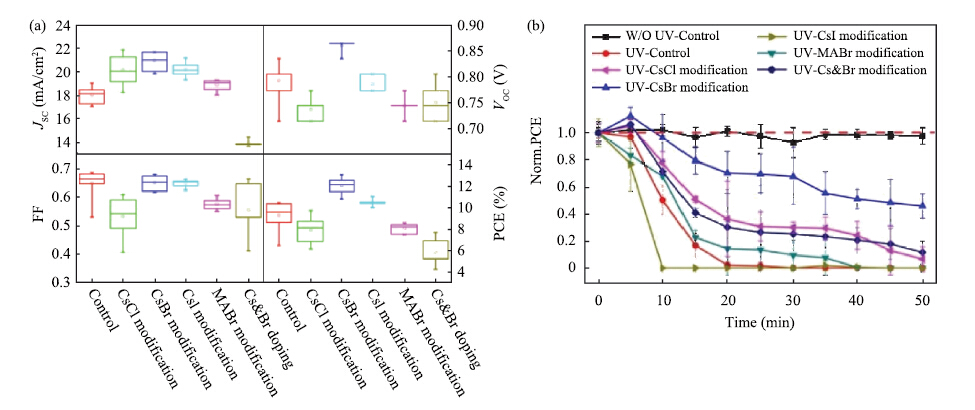
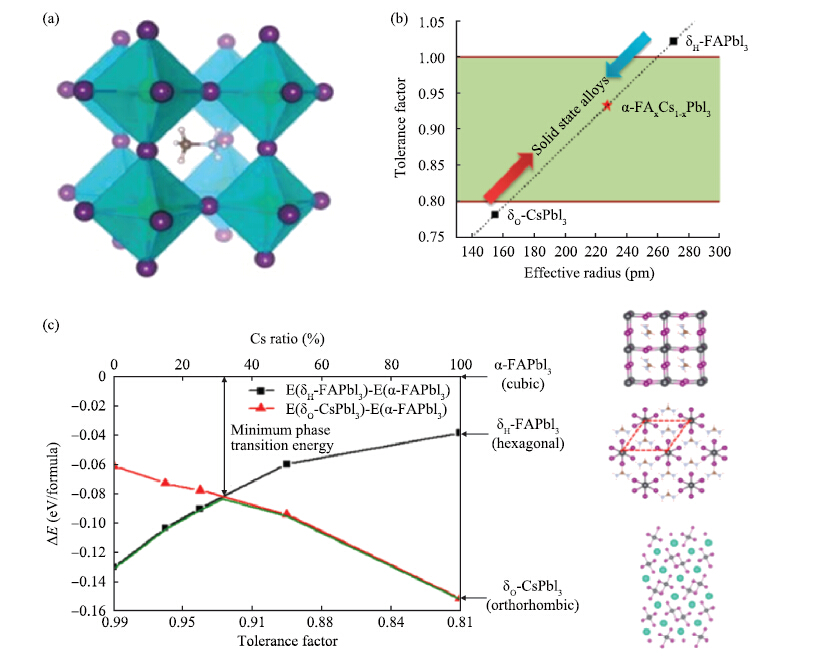
Perovskite solar cells have experienced an unprecedented rapid development in the power conversion efficiency (PCE) during the past 7 years, and the record PCE has been already comparable to the traditional polycrystalline silicon solar cells. Presently, it is more urgent to address the challenge on device stability for the future commercial application. Recently, the inorganic cesium lead halide perovskite has been intensively studied as one of the alternative candidates to improve device stability through controlling the phase transition. The cesium (Cs)-doped perovskites show more superior stability comparing with organic methylammonium (MA) lead halide perovskite or formamidinium (FA) lead halide perovskite. Here, recent progress of the inorganic cesium application in organic-inorganic perovskite solar cells (PSCs) is highlighted from the viewpoints of the device efficiency and the device stability.-
Keywords:
- cesium,
- perovskite solar cells,
- device efficiency,
- device stability
-
References
[1] Stranks S D, Eperon G E, Grancini G, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science, 2013, 342(6156): 341 doi: 10.1126/science.1243982[2] Xing G, Mathews N, Sun S, et al. Long-range balanced electronand hole-transport lengths in organic-inorganic CH3NH3PbI3. Science, 2013, 342(6156): 344 doi: 10.1126/science.1243167[3] Yin W J, Shi T, Yan Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv Mater, 2014, 26(27): 4653 doi: 10.1002/adma.v26.27[4] Zhao Y, Zhu K. Solution chemistry engineering toward highefficiency perovskite solar cells. J Phys Chem Lett, 2014, 5(23): 4175 doi: 10.1021/jz501983v[5] NREL chart, www.nrel.gov/ncpv/images/efficiency_chart.jpg[6] Kojima A, Teshima K, Shirai Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc, 2009, 131(17): 6050 doi: 10.1021/ja809598r[7] Chen W, Wu Y, Yue Y, et al. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science, 2015, 350(6263): 944 doi: 10.1126/science.aad1015[8] Jeng J Y, Chen K C, Chiang T Y, et al. Nickel oxide electrode interlayer in CH3NH3PbI3 perovskite/PCBM planarheterojunction hybrid solar cells. Adv Mater, 2014, 26(24): 4107[9] Shao Y, Yuan Y, Huang J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nat Energy, 2016, 1: 15001 doi: 10.1038/nenergy.2015.1[10] Sun S, Salim T, Mathews N, et al. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ Sci, 2014, 7(1): 399 doi: 10.1039/C3EE43161D[11] Xiao Z, Bi C, Shao Y, et al. Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers. Energy Environ Sci, 2014, 7(8): 2619 doi: 10.1039/C4EE01138D[12] You J, Meng L, Song T B, et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat Nanotechnol, 2016, 11(1): 75 http://cn.bing.com/academic/profile?id=cfa525fe83a9f619904826ac20a799fb&encoded=0&v=paper_preview&mkt=zh-cn[13] Burschka J, Pellet N, Moon S J, et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature, 2013, 499(7458): 316 doi: 10.1038/nature12340[14] Liu D, Kelly T L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat Photonics, 2014, 8(2): 133 http://cn.bing.com/academic/profile?id=e544adea4a5a7d33adfd0597fb77b8d6&encoded=0&v=paper_preview&mkt=zh-cn[15] Liu M, Johnston M B, Snaith H J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature, 2013, 501(7467): 395 doi: 10.1038/nature12509[16] Brivio F, Frost J M, Skelton J M, et al. Lattice dynamics and vibrational spectra of the orthorhombic, tetragonal, and cubic phases of methylammonium lead iodide. Phys Rev B, 2015, 92(14): 144308 doi: 10.1103/PhysRevB.92.144308[17] Stoumpos C C, Malliakas C D, Kanatzidis M G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg Chem, 2013, 52(15): 9019 doi: 10.1021/ic401215x[18] Conings B, Drijkoningen J, Gauquelin N, et al. Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv Energy Mater, 2015, 5(15): 1500477 doi: 10.1002/aenm.201500477[19] Han Y, Meyer S, Dkhissi Y, et al. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J Mater Chem A, 2015, 3(15): 8139 doi: 10.1039/C5TA00358J[20] Misra R K, Aharon S, Li B, et al. Temperature-and componentdependent degradation of perovskite photovoltaic materials under concentrated sunlight. J Phys Chem Lett, 2015, 6(3): 326 doi: 10.1021/jz502642b[21] Hailegnaw B, Kirmayer S, Edri E, et al. Rain on methylammonium lead iodide based perovskites: possible environmental effects of perovskite solar cells. J Phys Chem Lett, 2015, 6(9): 1543 doi: 10.1021/acs.jpclett.5b00504[22] Leguy A l M, Hu Y, Campoy-Quiles M, et al. Reversible hydration of CH3NH3PbI3 in films, single crystals, and solar cells. Chem Mater, 2015, 27(9): 3397 doi: 10.1021/acs.chemmater.5b00660[23] Yang J, Siempelkamp B D, Liu D, et al. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano, 2015, 9(2): 1955 doi: 10.1021/nn506864k[24] Binek A, Hanusch F C, Docampo P, et al. Stabilization of the trigonal high-temperature phase of formamidinium lead iodide. J Phys Chem Lett, 2015, 6(7): 1249 doi: 10.1021/acs.jpclett.5b00380[25] Eperon G E, Stranks S D, Menelaou C, et al. Formamidinium lead trihalide: a broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ Sci, 2014, 7(3): 982 doi: 10.1039/c3ee43822h[26] Koh T M, Fu K, Fang Y, et al. Formamidinium-containing metalhalide: an alternative material for near-IR absorption perovskite solar cells. J Phys Chem C, 2013, 118(30): 16458 https://www.researchgate.net/profile/Kunwu_Fu/publication/272135572_Formamidinium-Containing_Metal-Halide_An_Alternative_Material_for_Near-IR_Absorption_Perovskite_Solar_Cells/links/55d52d9308ae6788fa352b82.pdf?inViewer=true&pdfJsDownload=true&disableCoverPage=true&origin=publication_detail[27] Lee J W, Seol D J, Cho A N, et al. High-efficiency perovskite solar cells based on the black polymorph of HC(NH2)2PbI3. Adv Mater, 2014, 26(29): 4991 doi: 10.1002/adma.201401137[28] Lee D, Lee Y L, Hong W T, et al. Oxygen surface exchange kinetics and stability of (La, Sr)2CoO4±δ/La1-xSrxMO3-δ(MD=Co and Fe) hetero-interfaces at intermediate temperatures. J Mater Chem A, 2015, 3(5): 2144 doi: 10.1039/C4TA05795C[29] Pang S, Hu H, Zhang J, et al. NH2CH=NH2PbI3: an alternative organolead iodide perovskite sensitizer for mesoscopic solar cells. Chem Mater, 2014, 26(3): 1485 doi: 10.1021/cm404006p[30] Pellet N, Gao P, Gregori G, et al. Mixed-organic-cation Perovskite photovoltaics for enhanced solar-light harvesting. Angew Chem Int Edit, 2014, 53(12): 3151 doi: 10.1002/anie.201309361[31] Seol D J, Lee J W, Park N G. On the role of interfaces in planarstructured HC(NH2)2PbI3 perovskite solar Cells. Chem Sus Chem, 2015, 8(14): 2414 doi: 10.1002/cssc.v8.14[32] Kulbak M, Gupta S, Kedem N, et al. Cesium enhances longterm stability of lead bromide perovskite-based solar cells. J Phys Chem Lett, 2015, 7(1): 167 http://cn.bing.com/academic/profile?id=fb82ad0d1a1403fb8a886aa8347a7c8a&encoded=0&v=paper_preview&mkt=zh-cn[33] Lee J W, Kim D H, Kim H S, et al. Formamidinium and cesium hybridization for photo-and moisture-stable perovskite solar cell. Adv Energy Mater, 2015, 5(20): 1501310 doi: 10.1002/aenm.201501310[34] McMeekin D P, Sadoughi G, Rehman W, et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science, 2016, 351(6269): 151 doi: 10.1126/science.aad5845[35] Kim H S, Lee C R, Im J H, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci Rep, 2012, 2: 591 https://www.researchgate.net/profile/Jacques-E_Moser/publication/230716542_Lead_iodide_perovskite_sensitized_all-solid-state_submicron_thin_film_mesoscopic_solar_cell_with_efficiency_exceeding_9/links/09e41506f09cb81b07000000.pdf[36] Lee M M, Teuscher J, Miyasaka T, et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science, 2012, 338(6107): 643 doi: 10.1126/science.1228604[37] Hao F, Stoumpos C C, Cao D H, et al. Lead-free solid-state organic-inorganic halide perovskite solar cells. Nat Photonics, 2014, 8(6): 489 doi: 10.1038/nphoton.2014.82[38] Goldschmidt V M. Die gesetze der krystallochemie. Naturwissenschaften, 1926, 14(21): 477 doi: 10.1007/BF01507527[39] Baikie T, Fang Y, Kadro J M, et al. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J Mater Chem A, 2013, 1(18): 5628 doi: 10.1039/c3ta10518k[40] Mitzi D B. Solution-processed inorganic semiconductors. J Mater Chem, 2004, 14(15): 2355 doi: 10.1039/b403482a[41] Choi H, Jeong J, Kim H B, et al. Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells. Nano Energy, 2014, 7: 80 doi: 10.1016/j.nanoen.2014.04.017[42] Jeon N J, Noh J H, Yang W S, et al. Compositional engineering of perovskite materials for high-performance solar cells. Nature, 2015, 517(7535): 476 doi: 10.1038/nature14133[43] Etgar L, Gao P, Xue Z, et al. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J Am Chem Soc, 2012, 134(42): 17396 doi: 10.1021/ja307789s[44] Ball J M, Lee M M, Hey A, et al. Low-temperature processed meso-superstructured to thin-film perovskite solar cells. Energy Environ Sci, 2013, 6(6): 1739 doi: 10.1039/c3ee40810h[45] Carnie M J, Charbonneau C, Davies M L, et al. A one-step low temperature processing route for organolead halide perovskite solar cells. Chem Commun, 2013, 49(72): 7893 doi: 10.1039/c3cc44177f[46] Hu Q, Wu J, Jiang C, et al. Engineering of electron-selective contact for perovskite solar cells with efficiency exceeding 15%. ACS Nano, 2014, 8(10): 10161 doi: 10.1021/nn5029828[47] Li W, Li J, Niu G, et al. Effect of cesium chloride modification on the film morphology and UV-induced stability of planar perovskite solar cells. J Mater Chem A, 2016, 4(30): 11688 doi: 10.1039/C5TA09165A[48] Li W, Zhang W, Van Reenen S, et al. Enhanced UV-light stability of planar heterojunction perovskite solar cells with caesium bromide interface modification. Energy Environ Sci, 2016, 9(2): 490 doi: 10.1039/C5EE03522H[49] Sutton R J, Eperon G E, Miranda L, et al. Bandgap-tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv Energy Mater, 2016, 6(8): 1502458 doi: 10.1002/aenm.201502458[50] Hao F, Stoumpos C C, Cao D H, et al. Lead-free solid-state organic-inorganic halide perovskite solar cells. Nat Photon, 2014, 8: 489 doi: 10.1038/nphoton.2014.82[51] Kumar M H, Dharani S, Leong W L, et al. Lead-free halide perovskite solar cells with high photocurrents realized through vacancy modulation. Adv Mater, 2014, 26: 7122 doi: 10.1002/adma.201401991[52] Sabba D, Mulmudi H K, Prabhakar R R, et al. Impact of anionic br substitution on open circuit voltage in lead free perovskite (CsSnI3xBrx/solar cells. J Phys Chem C, 2015, 119:1763 doi: 10.1021/jp5126624[53] Fujishima A, Rao T N, Tryk D A. Titanium dioxide photocatalysis. J Photochem Photobiol C, 2000, 1(1): 1 doi: 10.1016/S1389-5567(00)00002-2[54] Ito S, Tanaka S, Manabe K, et al. Effects of surface blocking layer of Sb2S3 on nanocrystalline TiO2 for CH3NH3PbI3 perovskite solar cells. J Phys Chem C, 2014, 118(30): 16995 doi: 10.1021/jp500449z[55] Leijtens T, Eperon G E, Pathak S, et al. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat Commun, 2013, 4: 1 https://www.researchgate.net/profile/Antonio_Abate/publication/259154549_Overcoming_ultraviolet_light_instability_of_sensitized_TiO2_with_meso-superstructured_organometal_tri-halide_perovskite_solar_cells/links/0a85e52e256d97db57000000.pdf?origin=publication_list[56] Noh J H, Im S H, Heo J H, et al. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett, 2013, 13(4): 1764 doi: 10.1021/nl400349b -
Proportional views





 DownLoad:
DownLoad: