| Citation: |
Nanjie Guo, Taiyang Zhang, Ge Li, Feng Xu, Xufang Qian, Yixin Zhao. A simple fabrication of CH3NH3PbI3 perovskite for solar cells using low-purity PbI2[J]. Journal of Semiconductors, 2017, 38(1): 014004. doi: 10.1088/1674-4926/38/1/014004
****
N J Guo, T Y Zhang, G Li, F Xu, X F Qian, Y X Zhao. A simple fabrication of CH3NH3PbI3 perovskite for solar cells using low-purity PbI2[J]. J. Semicond., 2017, 38(1): 014004. doi: 10.1088/1674-4926/38/1/014004.
|
A simple fabrication of CH3NH3PbI3 perovskite for solar cells using low-purity PbI2
DOI: 10.1088/1674-4926/38/1/014004
More Information
-
Abstract
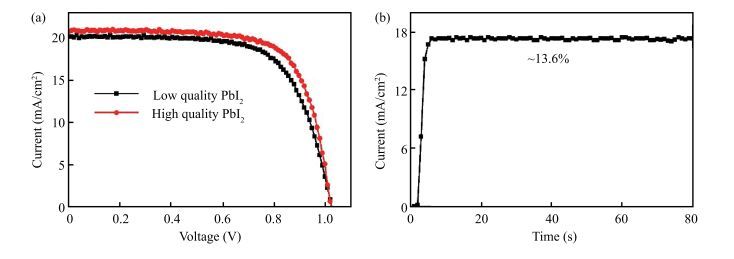
The CH3NH3PbI3 (MAPbI3) perovskite was usually prepared by high-purity PbI2 with high cost. The low cost and low-purity PbI2 was seldom reported for fabrication of MAPbI3 because it cannot even dissolve well in widely adopted solvent of DMF. We developed an easy method to adapt low-purity PbI2 for fabrication of high quality MAPbI3 just by the simple addition of some hydrochloric acid into the mixture of low-purity PbI2, MAI and DMF. This straightforward method can not only help dissolve the low quality PbI2 by reacting with some impurities in DMF, but also lead to a successful fabrication of high-quality perovskite solar cells with up to 14.80% efficiency comparable to the high quality PbI2 precursors.-
Keywords:
- perovskite material,
- low-quality PbI2,
- hydrochloric acid
-
References
[1] Kojima A, Teshima K, Shirai Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc, 2009, 131(17):6050 doi: 10.1021/ja809598r[2] Im J H, Lee C R, Lee J W, et al. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale, 2011, 3(10):4088 doi: 10.1039/c1nr10867k[3] Bi D, Yang L, Boschloo G, et al. Effect of different hole transport materials on recombination in CH3NH3PbI3 perovskitesensitized mesoscopic solar cells. J Phys Chem Lett, 2013, 4(9):1532 doi: 10.1021/jz400638x[4] Burschka J, Pellet N, Moon S J, et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature, 2013, 499(7458):316 doi: 10.1038/nature12340[5] Park N G. Organometal perovskite light absorbers toward a 20% efficiency low-cost solid-state mesoscopic solar cell. J Phys Chem Lett, 2013, 4(15):2423 doi: 10.1021/jz400892a[6] Stoumpos C C, Malliakas C D, Kanatzidis M G. Semiconducting tin and lead iodide perovskites with organic cations:phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg Chem, 2013, 52(15):9019 doi: 10.1021/ic401215x[7] Stranks S D, Eperon G E, Grancini G, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science, 2013, 342(6156):341 doi: 10.1126/science.1243982[8] Zhao Y, Zhu K. Charge transport and recombination in perovskite (CH3NH3)PbI3 sensitized TiO2 solar cells. J Phys Chem Lett, 2013, 4(17):2880 doi: 10.1021/jz401527q[9] Mei A, Li X, Liu L, et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science, 2014, 345(6194):295 doi: 10.1126/science.1254763[10] Zhao Y, Zhu K. Efficient planar perovskite solar cells based on 1.8 eV band gap CH3NH3PbI2Br nanosheets via thermal decomposition. J Am Chem Soc, 2014, 136(35):12241 doi: 10.1021/ja5071398[11] Dai X, Shi C, Zhang Y, et al. Hydrolysis preparation of the compact TiO2 layer using metastable TiCl4 isopropanol/water solution for inorganic-organic hybrid heterojunction perovskite solar cells. J Semicond, 2015, 36(7):074003 doi: 10.1088/1674-4926/36/7/074003[12] Li G, Zhang T, Zhao Y. Hydrochloric acid accelerated formation of planar CH3NH3PbI3 perovskite with high humidity tolerance. J Mater Chem A, 2015, 3(39):19674 doi: 10.1039/C5TA06172E[13] Nie W Y, Tsai H H, Asadpour R, et al. High-efficiency solutionprocessed perovskite solar cells with millimeter-scale grains. Science, 2015, 347(6221):522 doi: 10.1126/science.aaa0472[14] Yang W S, Noh J H, Jeon N J, et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science, 2015, 348(6240):1234 doi: 10.1126/science.aaa9272[15] Zhang T, Zhao Y. Recent progress of lead halide perovskite sensitized solar cells. Acta Chim Sinica, 2015, 73(3):202 doi: 10.6023/A14090656[16] Zhang T Y, Yang M J, Benson E E, et al. A facile solvothermal growth of single crystal mixed halide perovskite CH3NH3Pb(Br1-xClx)3. Chem Commun, 2015, 51(37):7820 doi: 10.1039/C5CC01835H[17] Si F, Tang F, Xue H, et al. Effects of defect states on the performance of perovskite solar cells. J Semicond, 2016, 37(7):072003 doi: 10.1088/1674-4926/37/7/072003[18] Wu Y, Yang R, Tian H, et al. Photoelectric characteristics of CH3NH3PbI3/p-Si heterojunction. J Semicond, 2016, 37(5):053002 doi: 10.1088/1674-4926/37/5/053002[19] You J, Meng L, Song T B, et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nature Nanotech, 2016, 11(1):75 http://cn.bing.com/academic/profile?id=cfa525fe83a9f619904826ac20a799fb&encoded=0&v=paper_preview&mkt=zh-cn[20] Zhang J, Shi C, Chen J, et al. Pyrolysis preparation of WO3 thin films using ammonium metatungstate DMF/water solution for efficient compact layers in planar perovskite solar cells. J Semicond, 2016, 37(3):033002 doi: 10.1088/1674-4926/37/3/033002[21] Zhao Y X, Zhu K. Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem Soc Rev, 2016, 45(3):655 doi: 10.1039/C4CS00458B[22] Wakamiya A, Endo M, Sasamori T, et al. Reproducible fabrication of efficient perovskite-based solar cells:X-ray crystallographic studies on the formation of CH3NH3PbI3 layers. Chem Lett, 2014, 43(5):711 doi: 10.1246/cl.140074[23] Baikie T, Fang Y, Kadro J M, et al. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J Mater Chem A, 2013, 1(18):5628 doi: 10.1039/c3ta10518k[24] Green M A, Ho-Baillie A, Snaith H J. The emergence of perovskite solar cells. Nat Photon, 2014, 8(7):506 doi: 10.1038/nphoton.2014.134[25] Chen Y N, Zhao Y X, Liang Z Q. Non-thermal annealing fabrication of efficient planar perovskite solar cells with inclusion of NH4Cl. Chem Mater, 2015, 27(5):1448 doi: 10.1021/acs.chemmater.5b00041[26] Dar M I, Arora N, Gao P, et al. Investigation regarding the role of chloride in organic-inorganic halide perovskites obtained from chloride containing precursors. Nano Lett, 2014, 14(12): 6991 doi: 10.1021/nl503279x[27] Dar M I, Ramos F J, Xue Z, et al. Photoanode based on (001)-oriented anatase nanoplatelets for organic-inorganic lead iodide perovskite solar cell. Chem Mater, 2014, 26(16):4675 doi: 10.1021/cm502185s[28] Lee M M, Teuscher J, Miyasaka T, et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science, 2012, 338(6107):643 doi: 10.1126/science.1228604[29] Li X, Dar M J, Yi C Y, et al. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid omega-ammonium chlorides. Nat Chem, 2015, 7(9):703 doi: 10.1038/nchem.2324[30] Lv S L, Pang S P, Zhou Y Y, et al. One-step, solution-processed formamidinium lead trihalide FAPbI3-xClx for mesoscopic perovskite-polymer solar cells. Phys Chem Chem Phys, 2014, 16(36):19206 doi: 10.1039/C4CP02113D[31] Qing J, Chandran H T, Cheng Y H, et al. Chlorine incorporation for enhanced performance of planar perovskite solar cell based on lead acetate precursor. ACS Appl Mater Interfaces, 2015, 7(41):23110 doi: 10.1021/acsami.5b06819[32] Wang D, Liu Z H, Zhou Z M, et al. Reproducible one-step fabrication of compact MAPbI3-xClx thin films derived from mixedlead-halide precursors. Chem Mater, 2014, 26(24):7145 doi: 10.1021/cm5037869[33] Wang Z W, Zhou Y Y, Pang S P, et al. Additive-modulated evolution of HC(NH2)(2)PbI3 black polymorph for mesoscopic perovskite solar cells. Chem Mater, 2015, 27(20):7149 doi: 10.1021/acs.chemmater.5b03169[34] Xu M, Rong Y, Ku Z, et al. Highly ordered mesoporous carbon for mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cell. J Mater Chem A, 2014, 2(23):8607 doi: 10.1039/c4ta00379a[35] Yan K Y, Long M Z, Zhang T K, et al. Hybrid halide perovskite solar cell precursors:colloidal chemistry and coordination engineering behind device processing for high efficiency. J Am Chem Soc, 2015, 137(13):4460 doi: 10.1021/jacs.5b00321[36] Zhao Y, Zhu K. CH3NH3Cl-assisted one-step solution growth of CH3NH3PbI3:structure, charge-carrier dynamics, and photovoltaic properties of perovskite solar cells. J Phys Chem C, 2014, 118(18):9412 doi: 10.1021/jp502696w[37] Zhang T, Guo N, Li G, et al. A controllable fabrication of grain boundary PbI2 nanoplates passivated lead halide perovskites for high performance solar cells. Nano Energy, 2016, 26:50 doi: 10.1016/j.nanoen.2016.05.003[38] Bi D Q, El-Zohry A M, Hagfeldt A, et al. Unraveling the effect of PbI2 concentration on charge recombination kinetics in perovskite solar cells. ACS Photonics, 2015, 2(5):589 doi: 10.1021/ph500255t[39] Cao D H, Stoumpos C C, Malliakas C D, et al. Remnant PbI2, an unforeseen necessity in high-efficiency hybrid perovskite-based solar cells. APL Mater, 2014, 2(9):091101 doi: 10.1063/1.4895038[40] Kim Y C, Jeon N J, Noh J H, et al. Beneficial effects of PbI2 incorporated in organo-lead halide perovskite solar cells. Adv Energy Mater, 2016, 6(4):8[41] Lee Y H, Luo J S, Humphry-Baker R, et al. Unraveling the reasons for efficiency loss in perovskite solar cells. Adv Funct Mater, 2015, 25(25):3925 doi: 10.1002/adfm.v25.25[42] Liu F, Dong Q, Wong M K, et al. Is excess PbI2 beneficial for perovskite solar cell performance. Adv Energy Mater, 2016, 6(7):1502206 doi: 10.1002/aenm.201502206[43] Wang L, McCleese C, Kovalsky A, et al. Femtosecond timeresolved transient absorption spectroscopy of CH3NH3PbI3 perovskite films:evidence for passivation effect of PbI2. J Am Chem Soc, 2014, 136(35):12205 doi: 10.1021/ja504632z[44] Wang S M, Dong W W, Fang X D, et al. Credible evidence for the passivation effect of remnant PbI2 in CH3NH3PbI3 films in improving the performance of perovskite solar cells. Nanoscale, 2016, 8(12):6600 doi: 10.1039/C5NR08344C[45] Li X, Bi D Q, Yi C Y, et al. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells. Science, 2016, 353(6294):58 doi: 10.1126/science.aaf8060 -
Proportional views






 DownLoad:
DownLoad: