| Citation: |
Van Nang Lam, Thi Bich Vu, Quang Dat Do, Thi Thanh Xuan Le, Tien Dai Nguyen, T.-Thanh-Bao Nguyen, Hoang Tung Do, Thi Tu Oanh Nguyen. One-step hydrothermal synthesis of Sn-doped α-Fe2O3 nanoparticles for enhanced photocatalytic degradation of Congo red[J]. Journal of Semiconductors, 2022, 43(12): 122001. doi: 10.1088/1674-4926/43/12/122001
V N Lam, T B Vu, Q D Do, T T X Le, T D Nguyen, T T B Nguyen, H T Do, T T O Nguyen. One-step hydrothermal synthesis of Sn-doped α-Fe2O3 nanoparticles for enhanced photocatalytic degradation of Congo red[J]. J. Semicond, 2022, 43(12): 122001. doi: 10.1088/1674-4926/43/12/122001
Export: BibTex EndNote
|
One-step hydrothermal synthesis of Sn-doped α-Fe2O3 nanoparticles for enhanced photocatalytic degradation of Congo red
doi: 10.1088/1674-4926/43/12/122001
More Information-
Abstract
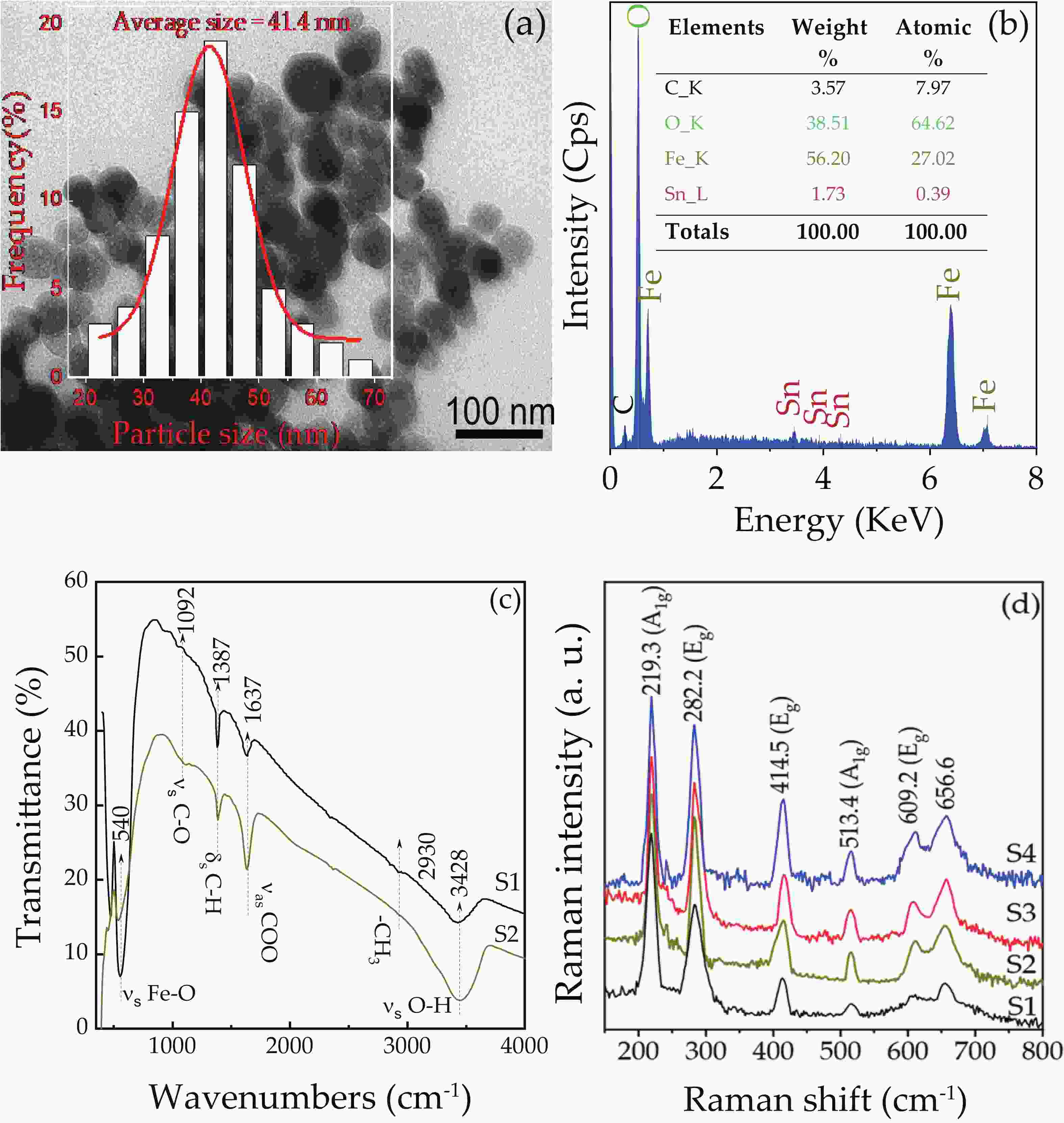
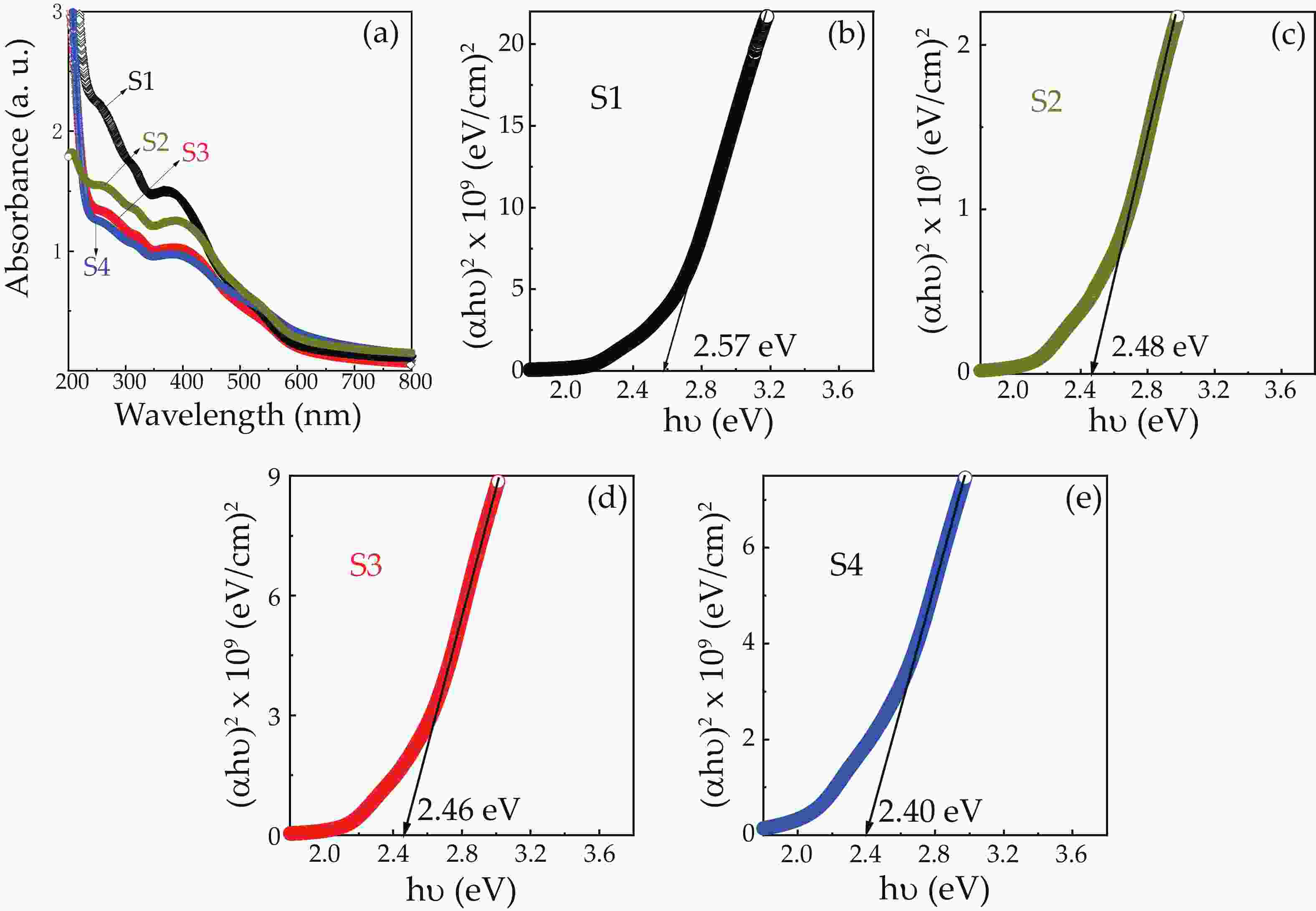
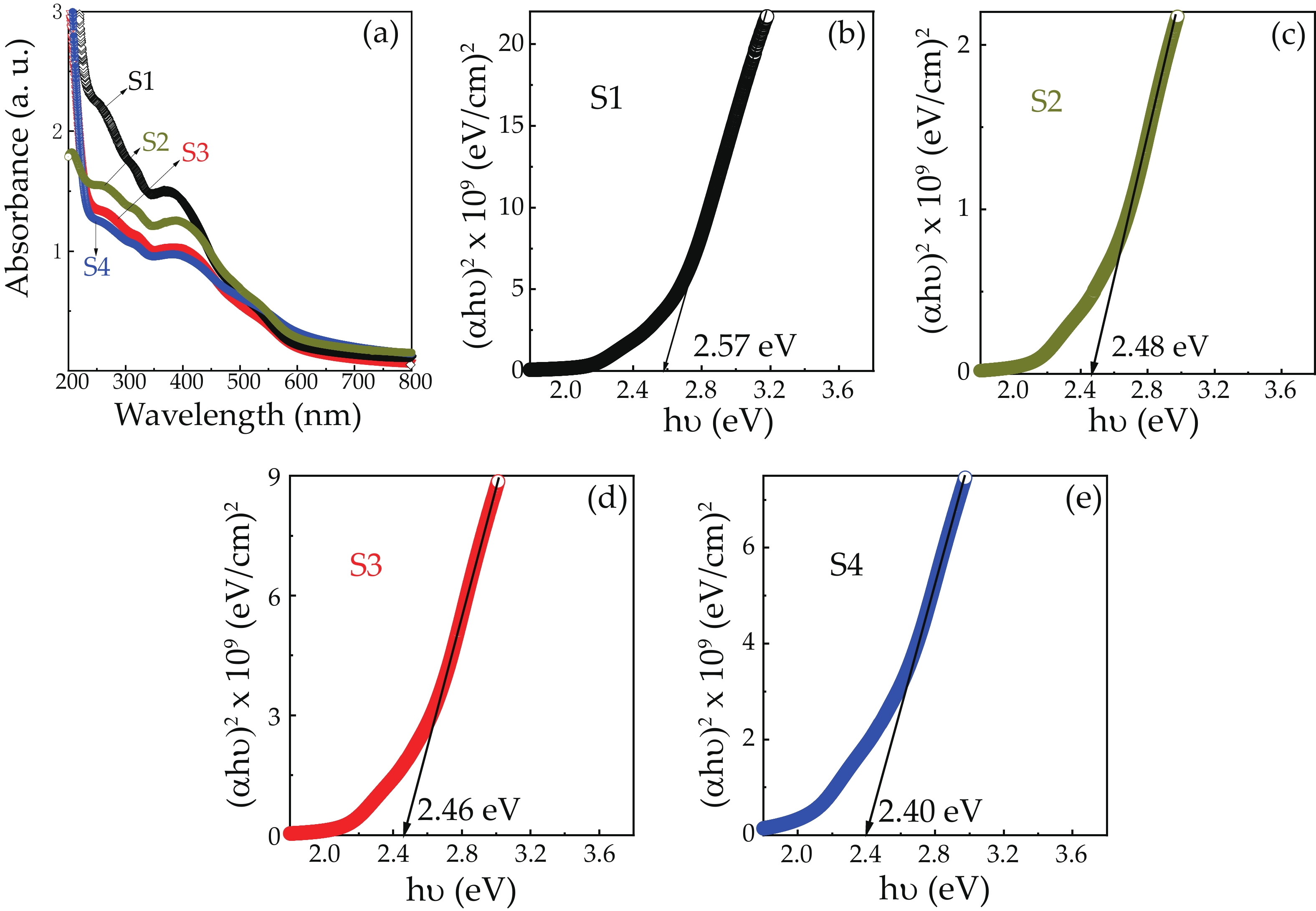
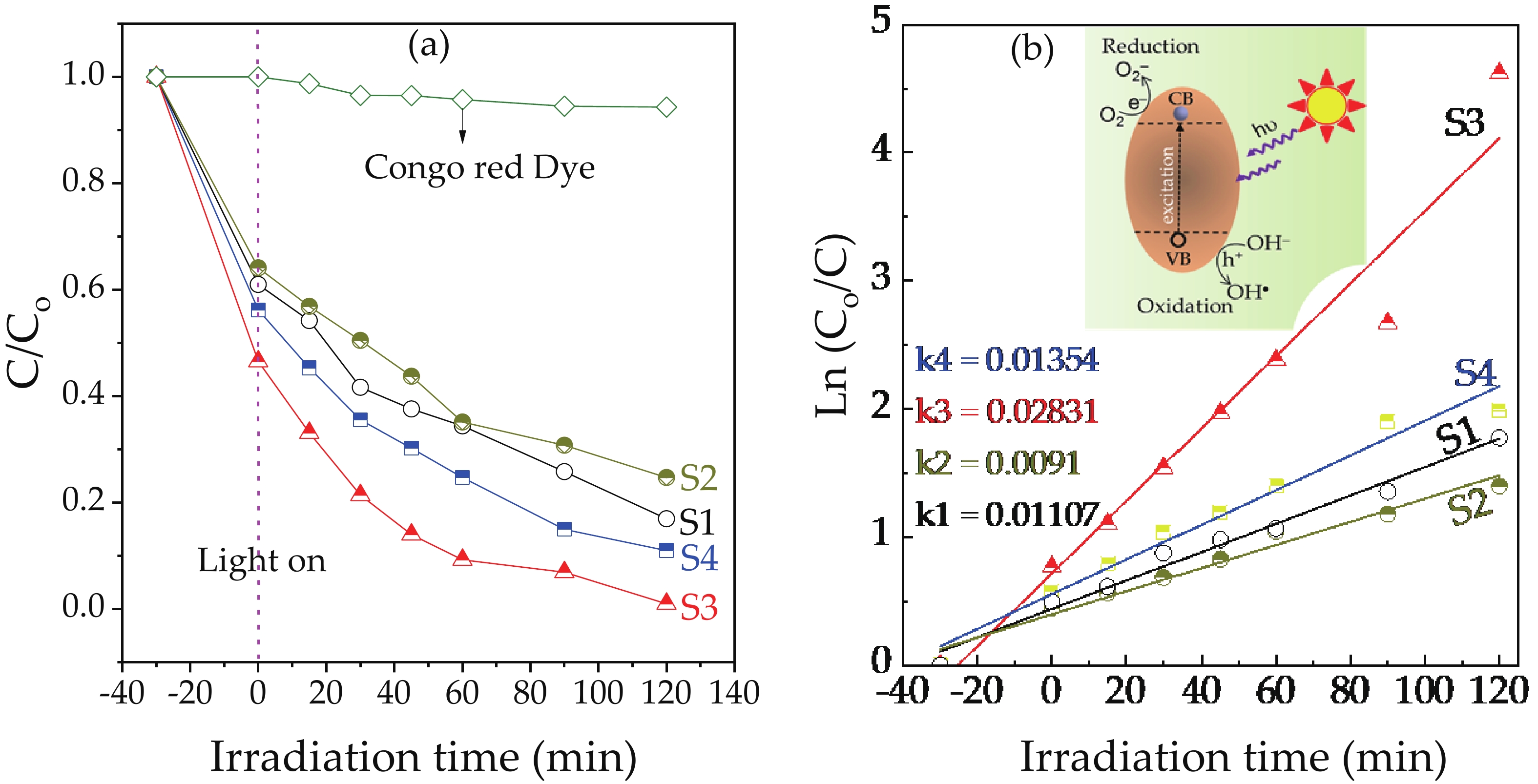
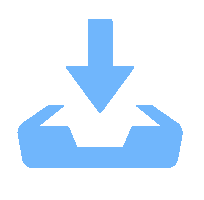
We report on the synthesis of Sn-doped hematite nanoparticles (Sn-α-Fe2O3 NPs) by the hydrothermal method. The prepared Sn-α-Fe2O3 NPs had a highly pure and well crystalline rhombohedral phase with an average particle size of 41.4 nm. The optical properties of as-synthesized α-Fe2O3 NPs show a higher bandgap energy (2.40–2.57 eV) than that of pure bulk α-Fe2O3 (2.1 eV). By doping Sn into α-Fe2O3 NPs, the Sn-doped hematite was observed a redshift toward a long wavelength with increasing Sn concentration from 0% to 4.0%. The photocatalytic activity of Sn-doped α-Fe2O3 NPs was evaluated by Congo red (CR) dye degradation. The degradation efficiency of CR dye using Sn-α-Fe2O3 NPs catalyst is higher than that of pure α-Fe2O3 NPs. The highest degradation efficiency of CR dye was 97.8% using 2.5% Sn-doped α-Fe2O3 NPs catalyst under visible-light irradiation. These results suggest that the synthesized Sn-doped α-Fe2O3 nanoparticles might be a suitable approach to develop a photocatalytic degradation of toxic inorganic dye in wastewater. -
References
[1] Zhang Y J, Kang L, Liu L C. Alkali-activated cements for photocatalytic degradation of organic dyes. In: Handbook of Alkali-Activated Cements, Mortars and Concretes. Amsterdam: Elsevier, 2015, 729 doi: https://doi.org/10.1533/9781782422884.5.729[2] Zheng Y Q, Cheng B, Fan J J, et al. Review on nickel-based adsorption materials for Congo red. J Hazard Mater, 2021, 403, 123559 doi: 10.1016/j.jhazmat.2020.123559[3] Waheed A, Mansha M, Kazi I W, et al. Synthesis of a novel 3, 5-diacrylamidobenzoic acid based hyper-cross-linked resin for the efficient adsorption of Congo Red and Rhodamine B. J Hazard Mater, 2019, 369, 528 doi: 10.1016/j.jhazmat.2019.02.058[4] Jiao C L, Liu D, Wei N N, et al. Efficient Congo red removal using porous cellulose/gelatin/sepiolite gel beads: Assembly, characterization, and adsorption mechanism. Polymers, 2021, 13, 3890 doi: 10.3390/polym13223890[5] Olivo-Alanis D, Garcia-Reyes R B, Alvarez L H, et al. Mechanism of anaerobic bio-reduction of azo dye assisted with lawsone-immobilized activated carbon. J Hazard Mater, 2018, 347, 423 doi: 10.1016/j.jhazmat.2018.01.019[6] Zhang Y J, Liu L C, Ni L L, et al. A facile and low-cost synthesis of granulated blast furnace slag-based cementitious material coupled with Fe2O3 catalyst for treatment of dye wastewater. Appl Catal B, 2013, 138/139, 9 doi: 10.1016/j.apcatb.2013.02.025[7] Souza J B Jr, Souza F L, Vayssieres L, et al. On the relevance of understanding and controlling the locations of dopants in hematite photoanodes for low-cost water splitting. Appl Phys Lett, 2021, 119, 200501 doi: 10.1063/5.0066931[8] Bonancêa C E, do Nascimento G M, de Souza M L, et al. Substrate development for surface-enhanced Raman study of photocatalytic degradation processes: Congo red over silver modified titanium dioxide films. Appl Catal B, 2006, 69, 34 doi: 10.1016/j.apcatb.2006.05.016[9] Zhao C W, Yang B, Han J L, et al. Preparation of carboxylic multiwalled-carbon-nanotube-modified poly(m-phenylene isophthalamide) hollow fiber nanofiltration membranes with improved performance and application for dye removal. Appl Surf Sci, 2018, 453, 502 doi: 10.1016/j.apsusc.2018.05.149[10] Dutta A K, Maji S K, Adhikary B. γ-Fe2O3 nanoparticles: An easily recoverable effective photo-catalyst for the degradation of rose Bengal and methylene blue dyes in the waste-water treatment plant. Mater Res Bull, 2014, 49, 28 doi: 10.1016/j.materresbull.2013.08.024[11] Li X, Liu Y, Zhang C L, et al. Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chem Eng J, 2018, 336, 241 doi: 10.1016/j.cej.2017.11.188[12] Guo S Q, Hu Z Z, Zhen M M, et al. Insights for optimum cation defects in photocatalysis: A case study of hematite nanostructures. Appl Catal B, 2020, 264, 118506 doi: 10.1016/j.apcatb.2019.118506[13] Suman, Chahal S, Singh S, et al. Understanding the role of Ni ions on the photocatalytic activity and dielectric properties of hematite nanostructures: An experimental and DFT approach. J Phys Chem Solids, 2021, 156, 110118 doi: 10.1016/j.jpcs.2021.110118[14] Lv K Z, Li J, Qing X X, et al. Synthesis and photo-degradation application of WO3/TiO2 hollow spheres. J Hazard Mater, 2011, 189, 329 doi: 10.1016/j.jhazmat.2011.02.038[15] Ling Y C, Wang G M, Wheeler D A, et al. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett, 2011, 11, 2119 doi: 10.1021/nl200708y[16] Valenzuela M A, Bosch P, Jiménez-Becerrill J, et al. Preparation, characterization and photocatalytic activity of ZnO, Fe2O3 and ZnFe2O4. J Photochem Photobiol A, 2002, 148, 177 doi: 10.1016/S1010-6030(02)00040-0[17] Ollis D. Heterogeneous photoassisted catalysis: Conversions of perchloroethylene, dichloroethane, chloroacetic acids, and chlorobenzenes. J Catal, 1984, 88, 89 doi: 10.1016/0021-9517(84)90053-8[18] Al-Ekabi H, Serpone N, Pelizzetti E, et al. Kinetic studies in heterogeneous photocatalysis. 2. Titania-mediated degradation of 4-chlorophenol alone and in a three-component mixture of 4-chlorophenol, 2,4-dichlorophenol, and 2,4,5-trichlorophenol in air-equilibrated aqueous media. Langmuir, 1989, 5, 250 doi: 10.1021/la00085a048[19] Sauer T, Cesconeto Neto G, José H J, et al. Kinetics of photocatalytic degradation of reactive dyes in a TiO2 slurry reactor. J Photochem Photobiol A, 2002, 149, 147 doi: 10.1016/S1010-6030(02)00015-1[20] Jang J S, Lee J, Ye H, et al. Rapid screening of effective dopants for Fe2O3 photocatalysts with scanning electrochemical microscopy and investigation of their photoelectrochemical properties. J Phys Chem C, 2009, 113, 6719 doi: 10.1021/jp8109429[21] Mahmoodi N M. Synthesis of magnetic carbon nanotube and photocatalytic dye degradation ability. Environ Monit Assess, 2014, 186, 5595 doi: 10.1007/s10661-014-3805-7[22] Rasheed R T, Al-Algawi S D, Kareem H H, et al. Preparation and characterization of hematite iron oxide (α-Fe2O3) by Sol-gel method. Chem Sci J, 2018, 9, 2 doi: 10.1039/C8SC90002G[23] Tsege E L, Atabaev T S, Hossain M A, et al. Cu-doped flower-like hematite nanostructures for efficient water splitting applications. J Phys Chem Solids, 2016, 98, 283 doi: 10.1016/j.jpcs.2016.07.014[24] Meng Q L, Wang Z B, Chai X Y, et al. Fabrication of hematite (α-Fe2O3) nanoparticles using electrochemical deposition. Appl Surf Sci, 2016, 368, 303 doi: 10.1016/j.apsusc.2016.02.007[25] Cao Z Q, Qin M L, Gu Y R, et al. Synthesis and characterization of Sn-doped hematite as visible light photocatalyst. Mater Res Bull, 2016, 77, 41 doi: 10.1016/j.materresbull.2016.01.004[26] Mahmoodi N M. Photocatalytic degradation of dyes using carbon nanotube and titania nanoparticle. Water Air Soil Pollut, 2013, 224, 1612 doi: 10.1007/s11270-013-1612-3[27] Oveisi M, Mahmoodi N M, Asli M A. Facile and green synthesis of metal-organic framework/inorganic nanofiber using electrospinning for recyclable visible-light photocatalysis. J Clean Prod, 2019, 222, 669 doi: 10.1016/j.jclepro.2019.03.066[28] Alagiri M, Hamid S B A. Sol-gel synthesis of α-Fe2O3 nanoparticles and its photocatalytic application. J Sol-Gel Sci Technol, 2015, 74, 783 doi: 10.1007/s10971-015-3663-y[29] Sarkar D, Mandal M, Mandal K. Design and synthesis of high performance multifunctional ultrathin hematite nanoribbons. ACS Appl Mater Interfaces, 2013, 5, 11995 doi: 10.1021/am403762d[30] Maji S K, Mukherjee N, Mondal A, et al. Synthesis, characterization and photocatalytic activity of α-Fe2O3 nanoparticles. Polyhedron, 2012, 33, 145 doi: 10.1016/j.poly.2011.11.017[31] Achouri F, Corbel S, Aboulaich A, et al. Aqueous synthesis and enhanced photocatalytic activity of ZnO/Fe2O3 heterostructures. J Phys Chem Solids, 2014, 75, 1081 doi: 10.1016/j.jpcs.2014.05.013[32] Zheng Q, Zhou B, Bai J, et al. Self-organized TiO2 nanotube array sensor for the determination of chemical oxygen demand. Adv Mater, 2008, 20, 1044 doi: 10.1002/adma.200701619[33] Sivula K, Zboril R, Le Formal F, et al. Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J Am Chem Soc, 2010, 132, 7436 doi: 10.1021/ja101564f[34] Cesar I, Sivula K, Kay A, et al. Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J Phys Chem C, 2009, 113, 772 doi: 10.1021/jp809060p[35] Cherepy N J, Liston D B, Lovejoy J A, et al. Ultrafast studies of photoexcited electron dynamics in γ- and α-Fe2O3 semiconductor nanoparticles. J Phys Chem B, 1998, 102, 770 doi: 10.1021/jp973149e[36] Dare-Edwards M P, Goodenough J B, Hamnett A, et al. Electrochemistry and photoelectrochemistry of iron(III) oxide. J Chem Soc, Faraday Trans 1, 1983, 79, 2027 doi: 10.1039/f19837902027[37] Gaudon M, Pailhé N, Majimel J, et al. Influence of Sn4+ and Sn4+/Mg2+ doping on structural features and visible absorption properties of α-Fe2O3 hematite. J Solid State Chem, 2010, 183, 2101 doi: 10.1016/j.jssc.2010.04.043[38] Krehula S, Štefanić G, Zadro K, et al. Synthesis and properties of iridium-doped hematite (α-Fe2O3). J Alloys Compd, 2012, 545, 200 doi: 10.1016/j.jallcom.2012.08.009[39] Ma Z L, Wen Z Y, Gu C P, et al. Doping of nonmetal Se in Fe2O3 nanowire array-based photoanodes for water oxidation. ACS Appl Nano Mater, 2021, 4, 13297 doi: 10.1021/acsanm.1c02807[40] Phuan Y W, Chong M N, Zhu T, et al. Effects of annealing temperature on the physicochemical, optical and photoelectrochemical properties of nanostructured hematite thin films prepared via electrodeposition method. Mater Res Bull, 2015, 69, 71 doi: 10.1016/j.materresbull.2014.12.059[41] Mohammadikish M. Hydrothermal synthesis, characterization and optical properties of ellipsoid shape α-Fe2O3 nanocrystals. Ceram Int, 2014, 40, 1351 doi: 10.1016/j.ceramint.2013.07.016[42] Piticescu R R, Motoc A M, Tudor A I, et al. Hydrothermal synthesis of nanostructured materials for energy harvesting applications. Int J Mater Chem Phys, 2015, 1, 31[43] Cai J G, Chen S Y, Ji M, et al. Organic additive-free synthesis of mesocrystalline hematite nanoplates via two-dimensional oriented attachment. CrystEngComm, 2014, 16, 1553 doi: 10.1039/C3CE41716F[44] Basavaraja S, Balaji D S, Bedre M D, et al. Solvothermal synthesis and characterization of acicular α-Fe2O3 nanoparticles. Bull Mater Sci, 2011, 34, 1313 doi: 10.1007/s12034-011-0321-z[45] Li G, Liu M Y, Kou H Z. Mesoporous α-Fe2O3 nanospheres: Structural evolution and investigation of magnetic properties. Chem Eur J, 2011, 17, 4323 doi: 10.1002/chem.201003068[46] Popov N, Ristić M, Bošković M, et al. Influence of Sn doping on the structural, magnetic, optical and photocatalytic properties of hematite (α-Fe2O3) nanoparticles. J Phys Chem Solids, 2022, 161, 110372 doi: 10.1016/j.jpcs.2021.110372[47] Chao J, Wang H T, Xia B, et al. Metal acetylacetonate domains grown on H-terminated porous silicon at room temperature and their specific I-V behavior. J Phys Chem B, 2006, 110, 24565 doi: 10.1021/jp0628481[48] Liang X, Wang X, Zhuang J, et al. Synthesis of nearly monodisperse iron oxide and oxyhydroxide nanocrystals. Adv Funct Mater, 2006, 16, 1805 doi: 10.1002/adfm.200500884[49] Jubb A M, Allen H C. Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. ACS Appl Mater Interfaces, 2010, 2, 2804 doi: 10.1021/am1004943[50] Hu Y S, Kleiman-Shwarsctein A, Forman A J, et al. Pt-doped α-Fe2O3 thin films active for photoelectrochemical water splitting. Chem Mater, 2008, 20, 3803 doi: 10.1021/cm800144q[51] Mansour H, Bargougui R, Autret-Lambert C, et al. Co-precipitation synthesis and characterization of tin-doped α-Fe2O3 nanoparticles with enhanced photocatalytic activities. J Phys Chem Solids, 2018, 114, 1 doi: 10.1016/j.jpcs.2017.11.013[52] Souza J C, Ribeiro R A P, da Trindade L G, et al. Unconventional disorder by femtosecond laser irradiation in Fe2O3. ACS Omega, 2021, 6, 28049 doi: 10.1021/acsomega.1c04079[53] Chen L Q, Yang X F, Chen J, et al. Continuous shape- and spectroscopy-tuning of hematite nanocrystals. Inorg Chem, 2010, 49, 8411 doi: 10.1021/ic100919a[54] Grätzel M. Photoelectrochemical cells. Nature, 2001, 414, 338 doi: 10.1038/35104607[55] Luo T, Hou X H, Liang Q, et al. The influence of Manganese ions doping on nanosheet assembly NiFe2O4 for the removal of Congo red. J Alloys Compd, 2018, 763, 780 doi: 10.1016/j.jallcom.2018.05.203[56] Ling Y, Li Y. Review of Sn-doped hematite nanostructures for photoelectrochemical water splitting. Part Part Syst Charact, 2014, 31, 1113 doi: 10.1002/ppsc.201400051[57] Em S, Yedigenov M, Khamkhash L, et al. Sn-doped hematite nanoparticles for potential photocatalytic dye degradation. IOP Conf Ser: Mater Sci Eng, 2020, 739, 012042 doi: 10.1088/1757-899X/739/1/012042[58] Qin D D, Li Y L, Wang T, et al. Sn-doped hematite films as photoanodes for efficient photoelectrochemical water oxidation. J Mater Chem A, 2015, 3, 6751 doi: 10.1039/C4TA06872F[59] Pan H J, Ao D B, Qin G W. Synergistic effects of dopant (Ti or Sn) and oxygen vacancy on the electronic properties of hematite: A DFT investigation. RSC Adv, 2020, 10, 23263 doi: 10.1039/D0RA01450H[60] Ravi G, Ravichandran S, Ameen F, et al. Sn doped α-Fe2O3 (Sn = 0, 10, 20, 30 wt%) photoanodes for photoelectrochemical water splitting applications. Renew Energy, 2018, 133, 566 doi: 10.1016/j.renene.2018.10.067[61] Barroso M, Pendlebury S R, Cowan A J, et al. Charge carrier trapping, recombination and transfer in hematite (α-Fe2O3) water splitting photoanodes. Chem Sci, 2013, 4, 2724 doi: 10.1039/c3sc50496d[62] Iordanova N, Dupuis M, Rosso K M. Charge transport in metal oxides: A theoretical study of hematite alpha-Fe2O3. J Chem Phys, 2005, 122, 144305 doi: 10.1063/1.1869492[63] Glasscock J A, Barnes P R F, Plumb I C, et al. Enhancement of photoelectrochemical hydrogen production from hematite thin films by the introduction of Ti and Si. J Phys Chem C, 2007, 111, 16477 doi: 10.1021/jp074556l[64] Zhang S Y, Hajiyani H, Hufnagel A G, et al. Sn-doped hematite for photoelectrochemical water splitting: The effect of Sn concentration. Z Phys Chem, 2020, 234, 683 doi: 10.1515/zpch-2019-1482 -
Proportional views




 DownLoad:
DownLoad: