| Citation: |
Li Zhong, Xiaobao Li, Wei Wang, Xinle Xiao. Electromechanical and photoelectric properties of a novel semiconducting Janus InGaSSe monolayer[J]. Journal of Semiconductors, 2023, 44(1): 012701. doi: 10.1088/1674-4926/44/1/012701
L Zhong, X B Li, W Wang, X L Xiao. Electromechanical and photoelectric properties of a novel semiconducting Janus InGaSSe monolayer[J]. J. Semicond, 2023, 44(1): 012701. doi: 10.1088/1674-4926/44/1/012701
Export: BibTex EndNote
|
Electromechanical and photoelectric properties of a novel semiconducting Janus InGaSSe monolayer
doi: 10.1088/1674-4926/44/1/012701
More Information-
Abstract
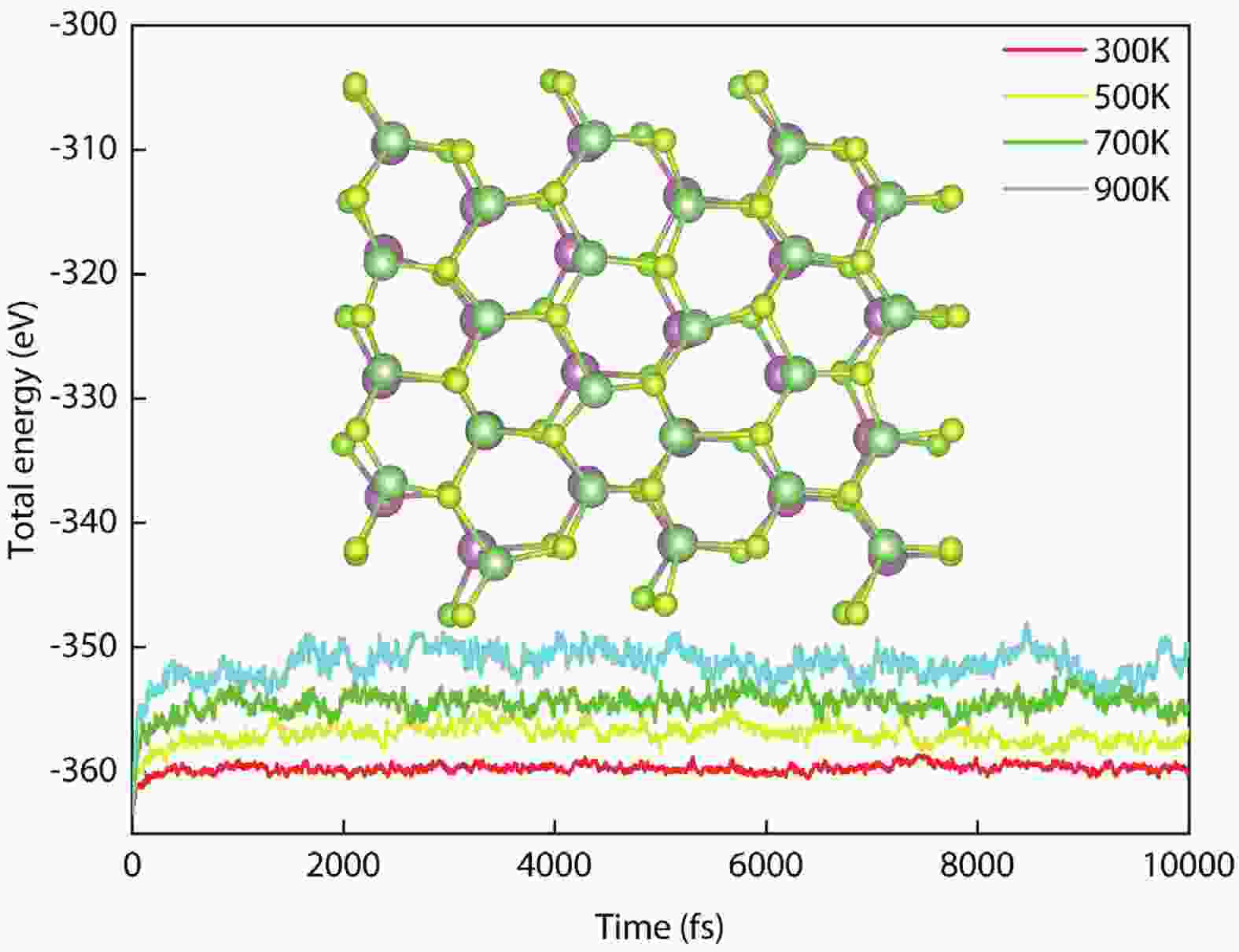
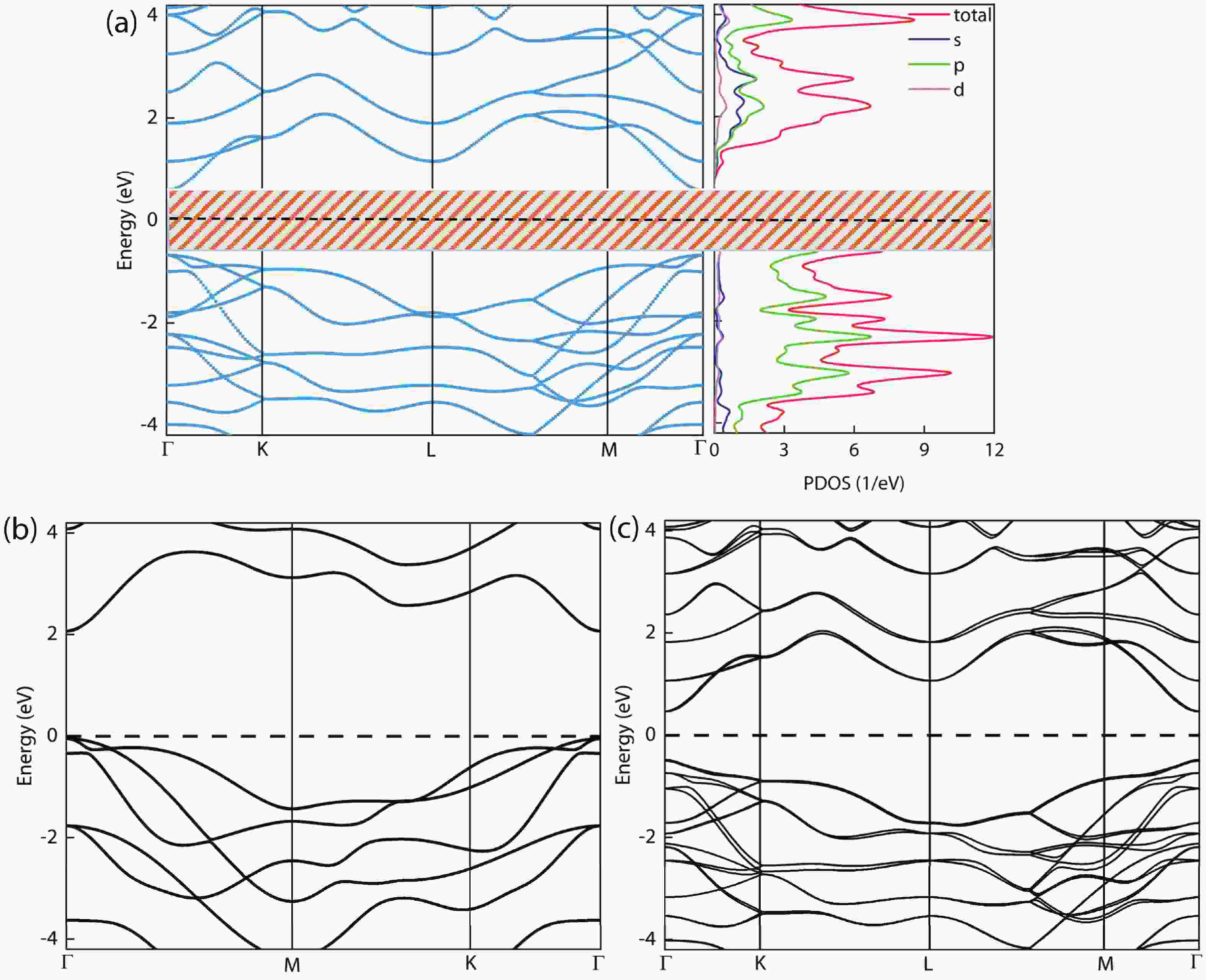
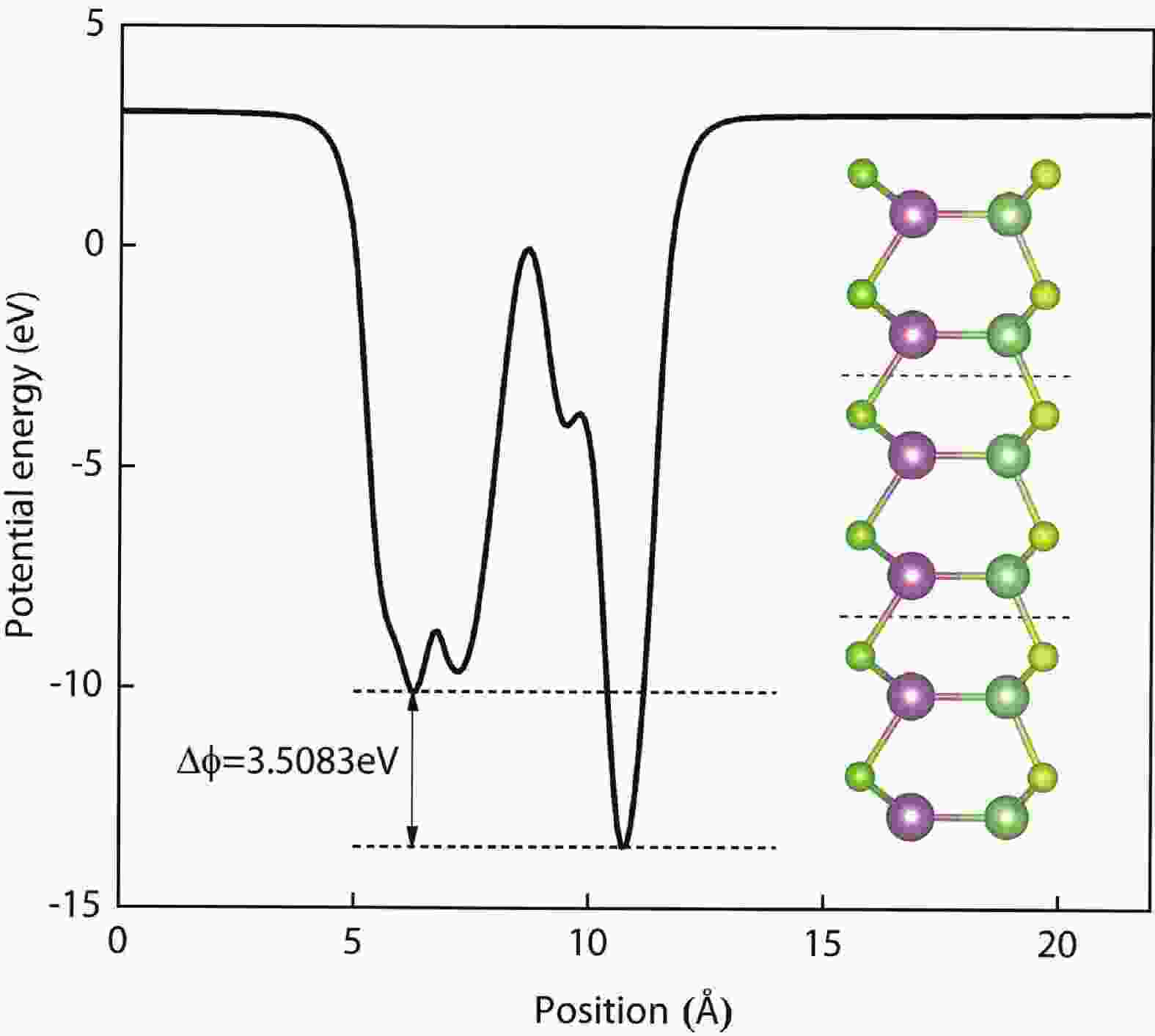
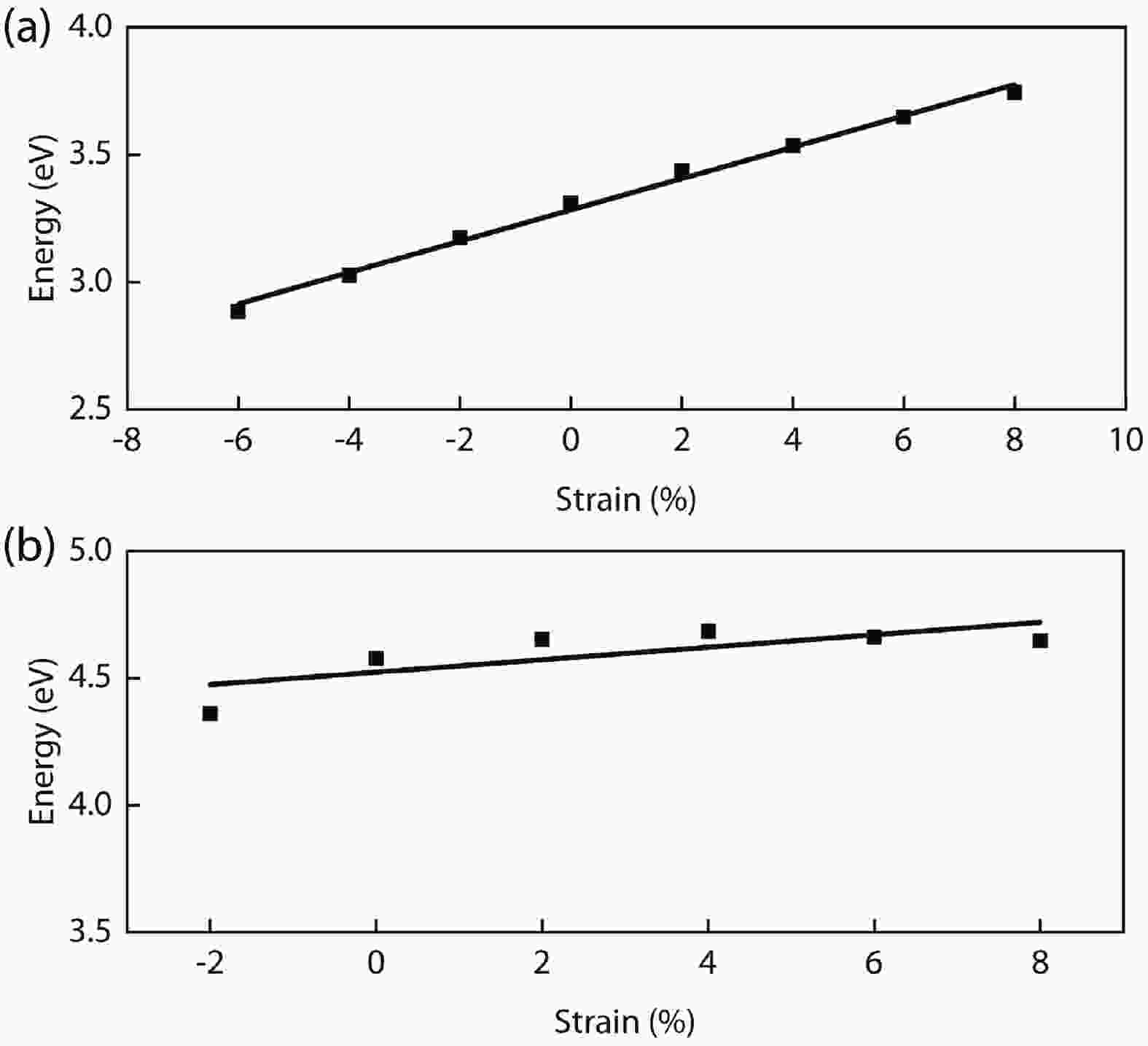
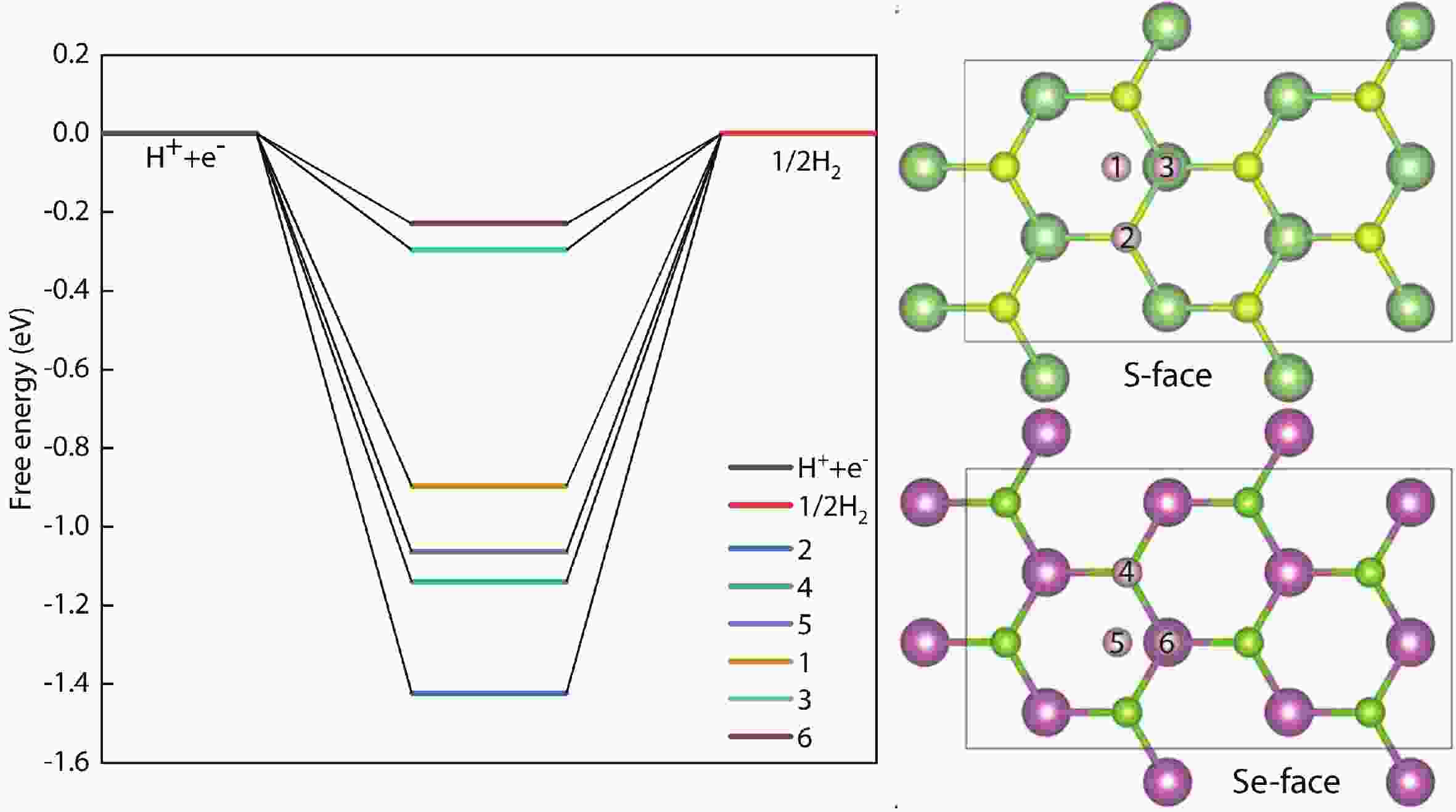
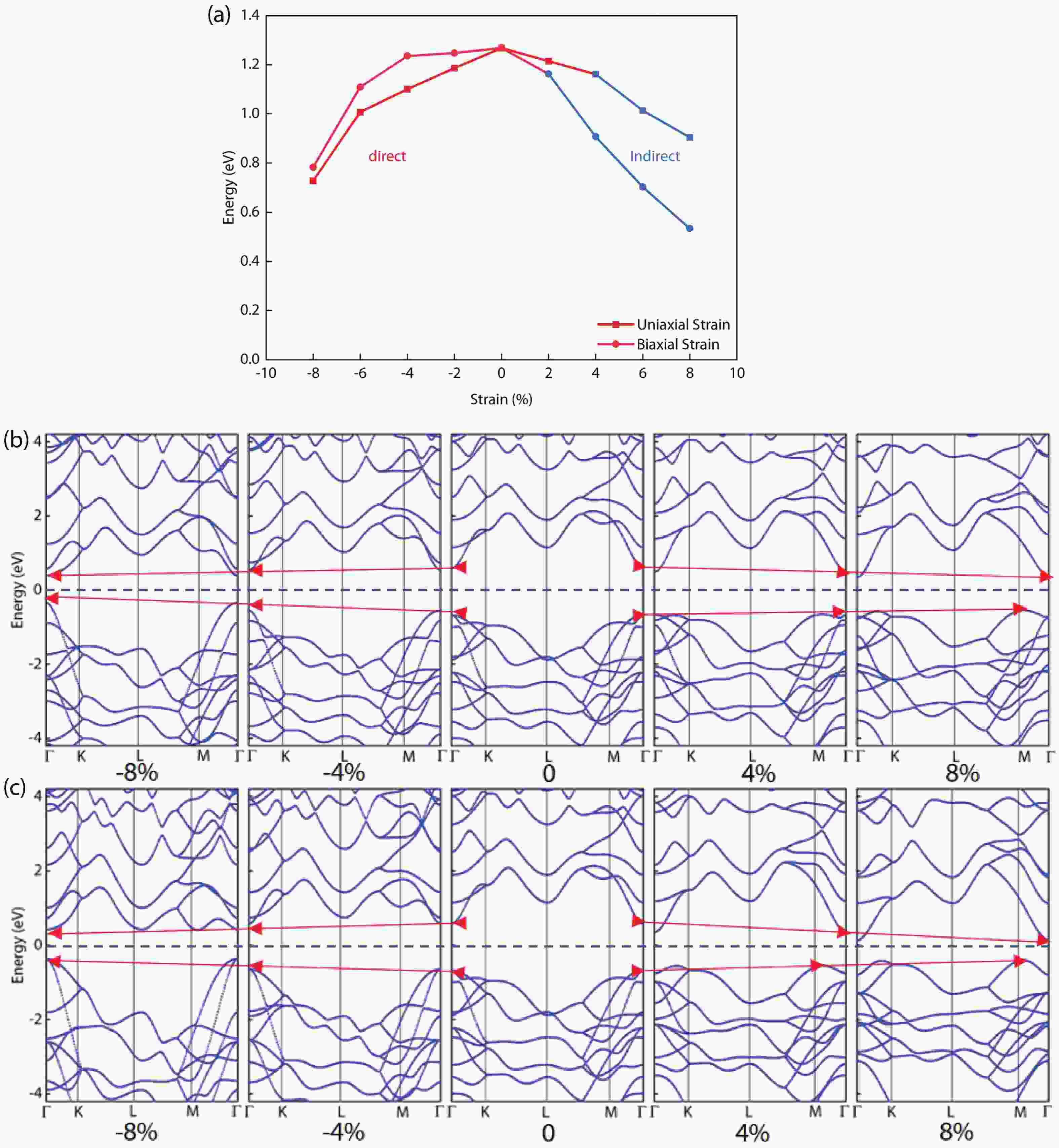
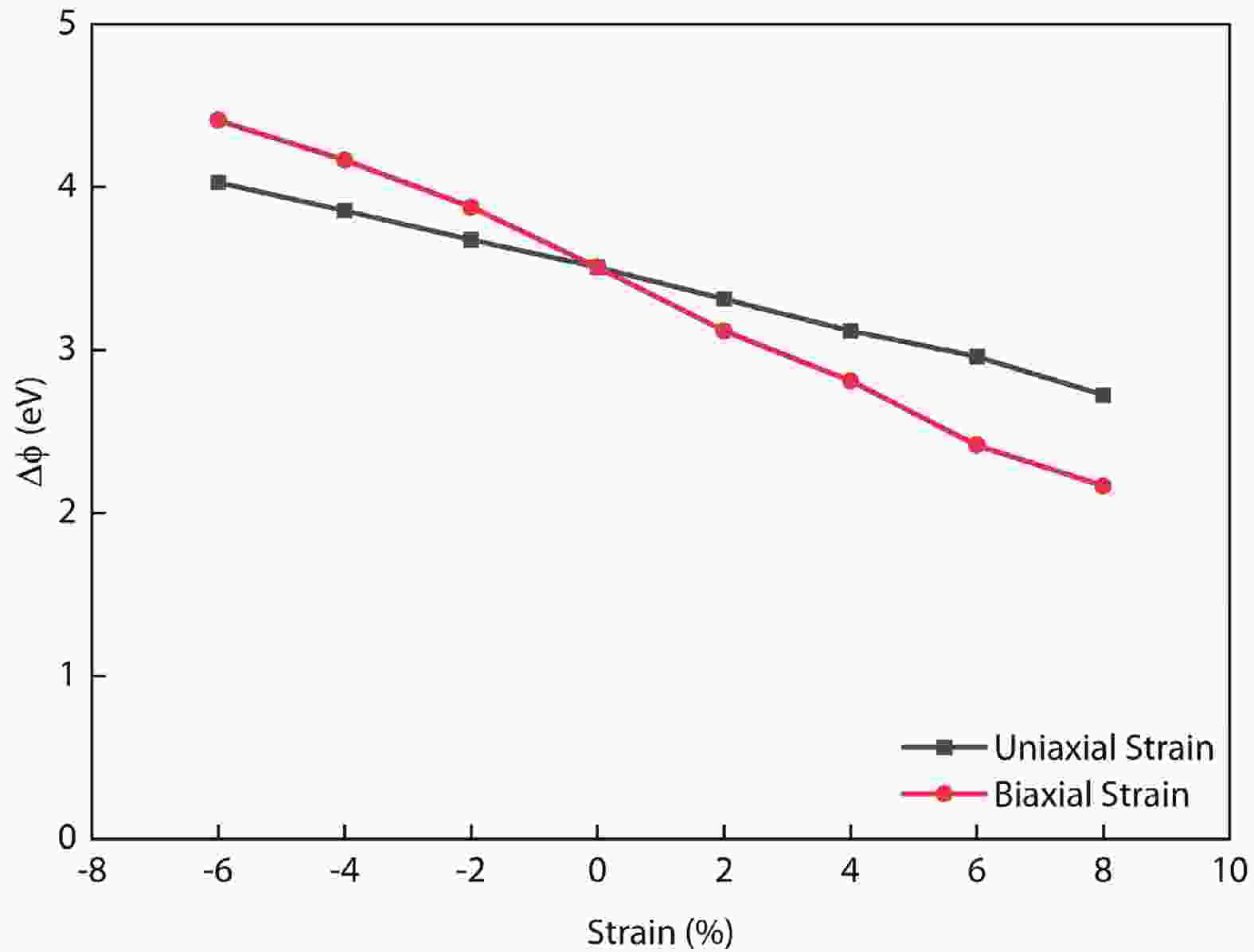
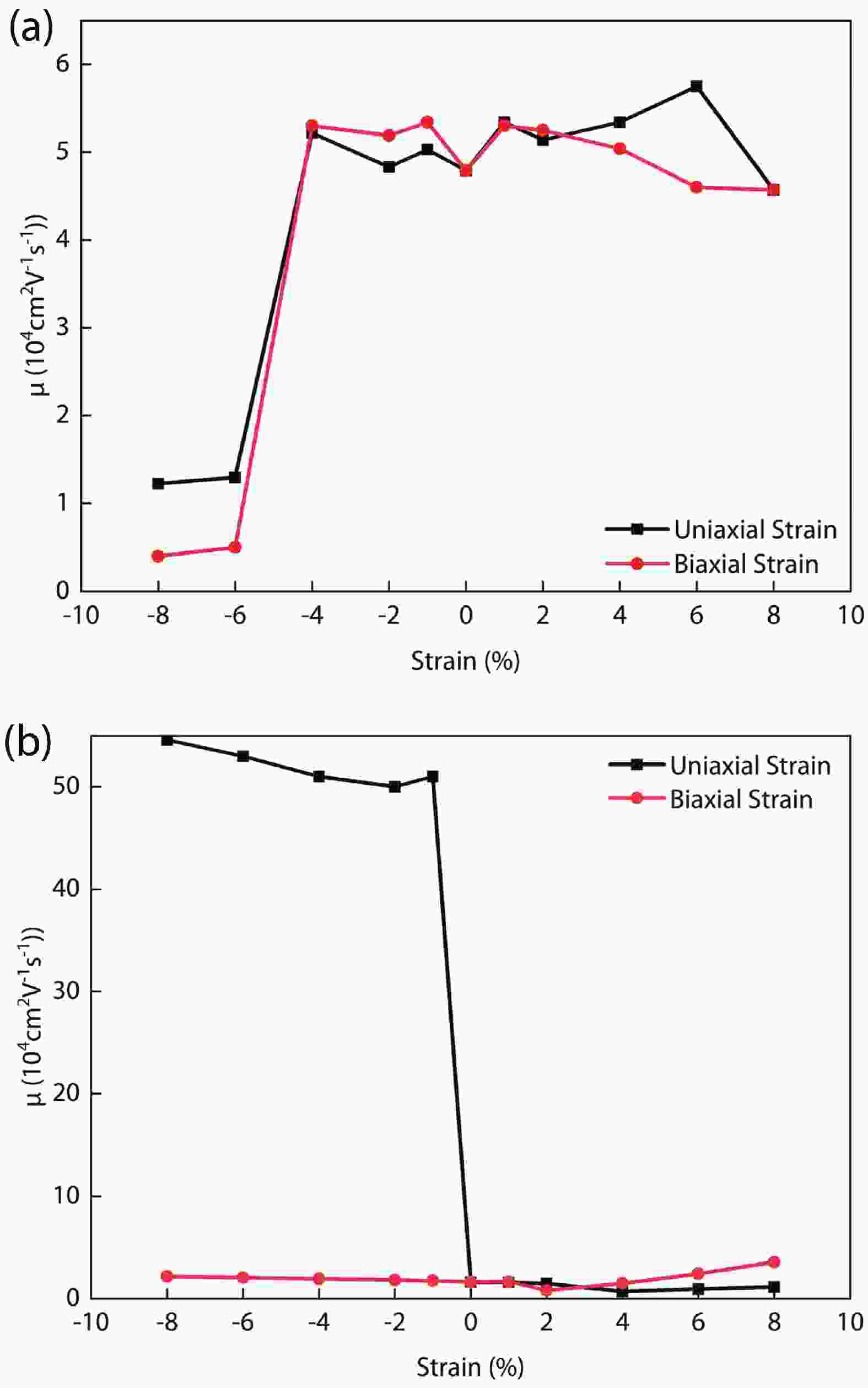
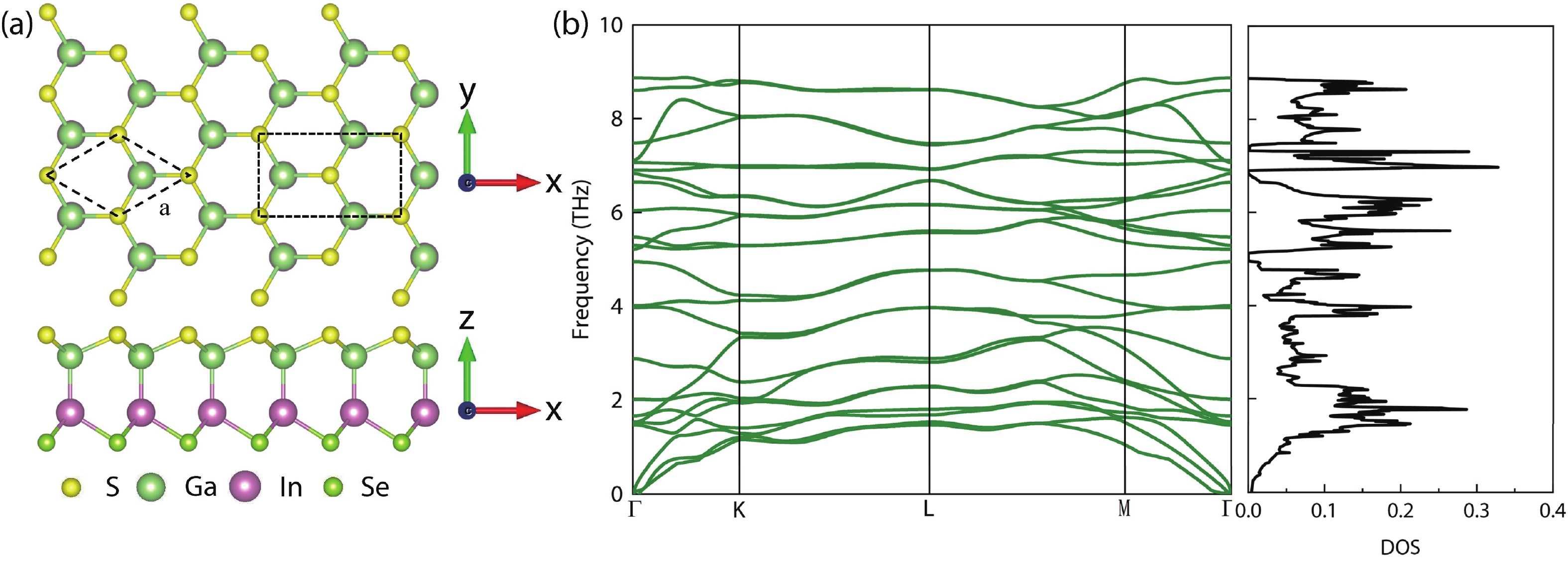
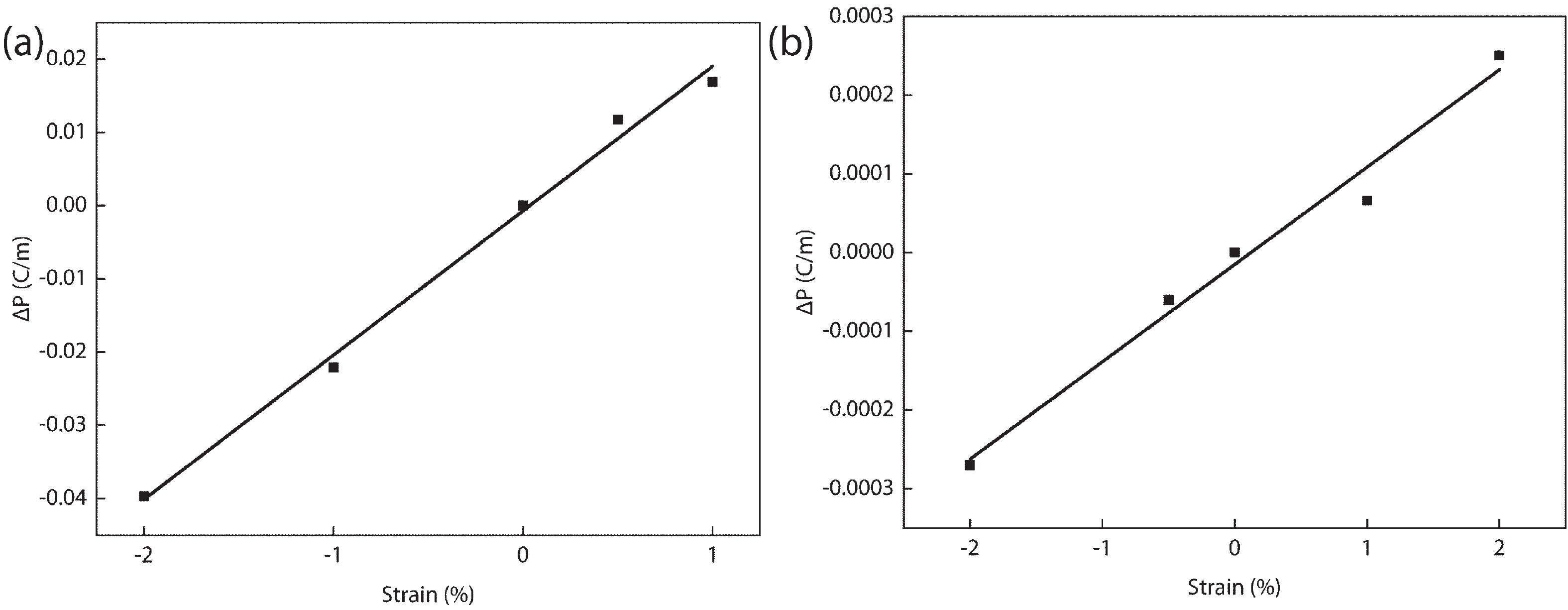
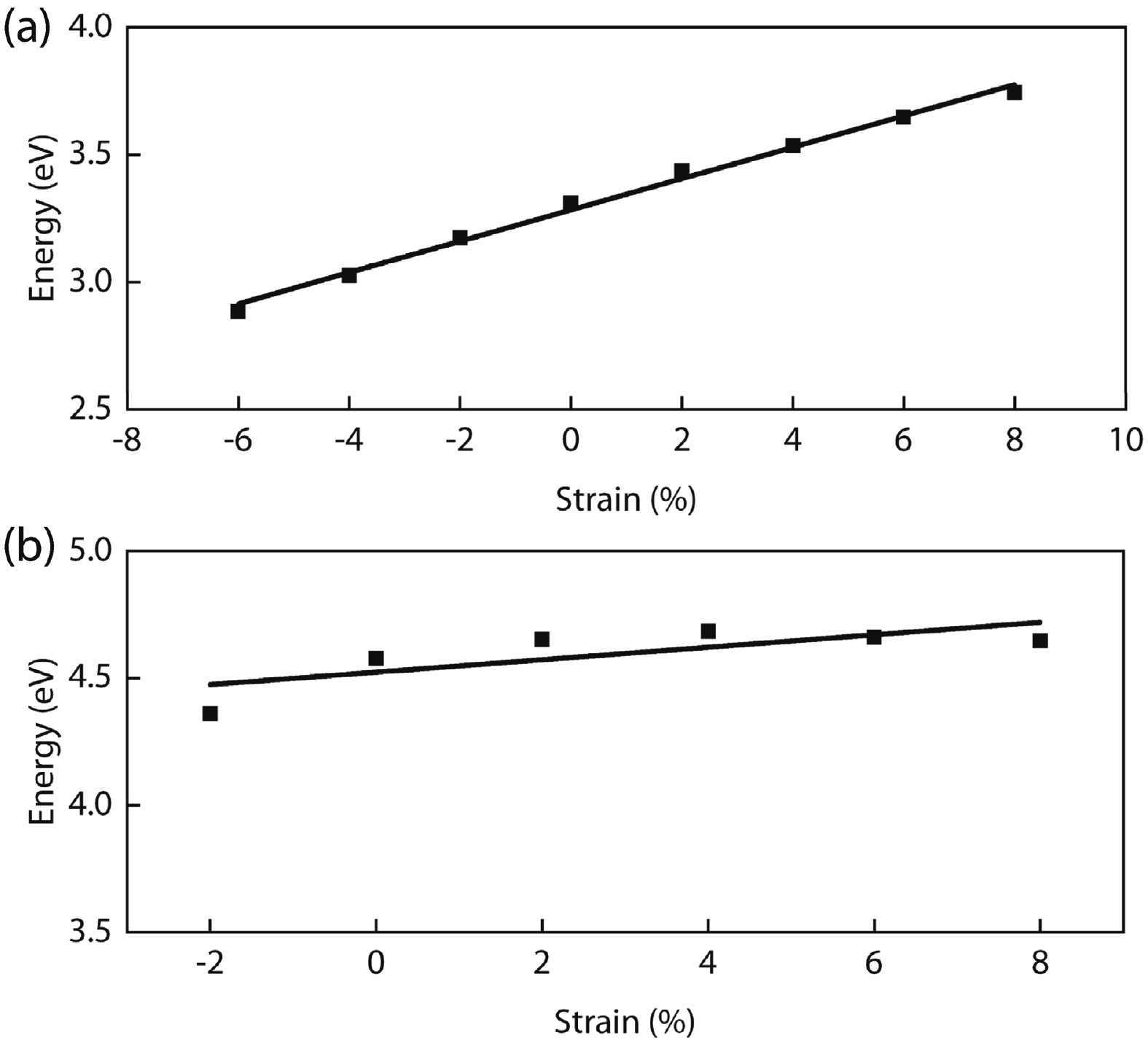
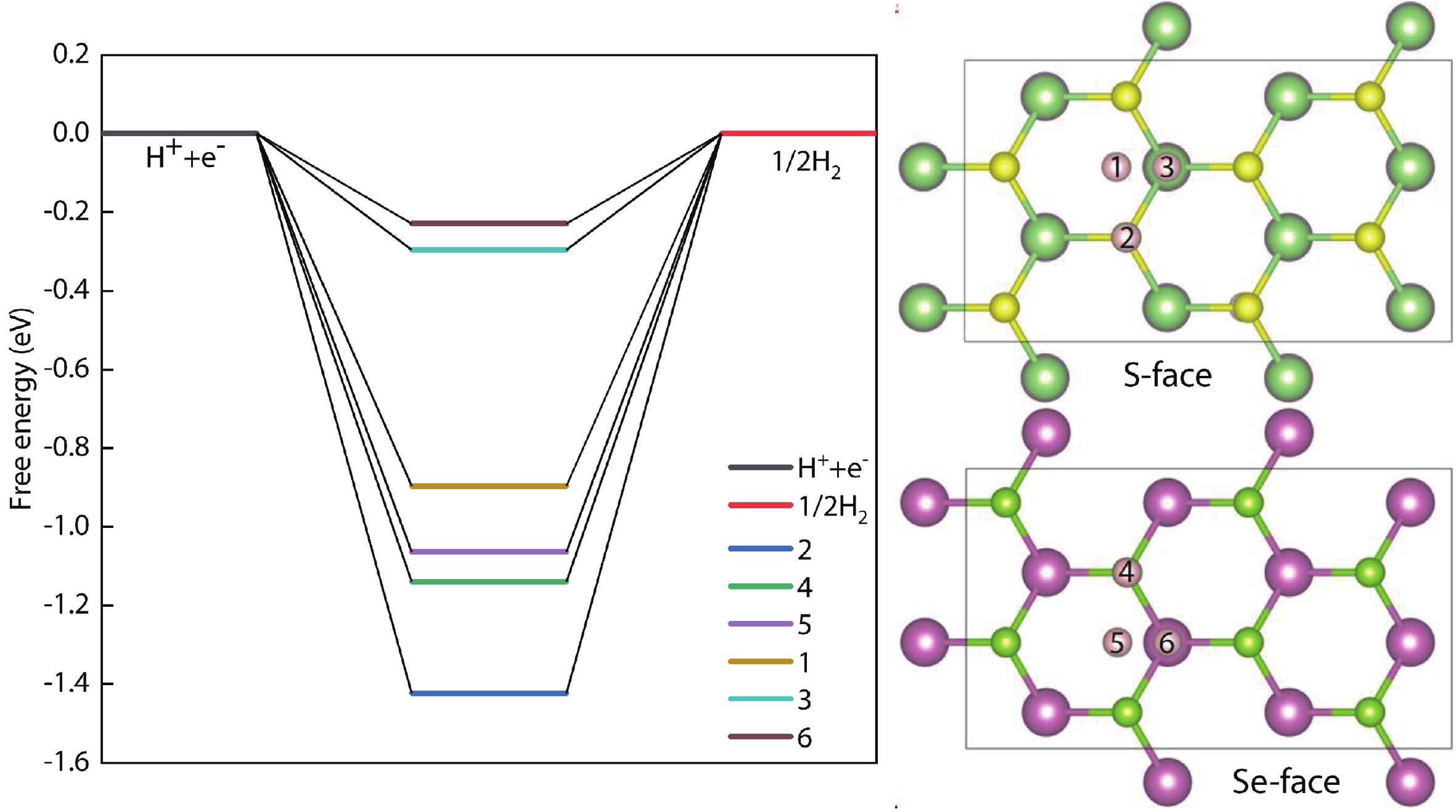
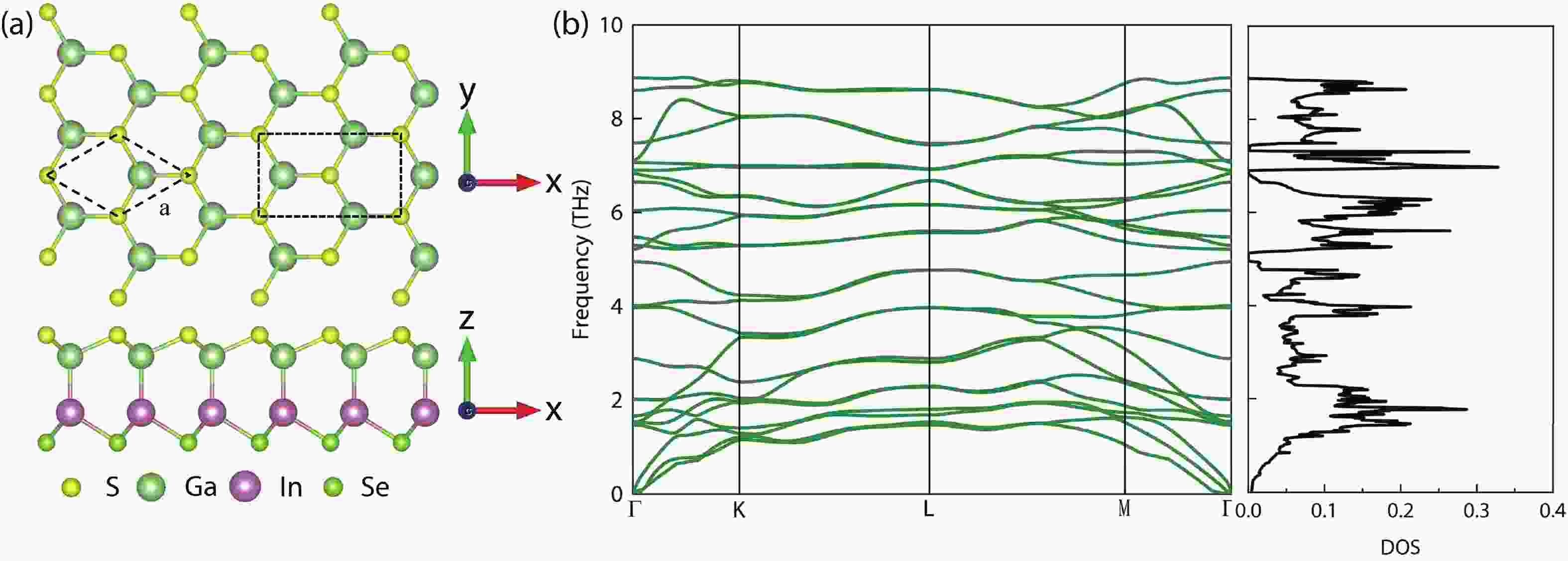
In recent years, Janus two-dimensional (2D) materials have received extensive research interests because of their outstanding electronic, mechanical, electromechanical, and optoelectronic properties. In this work, we explore the structural, electromechanical, and optoelectronic properties of a novel hypothesized Janus InGaSSe monolayer by means of first-principles calculations. It is confirmed that the Janus InGaSSe monolayer indeed show extraordinary charge transport properties with intrinsic electron mobility of 48 139 cm2/(V·s) and hole mobility of 16 311 cm2/(V·s). Both uniaxial and biaxial strains can effectively tune its electronic property. Moreover, the Janus InGaSSe monolayer possesses excellent piezoelectric property along both in-plane and out-of-plane directions. The results of this work imply that the Janus InGaSSe monolayer is in fact an efficient photocatalyst candidate, and may provide useful guidelines for the discovery of other new 2D photocatalytic and piezoelectric materials.
-
References
[1] Zhang B T, Liu J, Yue S Z, et al. Hot electron injection: An efficacious approach to charge LaCoO3 for improving the water splitting efficiency. Appl Catal B, 2017, 219, 432 doi: 10.1016/j.apcatb.2017.07.033[2] Wang W, Tade M O, Shao Z P. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem Soc Rev, 2015, 44, 5371 doi: 10.1039/C5CS00113G[3] Ma M M, Huang Y B, Liu J, et al. Engineering the photoelectrochemical behaviors of ZnO for efficient solar water splitting. J Semicond, 2020, 41, 091702 doi: 10.1088/1674-4926/41/9/091702[4] Concina I, Ibupoto Z H, Vomiero A. Electrochemical water splitting: Semiconducting metal oxide nanostructures for water splitting and photovoltaics. Adv Energy Mater, 2017, 7, 1770138 doi: 10.1002/aenm.201770138[5] Zhao H, Dai Z Y, Xu X Y, et al. Integrating semiconducting catalyst of ReS2 nanosheets into P-silicon photocathode for enhanced solar water reduction. ACS Appl Mater Interfaces, 2018, 10, 23074 doi: 10.1021/acsami.8b04740[6] Reece S Y, Hamel J A, Sung K, et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science, 2011, 334, 645 doi: 10.1126/science.1209816[7] Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films. Science, 2004, 306, 666 doi: 10.1126/science.1102896[8] Alyörük M M. Piezoelectric properties of monolayer II-VI group oxides by first-principles calculations. Phys Status Solidi B, 2016, 253, 2534 doi: 10.1002/pssb.201600387[9] Li X M, Zhang X, Park H, et al. Editorial: Electronics and optoelectronics of graphene and related 2D materials. Front Mater, 2020, 7, 235 doi: 10.3389/fmats.2020.00235[10] Liu B, Zhou K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog Mater Sci, 2019, 100, 99 doi: 10.1016/j.pmatsci.2018.09.004[11] Li C, Li J, Huang Y B, et al. Recent development in electronic structure tuning of graphitic carbon nitride for highly efficient photocatalysis. J Semicond, 2022, 43, 021701 doi: 10.1088/1674-4926/43/2/021701[12] Taghinejad H, Rehn D A, Muccianti C, et al. Defect-mediated alloying of monolayer transition-metal dichalcogenides. ACS Nano, 2018, 12, 12795 doi: 10.1021/acsnano.8b07920[13] Qiao M, Liu J, Wang Y, et al. PdSeO3 monolayer: Promising inorganic 2D photocatalyst for direct overall water splitting without using sacrificial reagents and cocatalysts. J Am Chem Soc, 2018, 140, 12256 doi: 10.1021/jacs.8b07855[14] Sun Y H, Wang X J, Zhao X G, et al. First-principle high-throughput calculations of carrier effective masses of two-dimensional transition metal dichalcogenides. J Semicond, 2018, 39, 072001 doi: 10.1088/1674-4926/39/7/072001[15] Lu A Y, Zhu H Y, Xiao J, et al. Janus monolayers of transition metal dichalcogenides. Nat Nanotechnol, 2017, 12, 744 doi: 10.1038/nnano.2017.100[16] Zhang J, Jia S, Kholmanov I, et al. Janus monolayer transition-metal dichalcogenides. ACS Nano, 2017, 11, 8192 doi: 10.1021/acsnano.7b03186[17] Ju L, Bie M, Shang J, et al. Janus transition metal dichalcogenides: A superior platform for photocatalytic water splitting. J Phys Mater, 2020, 3, 022004 doi: 10.1088/2515-7639/ab7c57[18] Zhao P, Ma Y D, Lv X S, et al. Two-dimensional III2-VI3 materials: Promising photocatalysts for overall water splitting under infrared light spectrum. Nano Energy, 2018, 51, 533 doi: 10.1016/j.nanoen.2018.07.010[19] Zhou X F, Shen B, Lyubartsev A, et al. Semiconducting piezoelectric heterostructures for piezo- and piezophotocatalysis. Nano Energy, 2022, 96, 107141 doi: 10.1016/j.nanoen.2022.107141[20] Schneider J, Matsuoka M, Takeuchi M, et al. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem Rev, 2014, 114, 9919 doi: 10.1021/cr5001892[21] Liao G F, Gong Y, Zhang L, et al. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ Sci, 2019, 12, 2080 doi: 10.1039/C9EE00717B[22] Wang P, Zong Y X, Liu H, et al. Highly efficient photocatalytic water splitting and enhanced piezoelectric properties of 2D Janus group-III chalcogenides. J Mater Chem C, 2021, 9, 4989 doi: 10.1039/D1TC00318F[23] Rahaman R, Sharmin M, Podder J. Band gap tuning and p to n-type transition in Mn-doped CuO nanostructured thin films. J Semicond, 2022, 43, 012801 doi: 10.1088/1674-4926/43/1/012801[24] Yin Z, Hu M, Liu J, et al. Tunable crystal structure of Cu-Zn-Sn-S nanocrystals for improving photocatalytic hydrogen evolution enabled by copper element regulation. J Semicond, 2022, 43, 032701 doi: 10.1088/1674-4926/43/3/032701[25] Lee H J, Lee S W, Hwang H, et al. Vertically oriented MoS2/WS2 heterostructures on reduced graphene oxide sheets as electrocatalysts for hydrogen evolution reaction. Mater Chem Front, 2021, 5, 3396 doi: 10.1039/D1QM00051A[26] Wang S K, Ren C D, Tian H Y, et al. MoS2/ZnO van der Waals heterostructure as a high-efficiency water splitting photocatalyst: A first-principles study. Phys Chem Chem Phys, 2018, 20, 13394 doi: 10.1039/C8CP00808F[27] Dang C Q, Lu A L, Wang H Y, et al. Diamond semiconductor and elastic strain engineering. J Semicond, 2022, 43, 021801 doi: 10.1088/1674-4926/43/2/021801[28] Thulin L, Guerra J. Calculations of strain-modified anatase TiO2 band structures. Phys Rev B, 2008, 77, 195112 doi: 10.1103/PhysRevB.77.195112[29] Gao Q, Sahin H, Kang J. Strain tunable band structure of a new 2D carbon allotrope C568. J Semicond, 2020, 41, 082005 doi: 10.1088/1674-4926/41/8/082005[30] Giannozzi P, Baroni S, Bonini N, et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J Phys: Condens Matter, 2009, 21, 395502 doi: 10.1088/0953-8984/21/39/395502[31] Giannozzi P, Andreussi O, Brumme T, et al. Advanced capabilities for materials modelling with QUA NTUM ESPRESSO. J Phys: Condens Matter, 2017, 29, 465901 doi: 10.1088/1361-648X/aa8f79[32] Heyd J, Scuseria G E, Ernzerhof M. Hybrid functionals based on a screened Coulomb potential. J Chem Phys, 2003, 118, 8207 doi: 10.1063/1.1564060[33] Heyd J, Scuseria G E, Ernzerhof M. Erratum: "Hybrid functionals based on a screened Coulomb potential" [J. Chem. Phys. 118, 8207 (2003)]. J Chem Phys, 2006, 124, 219906 doi: 10.1063/1.2204597[34] Vanderbilt D. Berry-phase theory of proper piezoelectric response. J Phys Chem Solids, 2000, 61, 147 doi: 10.1016/S0022-3697(99)00273-5[35] Guo S D. Phonon transport in Janus monolayer MoSSe: A first-principles study. Phys Chem Chem Phys, 2018, 20, 7236 doi: 10.1039/C8CP00350E[36] Dong L, Lou J, Shenoy V B. Large In-plane and vertical piezoelectricity in Janus transition metal dichalchogenides. ACS Nano, 2017, 11, 8242 doi: 10.1021/acsnano.7b03313[37] Ericksen J L. On the cauchy—Born rule. Math Mech Solids, 2008, 13, 199 doi: 10.1177/1081286507086898[38] Born M, Huang K. Dynamical theory of crystal lattices. Oxford: Clarendon Press, 1954[39] Peng R, Ma Y D, Huang B B, et al. Two-dimensional Janus PtSSe for photocatalytic water splitting under the visible or infrared light. J Mater Chem A, 2019, 7, 603 doi: 10.1039/C8TA09177C[40] Kaasbjerg K, Thygesen K S, Jauho A P. Acoustic phonon limited mobility in two-dimensional semiconductors: Deformation potential and piezoelectric scattering in monolayer MoS2 from first principles. Phys Rev B, 2013, 87, 235312 doi: 10.1103/PhysRevB.87.235312[41] Xi J Y, Long M Q, Tang L, et al. First-principles prediction of charge mobility in carbon and organic nanomaterials. Nanoscale, 2012, 4, 4348 doi: 10.1039/c2nr30585b[42] Yin W J, Wen B, Nie G Z, et al. Tunable dipole and carrier mobility for a few layer Janus MoSSe structure. J Mater Chem C, 2018, 6, 1693 doi: 10.1039/C7TC05225A[43] Zhang Y Z, Ye H, Yu Z Y, et al. First-principles study of square phase MX2 and Janus$MX\varUpsilon $ (M = Mo, W;$X $ ,$\varUpsilon $ = S, Se, Te) transition metal dichalcogenide monolayers under biaxial strain. Phys E, 2019, 110, 134 doi: 10.1016/j.physe.2019.02.009[44] Chaves A, Azadani J G, Alsalman H, et al. Bandgap engineering of two-dimensional semiconductor materials. npj 2D Mater Appl, 2020, 4, 1 doi: 10.1038/s41699-019-0135-1 -
Proportional views
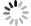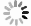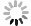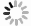







 DownLoad:
DownLoad: