| Citation: |
Yu Li, Shuaibing Wang, Jie Chen, Ouyang Lin, Zhe Yin, Chunhe Yang, Aiwei Tang. From kesterite 2D nanosheets to wurtzite 1D nanorods: controllable synthesis of Cu−Zn−Sn−S and their application in electrocatalytic hydrogen evolution[J]. Journal of Semiconductors, 2023, 44(12): 122701. doi: 10.1088/1674-4926/44/12/122701
Y Li, S B Wang, J Chen, O Y Lin, Z Yin, C H Yang, A W Tang. From kesterite 2D nanosheets to wurtzite 1D nanorods: controllable synthesis of Cu−Zn−Sn−S and their application in electrocatalytic hydrogen evolution[J]. J. Semicond, 2023, 44(12): 122701. doi: 10.1088/1674-4926/44/12/122701
Export: BibTex EndNote
|
From kesterite 2D nanosheets to wurtzite 1D nanorods: controllable synthesis of Cu−Zn−Sn−S and their application in electrocatalytic hydrogen evolution
doi: 10.1088/1674-4926/44/12/122701
More Information-
Abstract
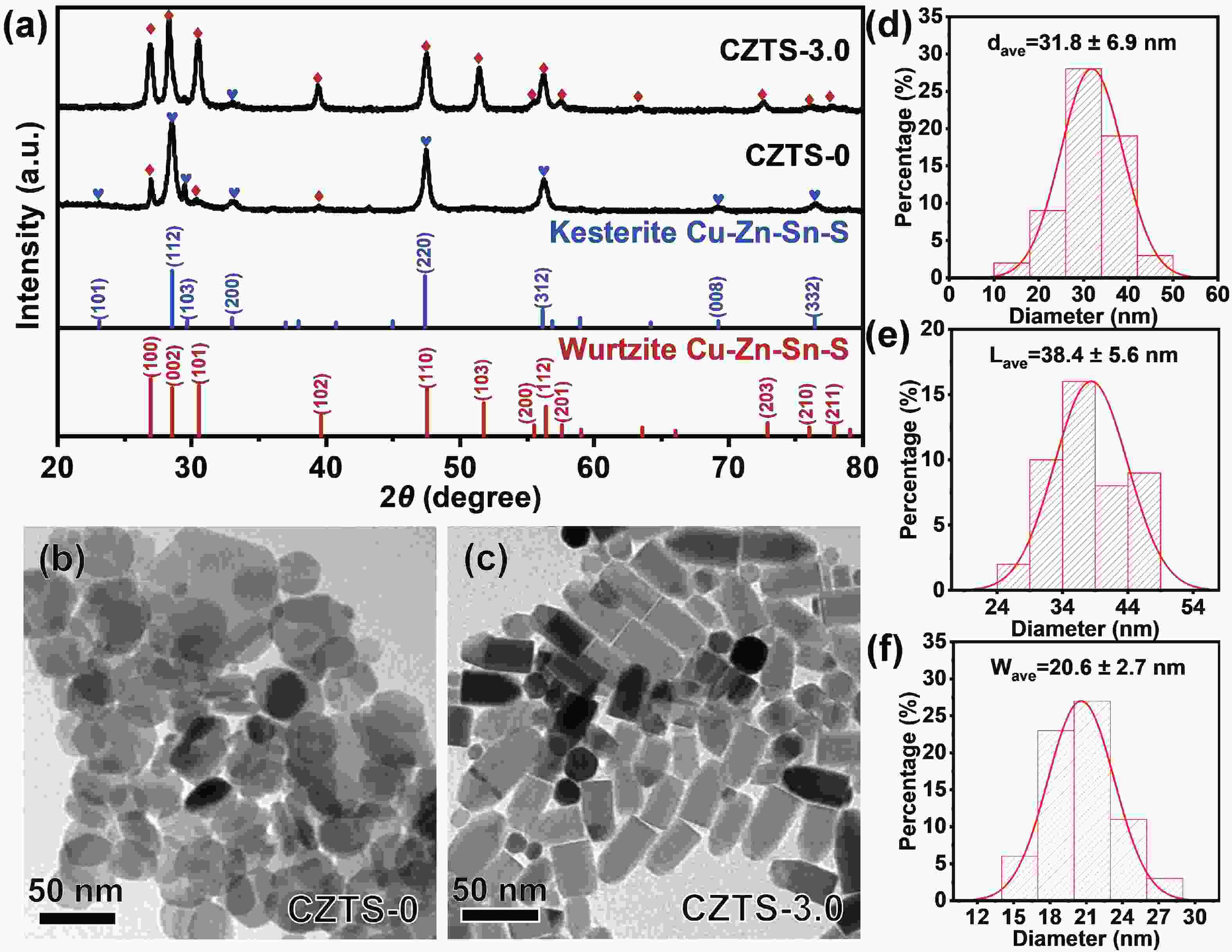
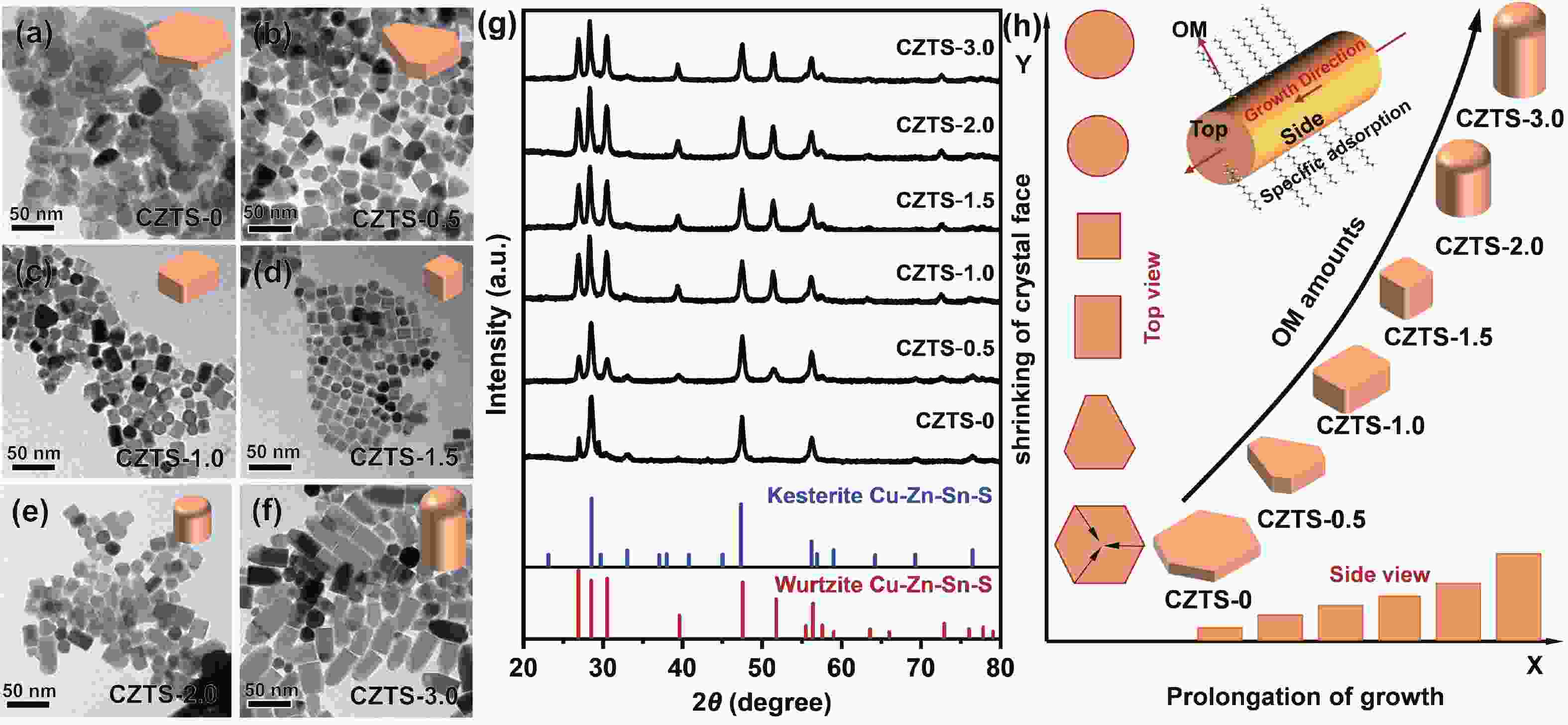
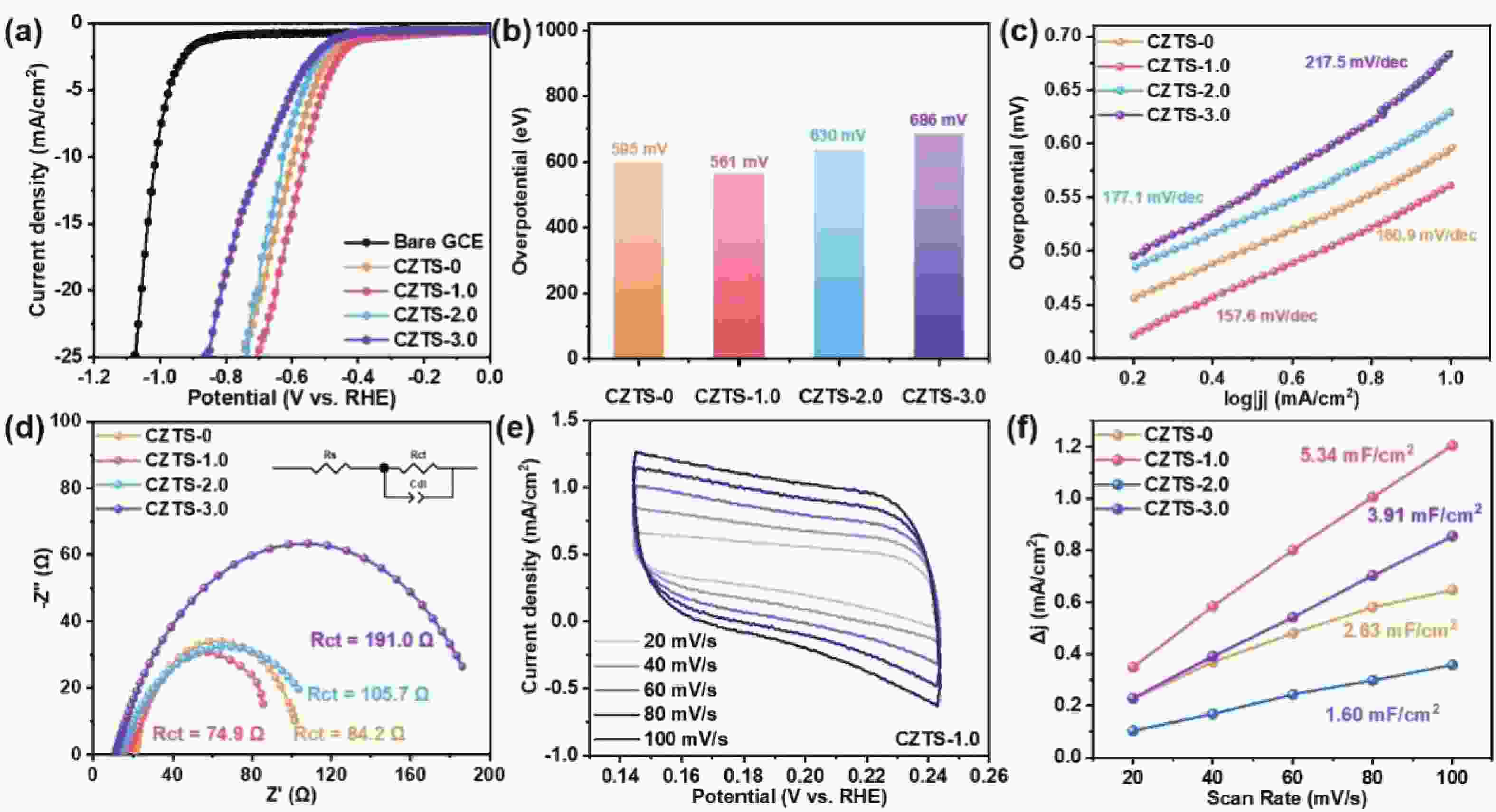
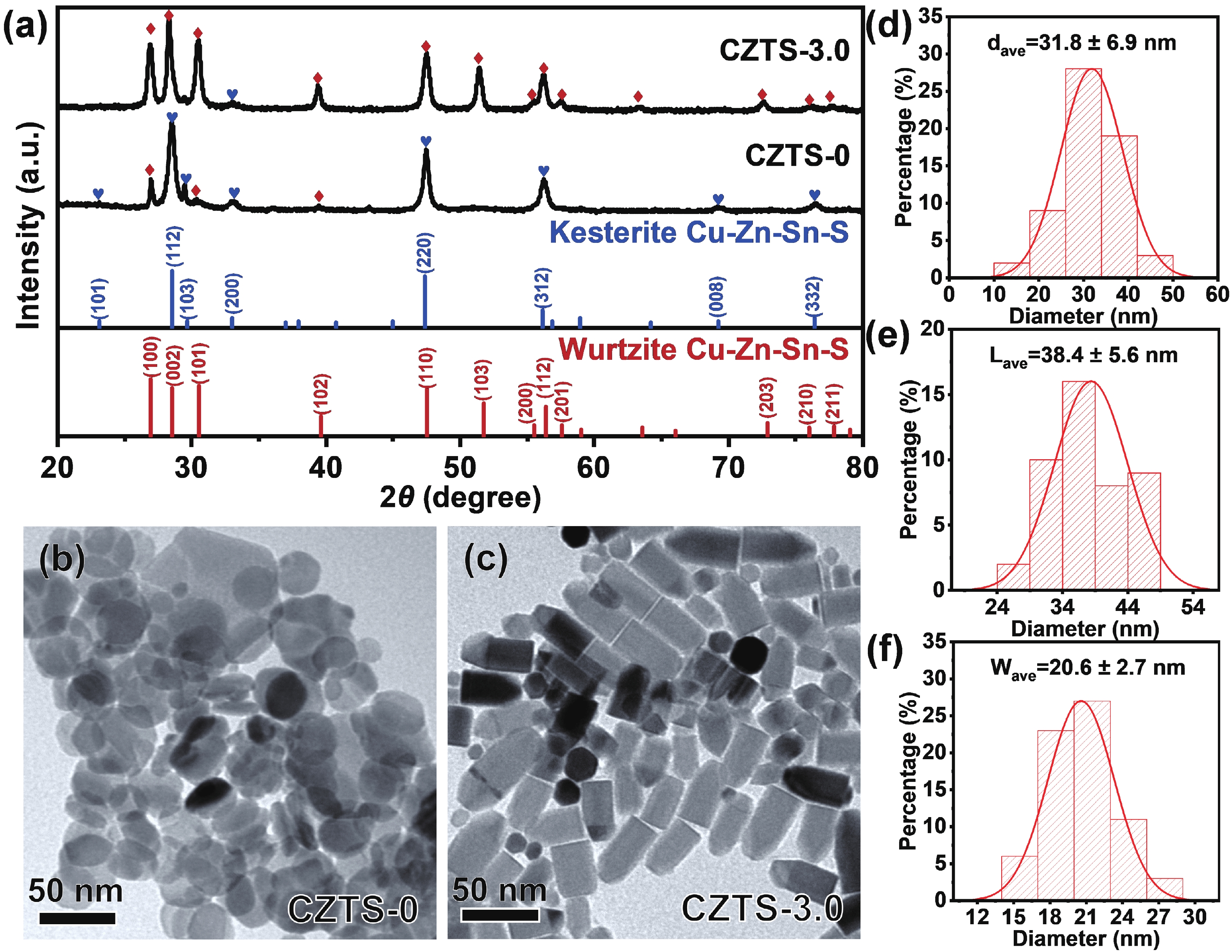
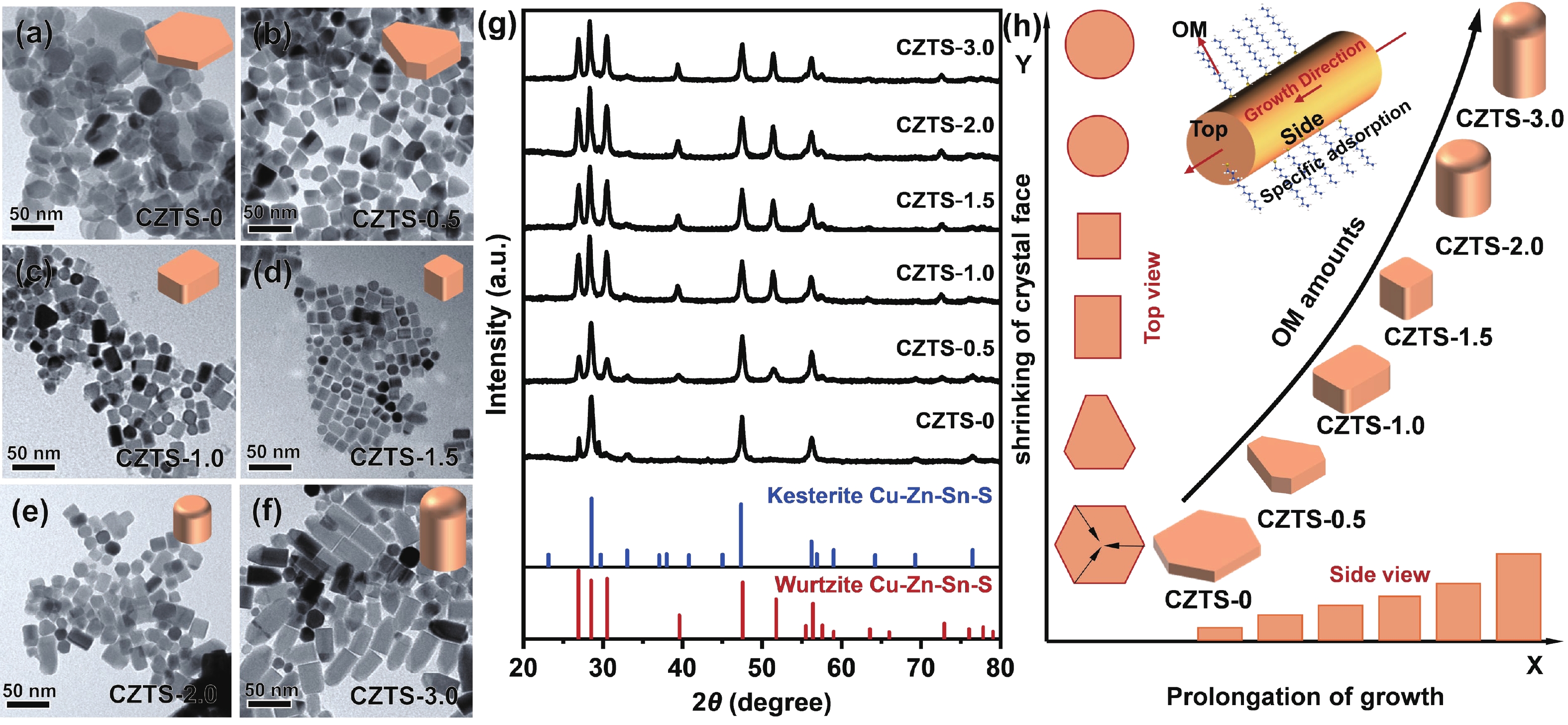
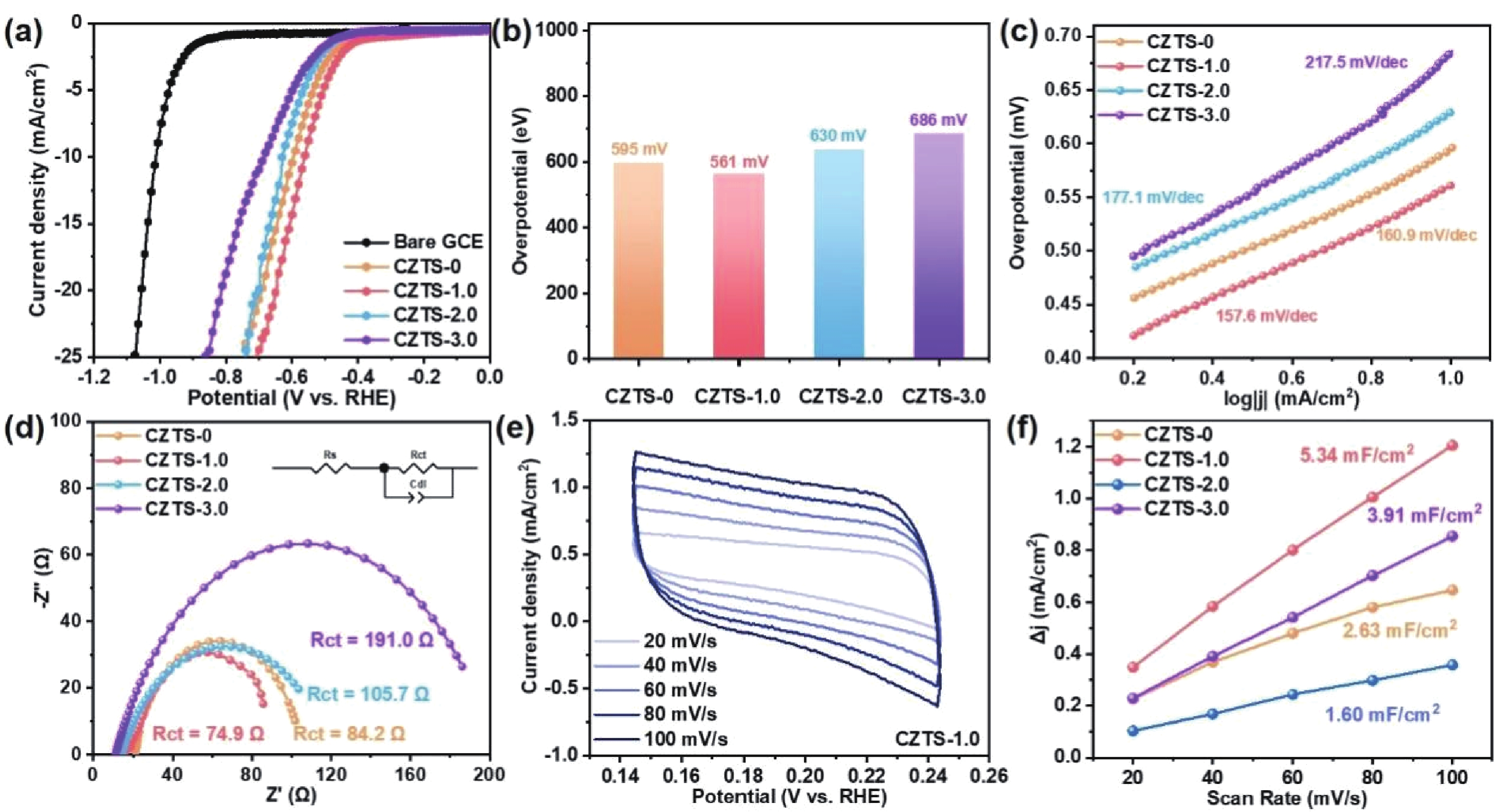
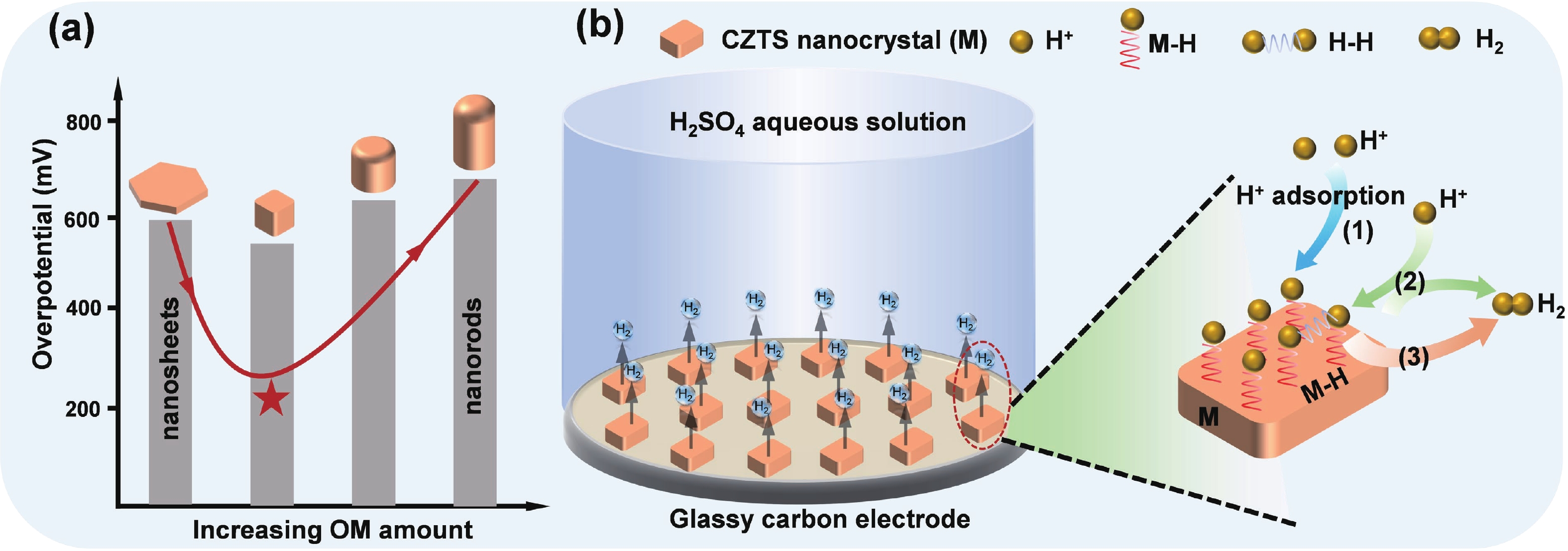
As typical quarternary copper-based chalcogenides, Cu–Zn–Sn–S nanocrystals (CZTS NCs) have emerged as a new-fashioned electrocatalyst in hydrogen evolution reactions (HERs). Oleylamine (OM), a reducing surfactant and solvent, plays a significant role in the assisting synthesis of CZTS NCs due to the ligand effect. Herein, we adopted a facile one-pot colloidal method for achieving the structure evolution of CZTS NCs from 2D nanosheets to 1D nanorods assisted through the continuous addition of OM. During the process, the mechanism of OM-induced morphology evolution was further discussed. When merely adding pure 1-dodecanethiol (DDT) as the solvent, the CZTS nanosheets were obtained. As OM was gradually added to the reaction, the CZTS NCs began to grow along the sides of the nanosheets and gradually shrink at the top, followed by the formation of stable nanorods. In acidic electrolytic conditions, the CZTS NCs with 1.0 OM addition display the optimal HER activity with a low overpotential of 561 mV at 10 mA/cm2 and a small Tafel slope of 157.6 mV/dec compared with other CZTS samples. The enhancement of HER activity could be attributed to the contribution of the synergistic effect of the diverse crystal facets to the reaction. -
References
[1] Zhu J, Hu L S, Zhao P X, et al. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem Rev, 2020, 120, 851 doi: 10.1021/acs.chemrev.9b00248[2] Zou X X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev, 2015, 44, 5148 doi: 10.1039/C4CS00448E[3] Ma M M, Huang Y B, Liu J, et al. Engineering the photoelectrochemical behaviors of ZnO for efficient solar water splitting. J Semicond, 2020, 41, 091702 doi: 10.1088/1674-4926/41/9/091702[4] Valentine S V. Emerging symbiosis: Renewable energy and energy security. Renew Sustain Energy Rev, 2011, 15, 4572 doi: 10.1016/j.rser.2011.07.095[5] Dawood F, Anda M, Shafiullah G M. Hydrogen production for energy: An overview. Int J Hydrog Energy, 2020, 45, 3847 doi: 10.1016/j.ijhydene.2019.12.059[6] Zhao S L, Yang Y C, Tang Z Y. Insight into structural evolution, active sites, and stability of heterogeneous electrocatalysts. Angew Chem Int Ed, 2022, 61, e202110186 doi: 10.1002/anie.202110186[7] Wu Y C, Wei W, Yu R H, et al. Anchoring sub-nanometer Pt clusters on crumpled paper-like MXene enables high hydrogen evolution mass activity. Adv Funct Materials, 2022, 32, 2110910 doi: 10.1002/adfm.202110910[8] Liu Z M, Tang A W, Liu J, et al. Non-injection synthesis of L-shaped wurtzite Cu–Ga–Zn–S alloyed nanorods and their advantageous application in photocatalytic hydrogen evolution. J Mater Chem A, 2018, 6, 18649 doi: 10.1039/C8TA05395B[9] Fu H, Tang A W. Rational design of multinary copper chalcogenide nanocrystals for photocatalytic hydrogen evolution. J Semicond, 2020, 41, 091706 doi: 10.1088/1674-4926/41/9/091706[10] Zheng X L, Yang Y J, Liu Y H, et al. Fundamentals and photocatalytic hydrogen evolution applications of quaternary chalcogenide semiconductor: Cu2ZnSnS4. Rare Met, 2022, 41, 2153 doi: 10.1007/s12598-021-01955-2[11] Digraskar R V, Mulik B B, Walke P S, et al. Enhanced hydrogen evolution reactions on nanostructured Cu2ZnSnS4 (CZTS) electrocatalyst. Appl Surf Sci, 2017, 412, 475 doi: 10.1016/j.apsusc.2017.03.262[12] Kush P, Deori K, Kumar A, et al. Efficient hydrogen/oxygen evolution and photocatalytic dye degradation and reduction of aqueous Cr(vi) by surfactant free hydrophilic Cu2ZnSnS4 nanoparticles. J Mater Chem A, 2015, 3, 8098 doi: 10.1039/C4TA06551D[13] Burhanuz Zaman M, Ahmad Mir R, Poolla R. Growth and properties of solvothermally derived highly crystalline Cu2ZnSnS4 nanoparticles for photocatalytic and electrocatalytic applications. Int J Hydrog Energy, 2019, 44, 23023 doi: 10.1016/j.ijhydene.2019.07.026[14] Ananthakumar S, Ram Kumar J, Moorthy Babu S. Colloidal synthesis and characterization of Cu2ZnSnS4 nanoplates. J Semicond, 2017, 38, 033007 doi: 10.1088/1674-4926/38/3/033007[15] Li M, Zhou W H, Guo J, et al. Synthesis of pure metastable wurtzite CZTS nanocrystals by facile one-pot method. J Phys Chem C, 2012, 116, 26507 doi: 10.1021/jp307346k[16] Digraskar R V, Sapner V S, Mali S M, et al. CZTS decorated on graphene oxide as an efficient electrocatalyst for high-performance hydrogen evolution reaction. ACS Omega, 2019, 4, 7650 doi: 10.1021/acsomega.8b03587[17] Digraskar R V, Sapner V S, Ghule A V, et al. Enhanced overall water-splitting performance: Oleylamine-functionalized GO/Cu2ZnSnS4 composite as a Nobel metal-free and nonprecious electrocatalyst. ACS Omega, 2019, 4, 18969 doi: 10.1021/acsomega.9b01680[18] Digraskar R V, Mali S M, Tayade S B, et al. Overall noble metal free Ni and Fe doped Cu2ZnSnS4 (CZTS) bifunctional electrocatalytic systems for enhanced water splitting reactions. Int J Hydrog Energy, 2019, 44, 8144 doi: 10.1016/j.ijhydene.2019.02.054[19] Digraskar R, Sapner V S, Narwade S S, et al. Enhanced electrocatalytic hydrogen generation from water via cobalt-doped Cu2ZnSnS4 nanoparticles. RSC Adv, 2018, 8, 20341 doi: 10.1039/C8RA01886C[20] Digraskar R V, Sapner V S, Ghule A V, et al. CZTS/MoS2-rGO heterostructures: An efficient and highly stable electrocatalyst for enhanced hydrogen generation reactions. J Electroanal Chem, 2021, 882, 114983 doi: 10.1016/j.jelechem.2021.114983[21] Zito J, Infante I. The future of ligand engineering in colloidal semiconductor nanocrystals. Acc Chem Res, 2021, 54, 1555 doi: 10.1021/acs.accounts.0c00765[22] Liu Z M, Liu J, Huang Y B, et al. From one-dimensional to two-dimensional wurtzite CuGaS2 nanocrystals: Non-injection synthesis and photocatalytic evolution. Nanoscale, 2019, 11, 158 doi: 10.1039/C8NR07353H[23] Mourdikoudis S, Liz-Marzán L M. Oleylamine in nanoparticle synthesis. Chem Mater, 2013, 25, 1465 doi: 10.1021/cm4000476[24] Liu L W, Li H, Liu Z R, et al. Structure and band gap tunable CuInS2 nanocrystal synthesized by hot-injection method with altering the dose of oleylamine. Mater Des, 2018, 149, 145 doi: 10.1016/j.matdes.2018.04.015[25] Yin Z, Hu M, Liu J, et al. Tunable crystal structure of Cu–Zn–Sn–S nanocrystals for improving photocatalytic hydrogen evolution enabled by copper element regulation. J Semicond, 2022, 43, 032701 doi: 10.1088/1674-4926/43/3/032701[26] Tang A W, Hu Z L, Yin Z, et al. One-pot synthesis of CuInS2 nanocrystals using different anions to engineer their morphology and crystal phase. Dalton Trans, 2015, 44, 9251 doi: 10.1039/C5DT01111F[27] Vivier V, Orazem M E. Impedance analysis of electrochemical systems. Chem Rev, 2022, 122, 11131 doi: 10.1021/acs.chemrev.1c00876[28] Zhang F, Zhao R, Wang Y R, et al. Superwettable Surface-Dependent efficiently electrocatalytic water splitting based on their excellent liquid adsorption and gas desorption. Chem Eng J, 2023, 452, 139513 doi: 10.1016/j.cej.2022.139513[29] Li Z S, Li B L, Yu M, et al. Amorphous metallic ultrathin nanostructures: A latent ultra-high-density atomic-level catalyst for electrochemical energy conversion. Int J Hydrog Energy, 2022, 47, 26956 doi: 10.1016/j.ijhydene.2022.06.049[30] Pakhira S, Kumar V, Ghosh S. Revealing the superior electrocatalytic performance of 2D monolayer WSe2 transition metal dichalcogenide for efficient H2 evolution reaction. Adv Materials Inter, 2023, 10, 2202075 doi: 10.1002/admi.202202075[31] Attarzadeh N, Das D, Chintalapalle S N, et al. Nature-inspired design of nano-architecture-aligned Ni5P4-Ni2P/NiS arrays for enhanced electrocatalytic activity of hydrogen evolution reaction (HER). ACS Appl Mater Interfaces, 2023, 15, 22036 doi: 10.1021/acsami.3c00781 -
Proportional views





 DownLoad:
DownLoad: