| Citation: |
Jiahao Zheng, Chunyan Feng, Songyin Qiu, Ke Xu, Caixia Wang, Xiaofei Liu, Jizhou Lv, Haoyang Yu, Shaoqiang Wu. Application and prospect of semiconductor biosensors in detection of viral zoonoses[J]. Journal of Semiconductors, 2023, 44(2): 023102. doi: 10.1088/1674-4926/44/2/023102
****
J H Zheng, C Y Feng, S Y Qiu, K Xu, C X Wang, X F Liu, J Z Lv, H Y Yu, S Q Wu. Application and prospect of semiconductor biosensors in detection of viral zoonoses[J]. J. Semicond, 2023, 44(2): 023102. doi: 10.1088/1674-4926/44/2/023102
|
Application and prospect of semiconductor biosensors in detection of viral zoonoses
DOI: 10.1088/1674-4926/44/2/023102
More Information
-
Abstract
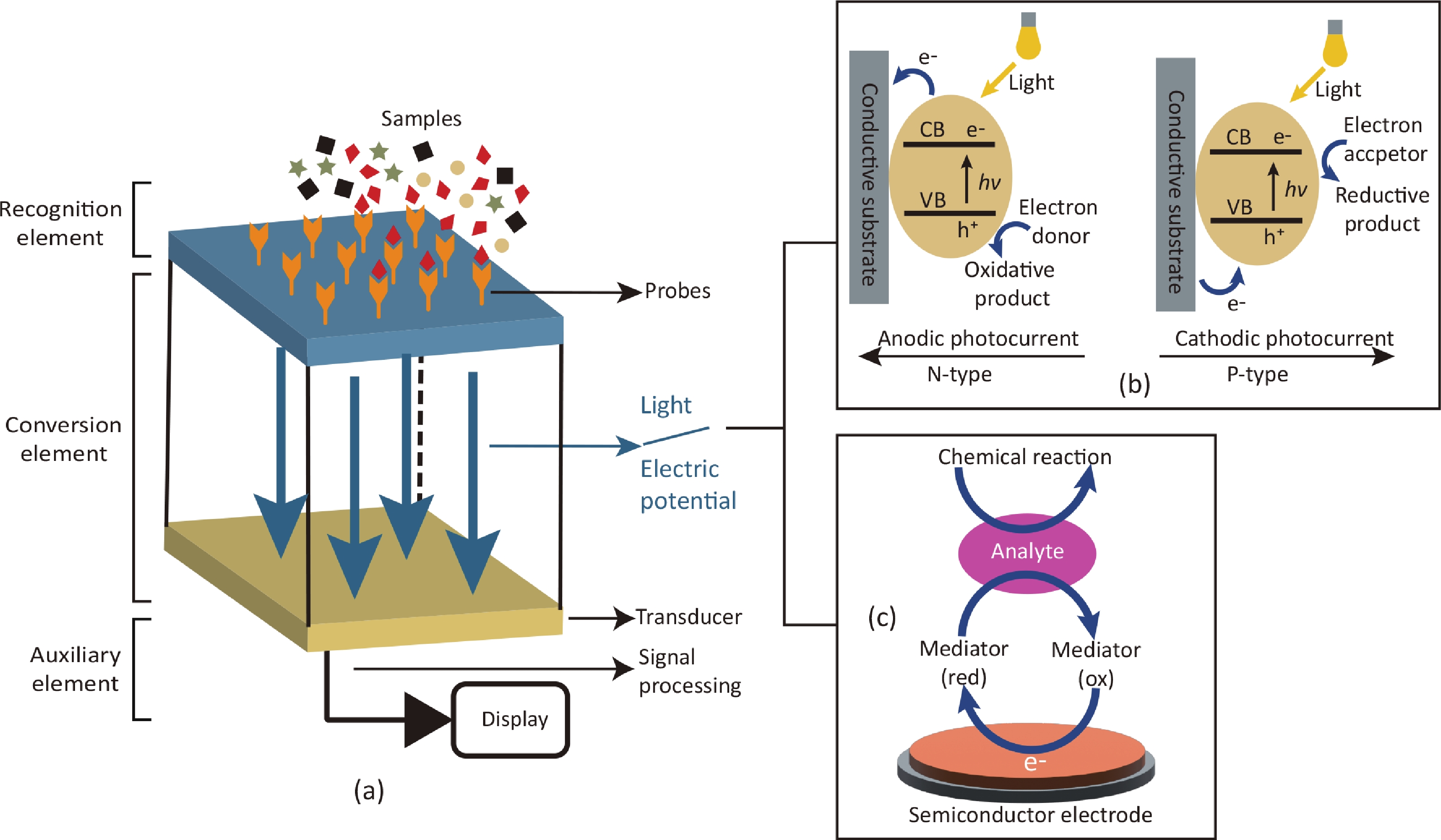
The rapid spread of viral zoonoses can cause severe consequences, including huge economic loss, public health problems or even global crisis of society. Clinical detection technology plays a very important role in the prevention and control of such zoonoses. The rapid and accurate detection of the pathogens of the diseases can directly lead to the early report and early successful control of the diseases. With the advantages of being easy to use, fast, portable, multiplexing and cost-effective, semiconductor biosensors are kinds of detection devices that play an important role in preventing epidemics, and thus have become one of the research hotspots. Here, we summarized the advances of semiconductor biosensors in viral zoonoses detection. By discussing the major principles and applications of each method for different pathogens, this review proposed the directions of designing semiconductor biosensors for clinical application and put forward perspectives in diagnostic of viral zoonoses. -
References
[1] Judson S D, Rabinowitz P M. Zoonoses and global epidemics. Curr Opin Infect Dis, 2021, 34, 385 doi: 10.1097/QCO.0000000000000749[2] Ciotti M, Ciccozzi M, Pieri M, et al. The COVID-19 pandemic: Viral variants and vaccine efficacy. Crit Rev Clin Lab Sci, 2022, 59, 66 doi: 10.1080/10408363.2021.1979462[3] Bernstein A S, Ando A W, Loch-Temzelides T, et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci Adv, 2022, 8, eabl4183 doi: 10.1126/sciadv.abl4183[4] Chen H, Liu K K, Li Z, et al. Point of care testing for infectious diseases. Clin Chim Acta, 2019, 493, 138 doi: 10.1016/j.cca.2019.03.008[5] Roychoudhury S, Das A, Sengupta P, et al. Viral pandemics of the last four decades: Pathophysiology, health impacts and perspectives. Int J Environ Res Public Health, 2020, 17, 9411 doi: 10.3390/ijerph17249411[6] The National Health Commission of PRC. China Health Statistics Yearbook 2019. Peking Union Medical College Press, 2020[7] Ravina R, Dalal A, Mohan H R, et al. Detection methods for influenza A H1N1 virus with special reference to biosensors: A review. Biosci Rep, 2020, 40, BSR20193852 doi: 10.1042/BSR20193852[8] Bu J Q, Deng Z W, Liu H, et al. Current methods and prospects of coronavirus detection. Talanta, 2021, 225, 121977 doi: 10.1016/j.talanta.2020.121977[9] Albertoni G, Girao M J, Schor N. Mini review: current molecular methods for the detection and quantification of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1. Int J Infect Dis, 2014, 25, 145 doi: 10.1016/j.ijid.2014.04.007[10] Kang J, Tahir A, Wang H J, et al. Applications of nanotechnology in virus detection, tracking, and infection mechanisms. WIREs Nanomed Nanobiotechnol, 2021, 13, e1700 doi: 10.1002/wnan.1700[11] Wang J, Wang Z F. Strengths, weaknesses, opportunities and threats (SWOT) analysis of China’s prevention and control strategy for the COVID-19 epidemic. Int J Environ Res Public Health, 2020, 17, 2235 doi: 10.3390/ijerph17072235[12] Coltart C E M, Lindsey B, Ghinai I, et al. The Ebola outbreak, 2013-2016: Old lessons for new epidemics. Philos Trans Royal Soc B, 2017, 372, 20160297 doi: 10.1098/rstb.2016.0297[13] Høiby N. Pandemics: Past, present, future: That is like choosing between cholera and plague. APMIS, 2021, 129, 352 doi: 10.1111/apm.13098[14] Davies H G, Bowman C, Luby S P. Cholera – management and prevention. J Infect, 2017, 74, S66 doi: 10.1016/S0163-4453(17)30194-9[15] Koedrith P, Thasiphu T, Weon J I, et al. Recent trends in rapid environmental monitoring of pathogens and toxicants: Potential of nanoparticle-based biosensor and applications. Sci World J, 2015, 2015, 510982 doi: 10.1155/2015/510982[16] Li Z, Wang P. Point-of-care drug of abuse testing in the opioid epidemic. Arch Pathol Lab Med, 2020, 144, 1325 doi: 10.5858/arpa.2020-0055-RA[17] Shen Y, Anwar T B, Mulchandani A. Current status, advances, challenges and perspectives on biosensors for COVID-19 diagnosis in resource-limited settings. Sens Actuat Rep, 2021, 3, 100025 doi: 10.1016/j.snr.2021.100025[18] Bhalla N, Jolly P, Formisano N, et al. Introduction to biosensors. Essays Biochem, 2016, 60, 1 doi: 10.1042/EBC20150001[19] Park K S. Nucleic acid aptamer-based methods for diagnosis of infections. Biosens Bioelectron, 2018, 102, 179 doi: 10.1016/j.bios.2017.11.028[20] Yoo S M, Lee S P. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol, 2016, 34, 7 doi: 10.1016/j.tibtech.2015.09.012[21] Seok Y, Batule B, Kim M G. Lab-on-paper for all-in-one molecular diagnostics (LAMDA) of zika, dengue, and chikungunya virus from human serum. Biosens Bioelectron, 2020, 165, 112400 doi: 10.1016/j.bios.2020.112400[22] Kutsuna S, Saito S, Ohmagari N. Simultaneous diagnosis of dengue virus, Chikungunya virus, and Zika virus infection using a new point-of-care testing (POCT) system based on the loop-mediated isothermal amplification (LAMP) method. J Infect Chemother, 2020, 26, 1249 doi: 10.1016/j.jiac.2020.07.001[23] Carinelli S, Kühnemund M, Nilsson M, et al. Yoctomole electrochemical genosensing of Ebola virus cDNA by rolling circle and circle to circle amplification. Biosens Bioelectron, 2017, 93, 65 doi: 10.1016/j.bios.2016.09.099[24] Yang L, Li M, Du F, et al. A novel colorimetric PCR-based biosensor for detection and quantification of hepatitis B virus. Methods Mol Biol, 2017, 1571, 357 doi: 10.1016/j.aca.2014.05.032[25] Eun H, Kim. Sensitive electrochemical biosensor combined with isothermal amplification for point-of-care COVID-19 tests. Biosens Bioelectron, 2021, 182, 113168 doi: 10.1016/j.bios.2021.113168[26] Cadoni E, Manicardi A, Madder A. PNA-based microRNA detection methodologies. Molecules, 2020, 25, 1296 doi: 10.3390/molecules25061296[27] Dixon R V, Skaria E, Lau W M, et al. Microneedle-based devices for point-of-care infectious disease diagnostics. Acta Pharm Sin B, 2021, 11, 2344 doi: 10.1016/j.apsb.2021.02.010[28] Ramakrishnan S G, Robert B, Salim A, et al. Nanotechnology based solutions to combat zoonotic viruses with special attention to SARS, MERS, and COVID 19: Detection, protection and medication. Microb Pathog, 2021, 159, 105133 doi: 10.1016/j.micpath.2021.105133[29] Dadina N, Tyson J, Zheng S, et al. Imaging organelle membranes in live cells at the nanoscale with lipid-based fluorescent probes. Curr Opin Chem Biol, 2021, 65, 154 doi: 10.1016/j.cbpa.2021.09.003[30] Elham, Sheikhzadeh. Nanomaterial application in bio/sensors for the detection of infectious diseases. Talanta, 2021, 230, 122026 doi: 10.1016/j.talanta.2020.122026[31] Ferrier D C, Honeychurch K C. Carbon nanotube (CNT)-based biosensors. Biosensors, 2021, 11, 486 doi: 10.3390/bios11120486[32] Silva M M S, Dias A C M S, Silva B V M, et al. Electrochemical detection of dengue virus NS1 protein with a poly(allylamine)/carbon nanotube layered immunoelectrode. J Chem Technol Biotechnol, 2015, 90, 194 doi: 10.1002/jctb.4305[33] Chen M, Hou C J, Huo D Q, et al. An ultrasensitive electrochemical DNA biosensor based on a copper oxide nanowires/single-walled carbon nanotubes nanocomposite. Appl Surf Sci, 2016, 364, 703 doi: 10.1016/j.apsusc.2015.12.203[34] Pinals R L, Ledesma F, Yang D, et al. Rapid SARS-CoV-2 spike protein detection by carbon nanotube-based near-infrared nanosensors. Nano Lett, 2021, 21, 2272 doi: 10.1021/acs.nanolett.1c00118[35] Daniel, Wasik. A heparin-functionalized carbon nanotube-based affinity biosensor for dengue virus. Biosens Bioelectron, 2017, 91, 811 doi: 10.1016/j.bios.2017.01.017[36] Fu Y X, Romay V, Liu Y, et al. Chemiresistive biosensors based on carbon nanotubes for label-free detection of DNA sequences derived from avian influenza virus H5N1. Sens Actuat B, 2017, 249, 691 doi: 10.1016/j.snb.2017.04.080[37] Shao W T, Shurin M R, Wheeler S E, et al. Rapid detection of SARS-CoV-2 antigens using high-purity semiconducting single-walled carbon nanotube-based field-effect transistors. ACS Appl Mater Interfaces, 2021, 13, 10321 doi: 10.1021/acsami.0c22589[38] Vermisoglou E, Panáček D, Jayaramulu K, et al. Human virus detection with graphene-based materials. Biosens Bioelectron, 2020, 166, 112436 doi: 10.1016/j.bios.2020.112436[39] Lee J, Takemura K, Park E Y. Plasmonic nanomaterial-based optical biosensing platforms for virus detection. Sensors, 2017, 17, 2332 doi: 10.3390/s17102332[40] Anik Ü, Tepeli Y, Sayhi M, et al. Towards the electrochemical diagnostic of influenza virus: Development of a graphene-Au hybrid nanocomposite modified influenza virus biosensor based on neuraminidase activity. Analyst, 2017, 143, 150 doi: 10.1039/C7AN01537B[41] Joshi S R, Sharma A, Kim G H, et al. Low cost synthesis of reduced graphene oxide using biopolymer for influenza virus sensor. Mater Sci Eng C Mater Biol Appl, 2020, 108, 110465 doi: 10.1016/j.msec.2019.110465[42] Jeong S, Kim D M, An S Y, et al. Fluorometric detection of influenza viral RNA using graphene oxide. Anal Biochem, 2018, 561/562, 66 doi: 10.1016/j.ab.2018.09.015[43] Jin X, Zhang H, Li Y T, et al. A field effect transistor modified with reduced graphene oxide for immunodetection of Ebola virus. Mikrochim Acta, 2019, 186, 223 doi: 10.1007/s00604-019-3256-5[44] Maity A, Sui X Y, Jin B, et al. Resonance-frequency modulation for rapid, point-of-care Ebola-glycoprotein diagnosis with a graphene-based field-effect biotransistor. Anal Chem, 2018, 90, 14230 doi: 10.1021/acs.analchem.8b03226[45] Omar N A S, Fen Y W, Abdullah J, et al. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci Rep, 2020, 10, 2374 doi: 10.1038/s41598-020-59388-3[46] Omar N A S, Fen Y W, Abdullah J, et al. Quantitative and selective surface plasmon resonance response based on a reduced graphene oxide-polyamidoamine nanocomposite for detection of dengue virus E-proteins. Nanomaterials, 2020, 10, 569 doi: 10.3390/nano10030569[47] Kanagavalli P, Veerapandian M. Opto-electrochemical functionality of Ru(II)-reinforced graphene oxide nanosheets for immunosensing of dengue virus non-structural 1 protein. Biosens Bioelectron, 2020, 150, 111878 doi: 10.1016/j.bios.2019.111878[48] Fan J L, Yuan L Q, Liu Q X, et al. An ultrasensitive and simple assay for the Hepatitis C virus using a reduced graphene oxide-assisted hybridization chain reaction. Analyst, 2019, 144, 3972 doi: 10.1039/C9AN00179D[49] Valipour A, Roushani M. Using silver nanoparticle and thiol graphene quantum dots nanocomposite as a substratum to load antibody for detection of hepatitis C virus core antigen: Electrochemical oxidation of riboflavin was used as redox probe. Biosens Bioelectron, 2017, 89, 946 doi: 10.1016/j.bios.2016.09.086[50] Ku J, Chauhan K, Hwang S H, et al. Enhanced specificity in loop-mediated isothermal amplification with poly(ethylene glycol)-engrafted graphene oxide for detection of viral genes. Biosensors, 2022, 12, 661 doi: 10.3390/bios12080661[51] Seo G, Lee G, Kim M J, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 2020, 14, 5135 doi: 10.1021/acsnano.0c02823[52] Afsahi S, Lerner M B, Goldstein J M, et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens Bioelectron, 2018, 100, 85 doi: 10.1016/j.bios.2017.08.051[53] Islam S, Shukla S, Bajpai V K, et al. A smart nanosensor for the detection of human immunodeficiency virus and associated cardiovascular and arthritis diseases using functionalized graphene-based transistors. Biosens Bioelectron, 2019, 126, 792 doi: 10.1016/j.bios.2018.11.041[54] Gogola J L, Martins G, Gevaerd A, et al. Label-free aptasensor for p24-HIV protein detection based on graphene quantum dots as an electrochemical signal amplifier. Anal Chim Acta, 2021, 1166, 338548 doi: 10.1016/j.aca.2021.338548[55] Abd Muain M F, Cheo K H, Omar M N, et al. Gold nanoparticle-decorated reduced-graphene oxide targeting anti hepatitis B virus core antigen. Bioelectrochemistry, 2018, 122, 199 doi: 10.1016/j.bioelechem.2018.04.004[56] Xiang Q, Huang J Y, Huang H Y, et al. A label-free electrochemical platform for the highly sensitive detection of hepatitis B virus DNA using graphene quantum dots. RSC Adv, 2018, 8, 1820 doi: 10.1039/C7RA11945C[57] Yong S K, Shen S K, Chiang C W, et al. Silicon nanowire field-effect transistor as label-free detection of hepatitis B virus proteins with opposite net charges. Biosensors, 2021, 11, 442 doi: 10.3390/bios11110442[58] Uhm M, Lee J M, Lee J, et al. Ultrasensitive electrical detection of hemagglutinin for point-of-care detection of influenza virus based on a CMP-NANA probe and top-down processed silicon nanowire field-effect transistors. Sensors, 2019, 19, 4502 doi: 10.3390/s19204502[59] Wu C C. Silicon nanowires length and numbers dependence on sensitivity of the field-effect transistor sensor for hepatitis B virus surface antigen detection. Biosensors, 2022, 12, 115 doi: 10.3390/bios12020115[60] Wasfi A, Awwad F, Gelovani J G, et al. COVID-19 detection via silicon nanowire field-effect transistor: Setup and modeling of its function. Nanomaterials, 2022, 12, 2638 doi: 10.3390/nano12152638[61] Gao B T, Rojas Chavez A A, Malkawi W I, et al. Sensitive detection of SARS-CoV-2 spike protein using vertically-oriented silicon nanowire array-based biosensor. Sens Biosensing Res, 2022, 36, 100487 doi: 10.1016/j.sbsr.2022.100487 -
Proportional views






 DownLoad:
DownLoad:












 Jiahao Zheng:got his BS from Tianjin University of Traditional Chinese Medicine in 2020. Now he is a MS student at King's College London. His research focuses on drug development science and virus detection
Jiahao Zheng:got his BS from Tianjin University of Traditional Chinese Medicine in 2020. Now he is a MS student at King's College London. His research focuses on drug development science and virus detection Shaoqiang Wu:got his PhD on vet-erinary parasitology in 2003 from China Agricultural University. His research interests include precision detection technologies for biosecurity risk factors, on-site detection techniques for shellfish parasites and aquatic animal diseases
Shaoqiang Wu:got his PhD on vet-erinary parasitology in 2003 from China Agricultural University. His research interests include precision detection technologies for biosecurity risk factors, on-site detection techniques for shellfish parasites and aquatic animal diseases



