| Citation: |
Wenbo Qiu, Zidong Wang, Shijiang He, Huaping Zhao, Yong Lei. Recent progress and future prospects of high-entropy materials for battery applications[J]. Journal of Semiconductors, 2024, 45(3): 030202. doi: 10.1088/1674-4926/45/3/030202
W B Qiu, Z D Wang, S J He, H P Zhao, Y Lei. Recent progress and future prospects of high-entropy materials for battery applications[J]. J. Semicond, 2024, 45(3): 030202. doi: 10.1088/1674-4926/45/3/030202
Export: BibTex EndNote
|
Recent progress and future prospects of high-entropy materials for battery applications
doi: 10.1088/1674-4926/45/3/030202
More Information-
References
[1] Qu J, Dai X X, Cui J S, et al. Hierarchical polyaromatic hydrocarbons (PAH) with superior sodium storage properties. J Mater Chem A, 2021, 9, 16554 doi: 10.1039/D1TA03101E[2] Yang S Q, Wang P B, Wei H X, et al. Li4V2Mn(PO4)4-stablized Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for lithium ion batteries. Nano Energy, 2019, 63, 103889 doi: 10.1016/j.nanoen.2019.103889[3] Jiang Z, Li Y H, Han C, et al. Raising lithium storage performances of NaTi2(PO4)3 by nitrogen and sulfur dual-doped carbon layer. J Electrochem Soc, 2020, 167, 020550 doi: 10.1149/1945-7111/ab6c5c[4] Chao X, Yan C Z, Zhao H P, et al. Micro-nano structural electrode architecture for high power energy storage. J Semicond, 2023, 44, 050201 doi: 10.1088/1674-4926/44/5/050201[5] Wu Y H, Chen G B, Wu X N, et al. Research progress on vanadium oxides for potassium-ion batteries. J Semicond, 2023, 44, 041701 doi: 10.1088/1674-4926/44/4/041701[6] Yin Z, Hu M, Liu J, et al. Tunable crystal structure of Cu–Zn–Sn–S nanocrystals for improving photocatalytic hydrogen evolution enabled by copper element regulation. J Semicond, 2022, 43, 032701 doi: 10.1088/1674-4926/43/3/032701[7] Li C, Li J, Huang Y B, et al. Recent development in electronic structure tuning of graphitic carbon nitride for highly efficient photocatalysis. J Semicond, 2022, 43, 021701 doi: 10.1088/1674-4926/43/2/021701[8] Wang Z D, Hong P, Peng S J, et al. Hierarchitecture Co2(OH)3 Cl@FeCo2O4 composite as a novel and high-performance electrode material applied in supercapacitor. Int J Energy Res, 2020, 44, 3122 doi: 10.1002/er.5152[9] Wang Z D, Hong P, Zhao H P, et al. Recent developments and future prospects of transition metal compounds as electrode materials for potassium-ion hybrid capacitors. Adv Mater Technol, 2023, 8, 2200515 doi: 10.1002/admt.202200515[10] Qu J, Sheng T, Wu Z G, et al. Unexpected effects of zirconium-doping in the high performance sodium manganese-based layer-tunnel cathode. J Mater Chem A, 2018, 6, 13934 doi: 10.1039/C8TA04818E[11] He S J, Wang Z D, Wang Z J, et al. Recent progress and future prospect of novel multi-ion storage devices. J Semicond, 2023, 44, 040201 doi: 10.1088/1674-4926/44/4/040201[12] Zhang Y Q, Wang D D, Wang S Y. High-entropy alloys for electrocatalysis: Design, characterization, and applications. Small, 2022, 18, e2104339 doi: 10.1002/smll.202104339[13] Sarkar A, Wang Q S, Schiele A, et al. High-entropy oxides: Fundamental aspects and electrochemical properties. Adv Mater, 2019, 31, e1806236 doi: 10.1002/adma.201806236[14] Sarkar A, Velasco L, Wang D, et al. High entropy oxides for reversible energy storage. Nat Commun, 2018, 9, 3400 doi: 10.1038/s41467-018-05774-5[15] Stygar M, Dąbrowa J, Moździerz M, et al. Formation and properties of high entropy oxides in Co-Cr-Fe-Mg-Mn-Ni-O system: Novel (Cr, Fe, Mg, Mn, Ni)3O4 and (Co, Cr, Fe, Mg, Mn)3O4 high entropy spinels. J Eur Ceram Soc, 2020, 40, 1644 doi: 10.1016/j.jeurceramsoc.2019.11.030[16] Rost C M, Sachet E, Borman T, et al. Entropy-stabilized oxides. Nat Commun, 2015, 6, 8485 doi: 10.1038/ncomms9485[17] Sukkurji P A, Cui Y Y, Lee S, et al. Mechanochemical synthesis of novel rutile-type high entropy fluorides for electrocatalysis. J Mater Chem A, 2021, 9, 8998 doi: 10.1039/D0TA10209A[18] Nguyen T X, Su Y H, Lin C C, et al. Self-reconstruction of sulfate-containing high entropy sulfide for exceptionally high-performance oxygen evolution reaction electrocatalyst. Adv Funct Materials, 2021, 31(48), 2106229 doi: 10.1002/adfm.202106229[19] Gao M C, Miracle D B, Maurice D, et al. High-entropy functional materials. J Mater Res, 2018, 33, 3138 doi: 10.1557/jmr.2018.323[20] Senkov O N. A critical review of high entropy alloys and related concepts. Acta Mater, 2017, 122, 448 doi: 10.1016/j.actamat.2016.08.081[21] Zhou P F, Che Z N, Liu J, et al. High-entropy P2/O3 biphasic cathode materials for wide-temperature rechargeable sodium-ion batteries. Energy Storage Mater, 2023, 57, 618 doi: 10.1016/j.ensm.2023.03.007[22] Joshi A, Chakrabarty S, Akella S H, et al. High-entropy co-free O3- type layered oxyfluoride: A promising air-stable cathode for sodium-ion batteries. Adv Mater, 2023, 35, e2304440 doi: 10.1002/adma.202304440[23] Huang Y, Zhang X, Ji L, et al. Boosting the sodium storage performance of Prussian blue analogs by single-crystal and high-entropy approach. Energy Storage Mater, 2023, 58, 1 doi: 10.1016/j.ensm.2023.03.011[24] Zhao X, Xing Z H, Huang C D. Investigation of high-entropy Prussian blue analog as cathode material for aqueous sodium-ion batteries. J Mater Chem A, 2023, 11, 22835 doi: 10.1039/D3TA04349E[25] Gu Z Y, Guo J Z, Cao J M, et al. An advanced high-entropy fluorophosphate cathode for sodium-ion batteries with increased working voltage and energy density. Adv Mater, 2022, 34, e2110108 doi: 10.1002/adma.202110108[26] Liu C, Bi J Q, Xie L L, et al. High entropy spinel oxides (CrFeMnNiCo x )3O4 (x = 2, 3, 4) nanoparticles as anode material towards electrochemical properties. J Energy Storage, 2023, 71, 108211 doi: 10.1016/j.est.2023.108211[27] Yang X B, Wang H Q, Song Y Y, et al. Low-temperature synthesis of a porous high-entropy transition-metal oxide as an anode for high-performance lithium-ion batteries. ACS Appl Mater Interfaces, 2022, 14(23), 26873 doi: 10.1021/acsami.2c07576[28] Brandt T G, Tuokkola A R, Yu M J, et al. Liquid-feed flame spray pyrolysis enabled synthesis of Co- and Cr-free, high-entropy spinel oxides as Li-ion anodes. Chem Eng J, 2023, 474, 145495 doi: 10.1016/j.cej.2023.145495[29] Lin L, Wang K, Sarkar A, et al. High-entropy sulfides as electrode materials for Li-ion batteries. Adv Energy Mater, 2022, 12, 2103090 doi: 10.1002/aenm.202103090[30] Zhao J, Zhang Y, Chen X, et al. Entropy-change driven highly reversible sodium storage for conversion-type sulfide. Adv Funct Materials, 2022, 32, 2206531 doi: 10.1002/adfm.202206531 -
Proportional views
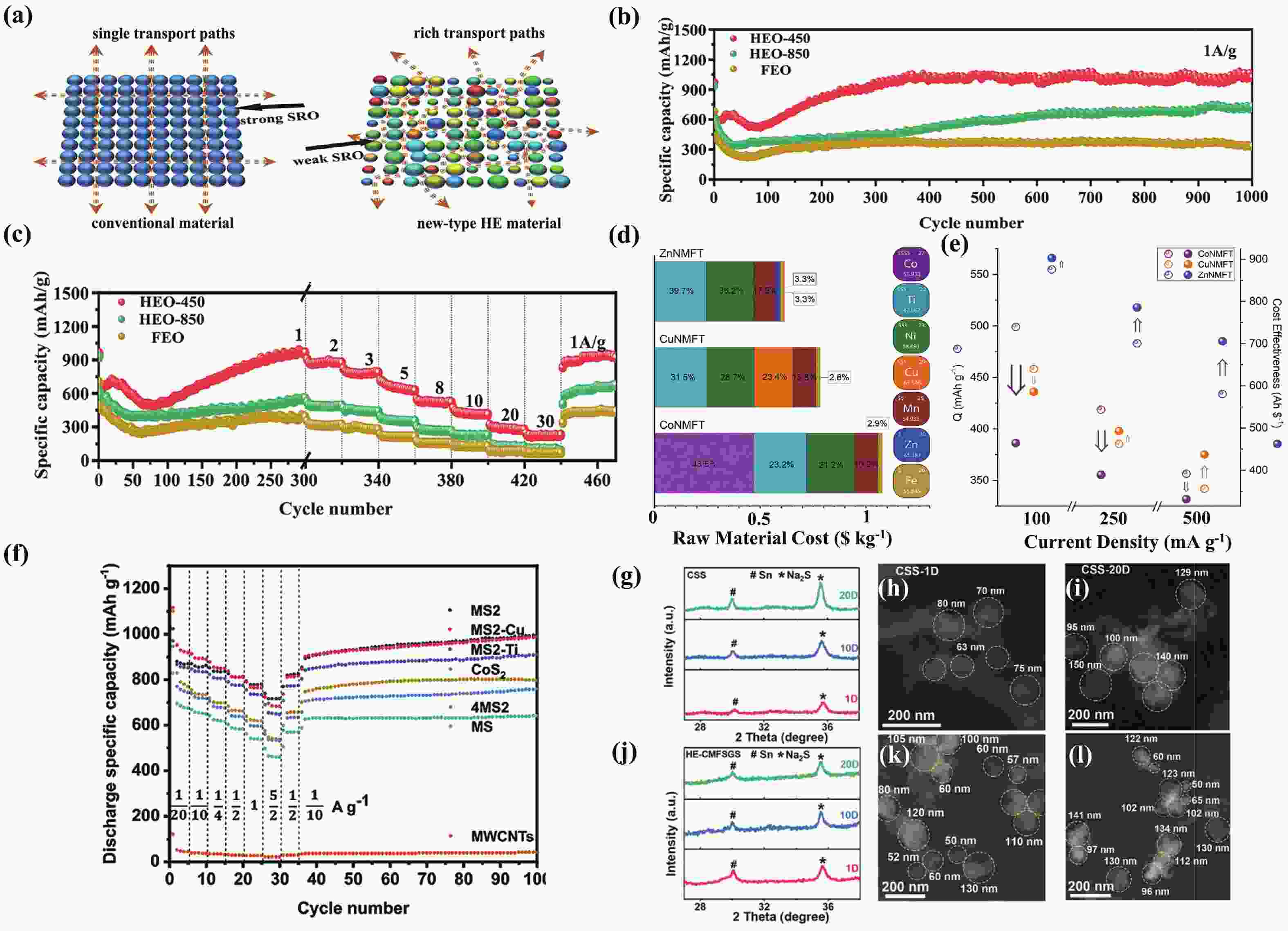
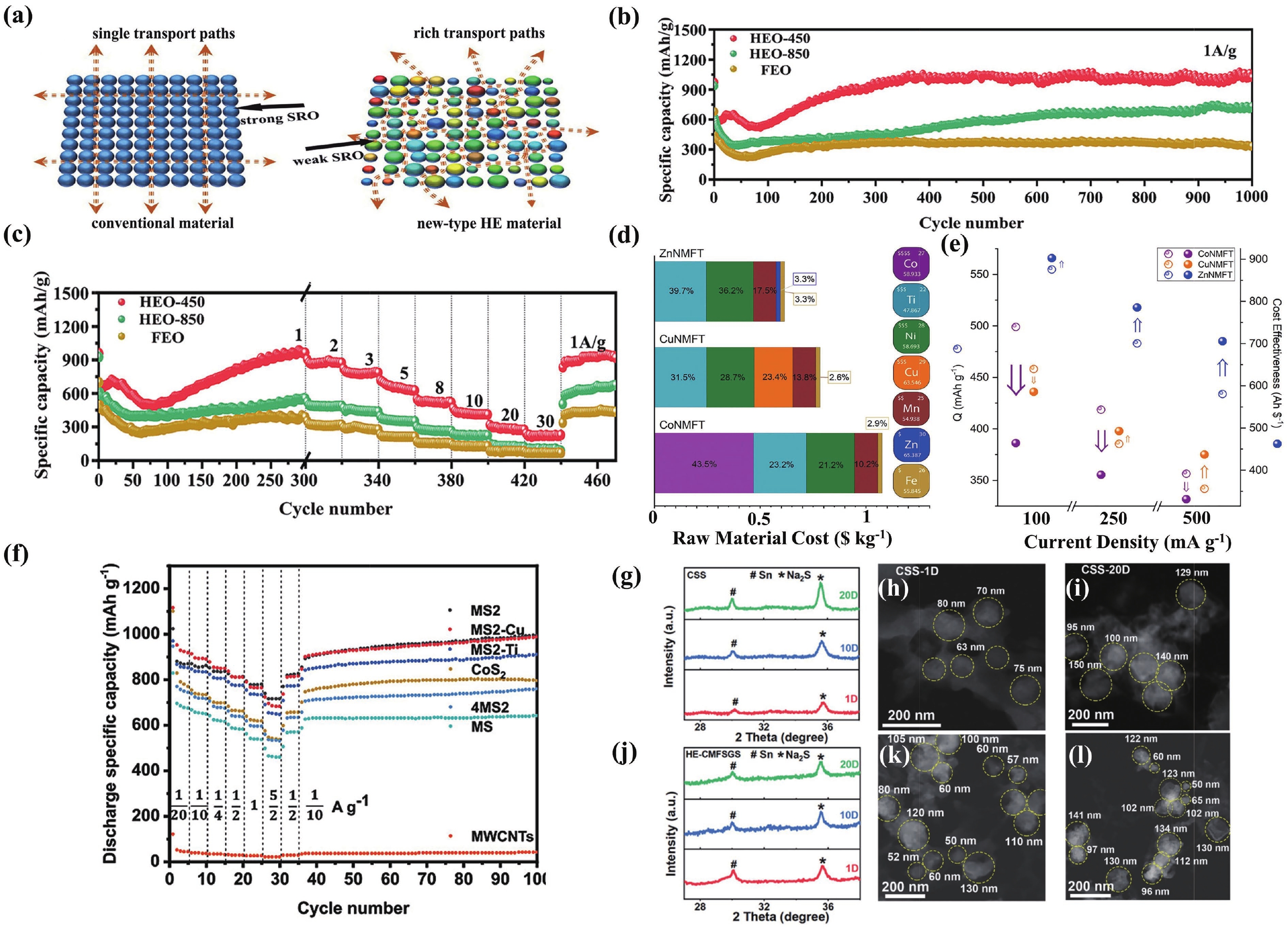
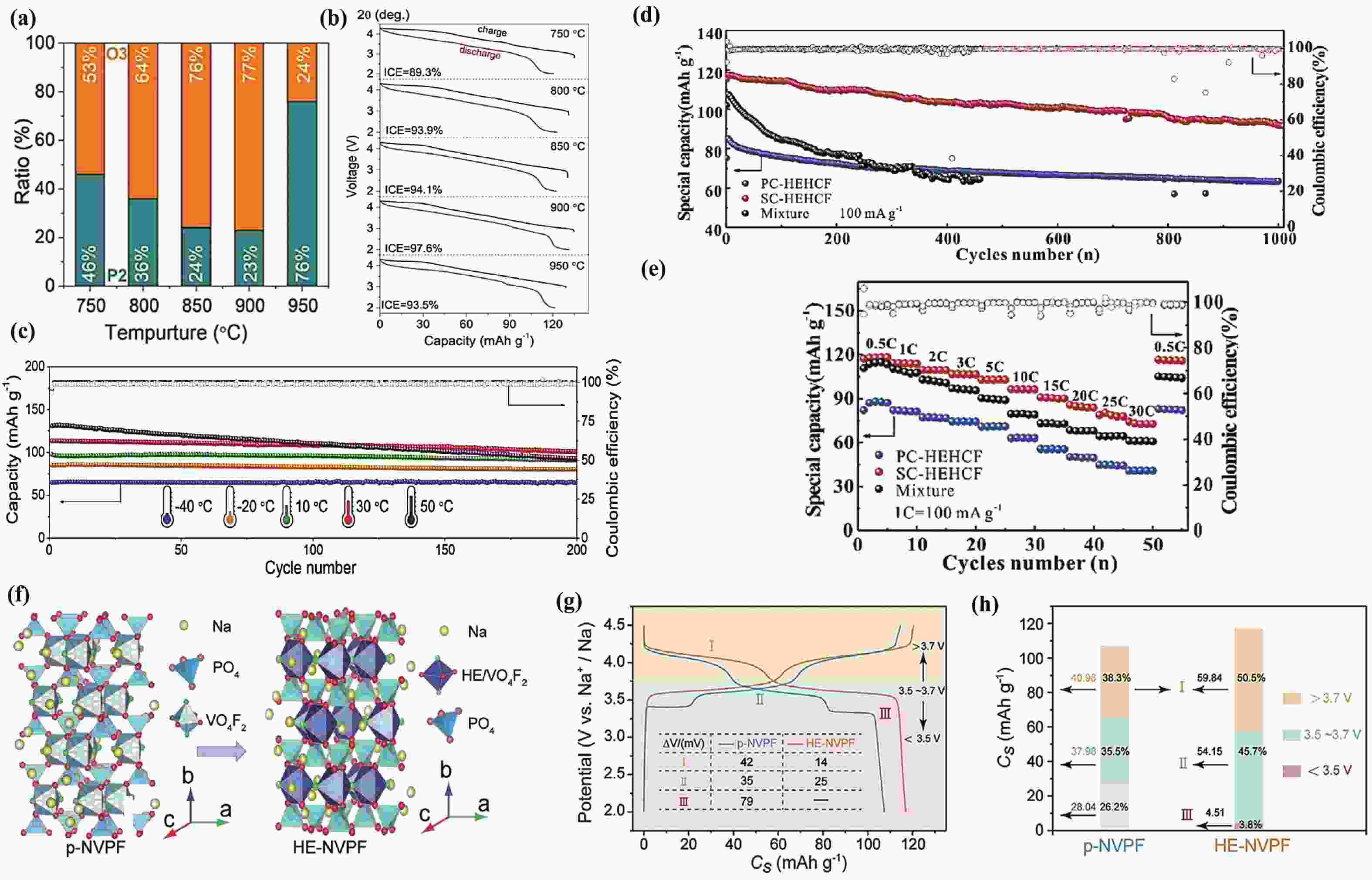
§Wenbo Qiu and Zidong Wang contributed equally to this work and should be considered as co-first authors.




 DownLoad:
DownLoad: