| Citation: |
Shengxun Cai, Jianqing Nie, Kun Wang, Yimin Guan, Demeng Liu. A multichannel thermal bubble-actuated impedance flow cytometer with on-chip TIA based on CMOS-MEMS[J]. Journal of Semiconductors, 2024, 45(5): 052201. doi: 10.1088/1674-4926/45/5/052201
S X Cai, J Q Nie, K Wang, Y M Guan, and D M Liu, A multichannel thermal bubble-actuated impedance flow cytometer with on-chip TIA based on CMOS-MEMS[J]. J. Semicond., 2024, 45(5), 052201 doi: 10.1088/1674-4926/45/5/052201
Export: BibTex EndNote
|
A multichannel thermal bubble-actuated impedance flow cytometer with on-chip TIA based on CMOS-MEMS
doi: 10.1088/1674-4926/45/5/052201
More Information-
Abstract
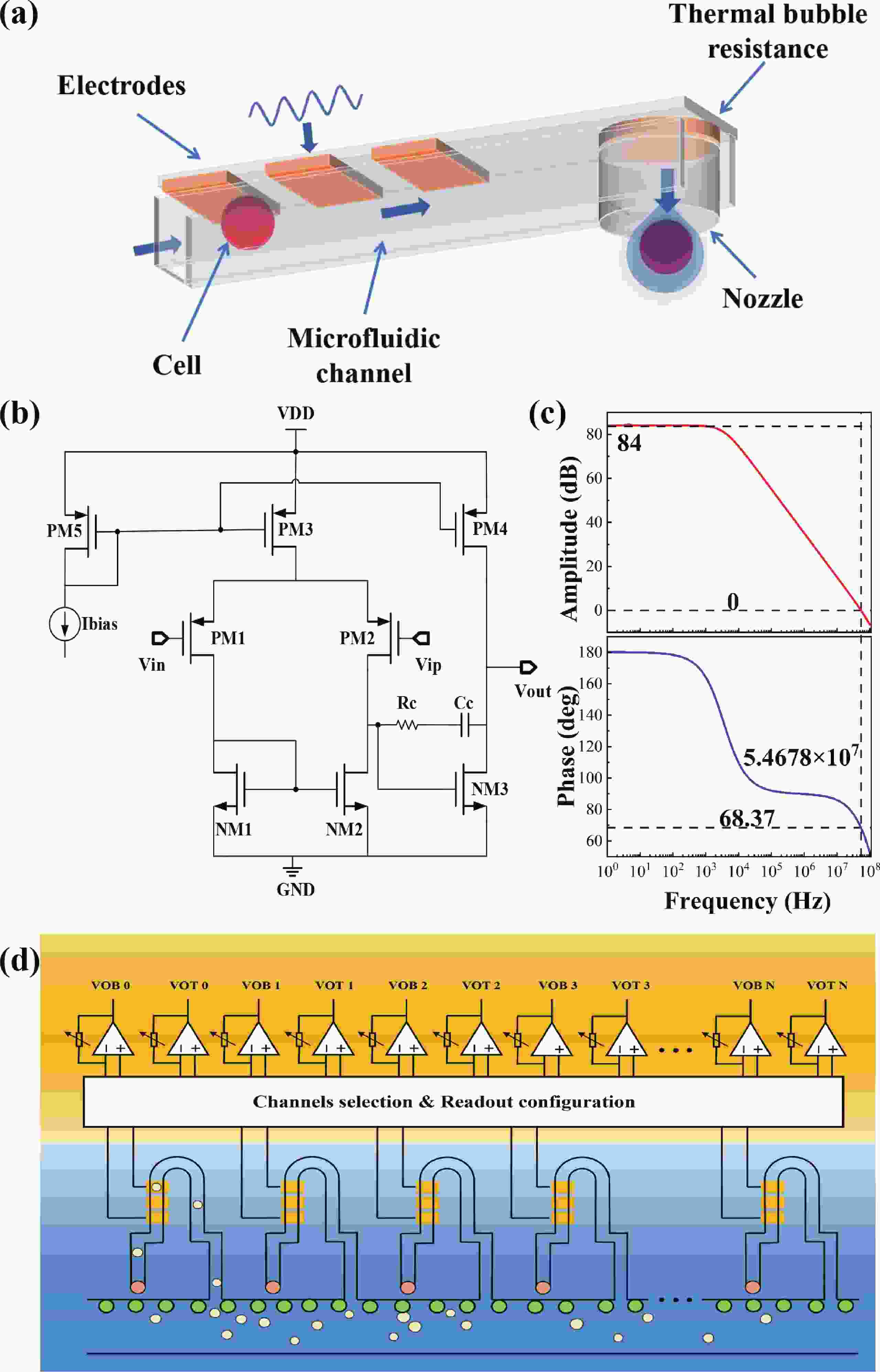
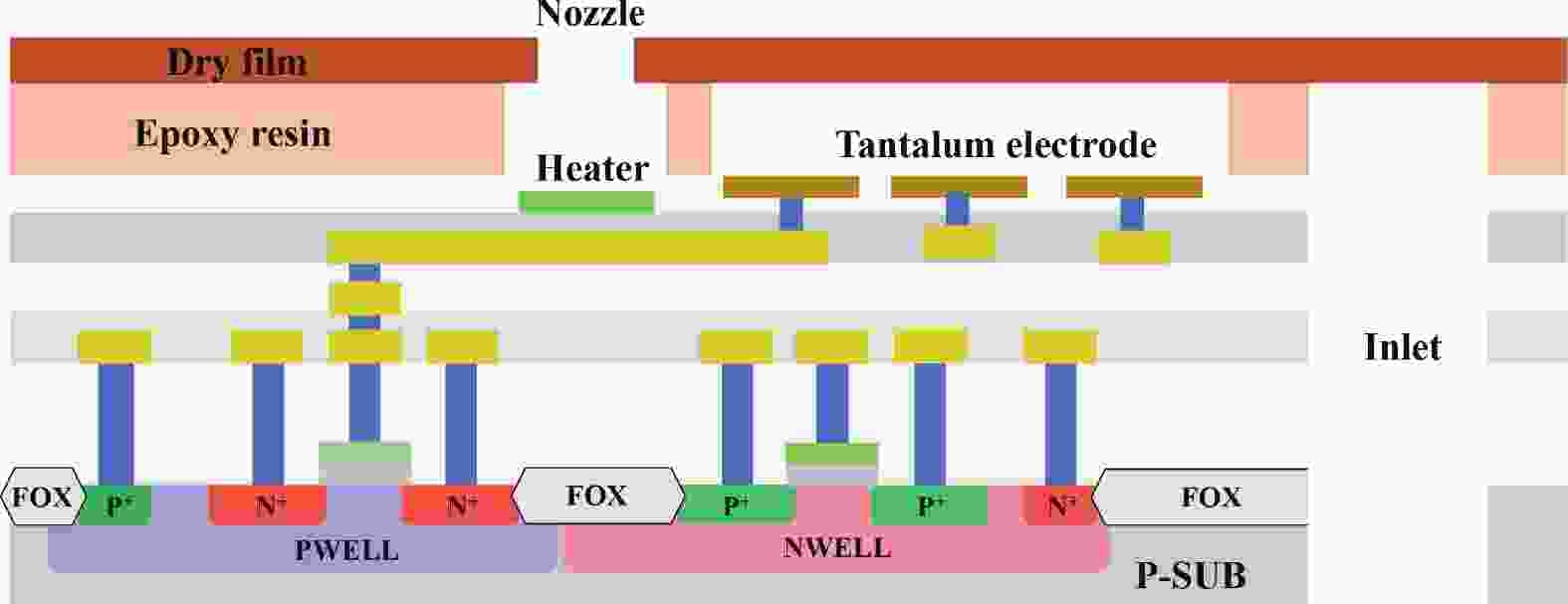
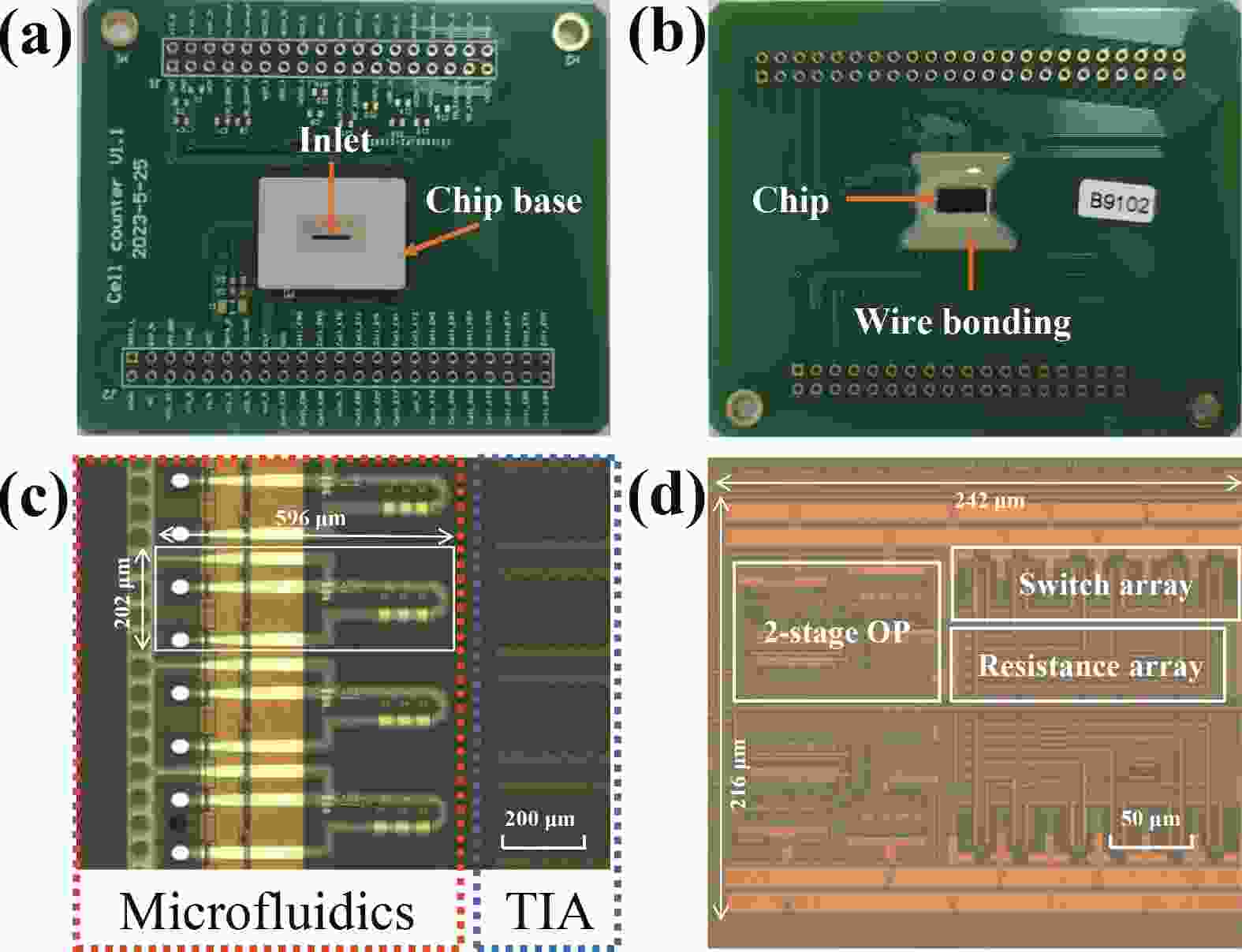
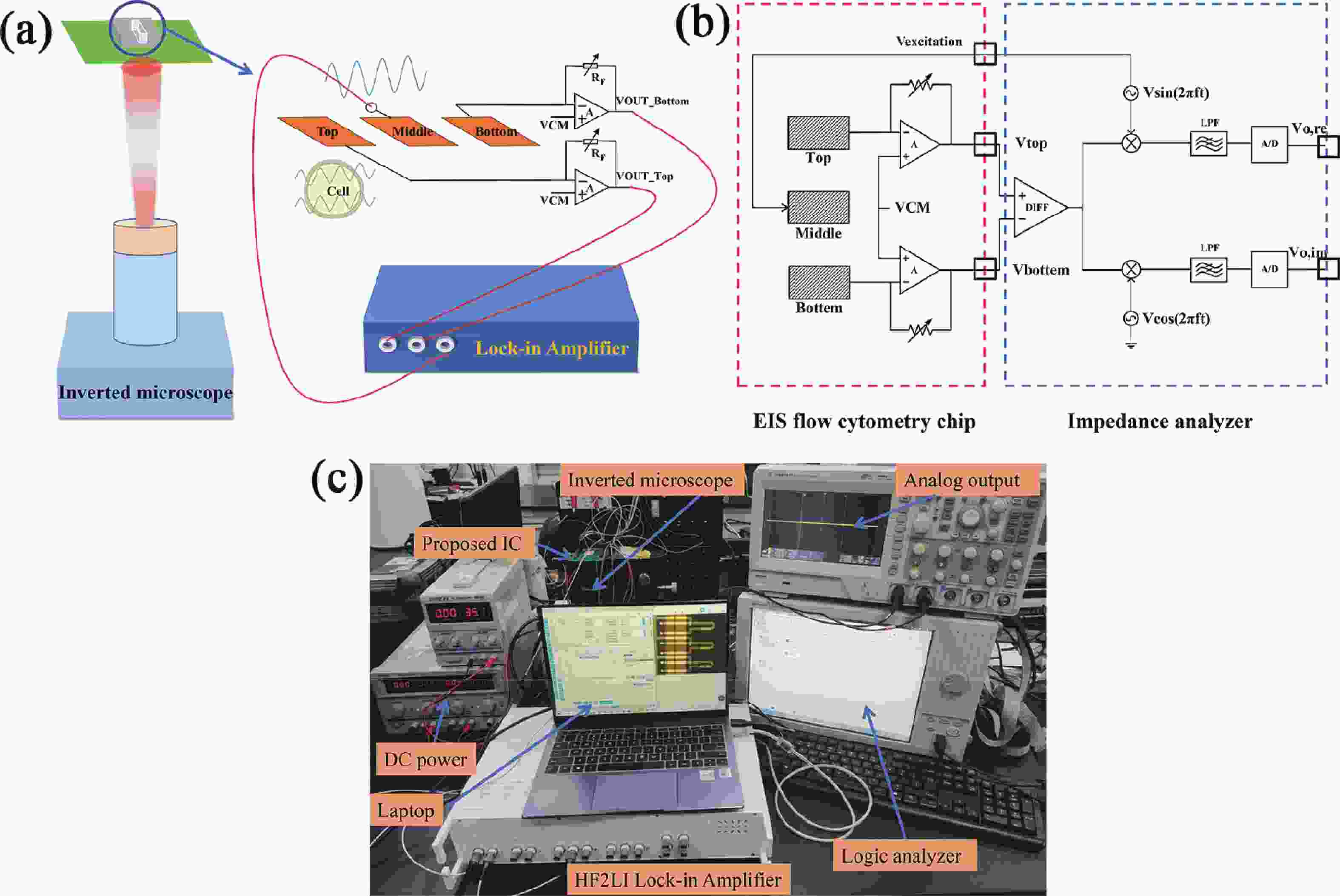
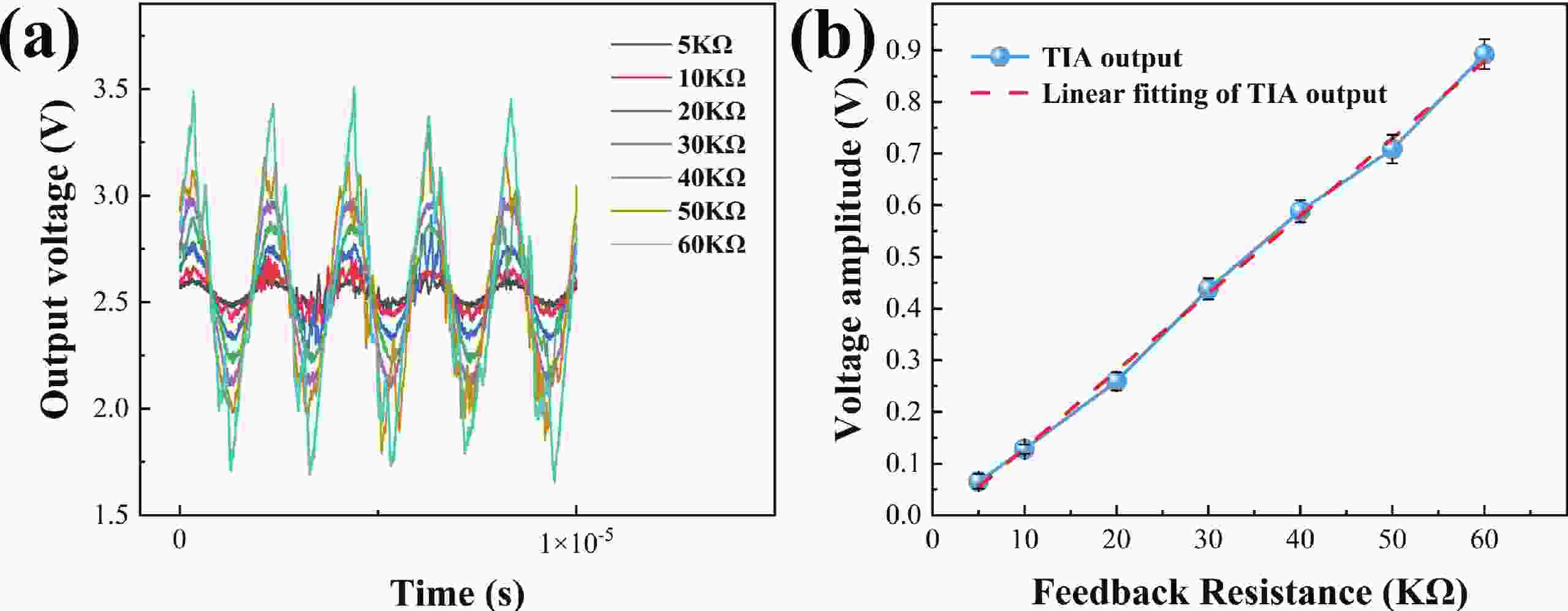
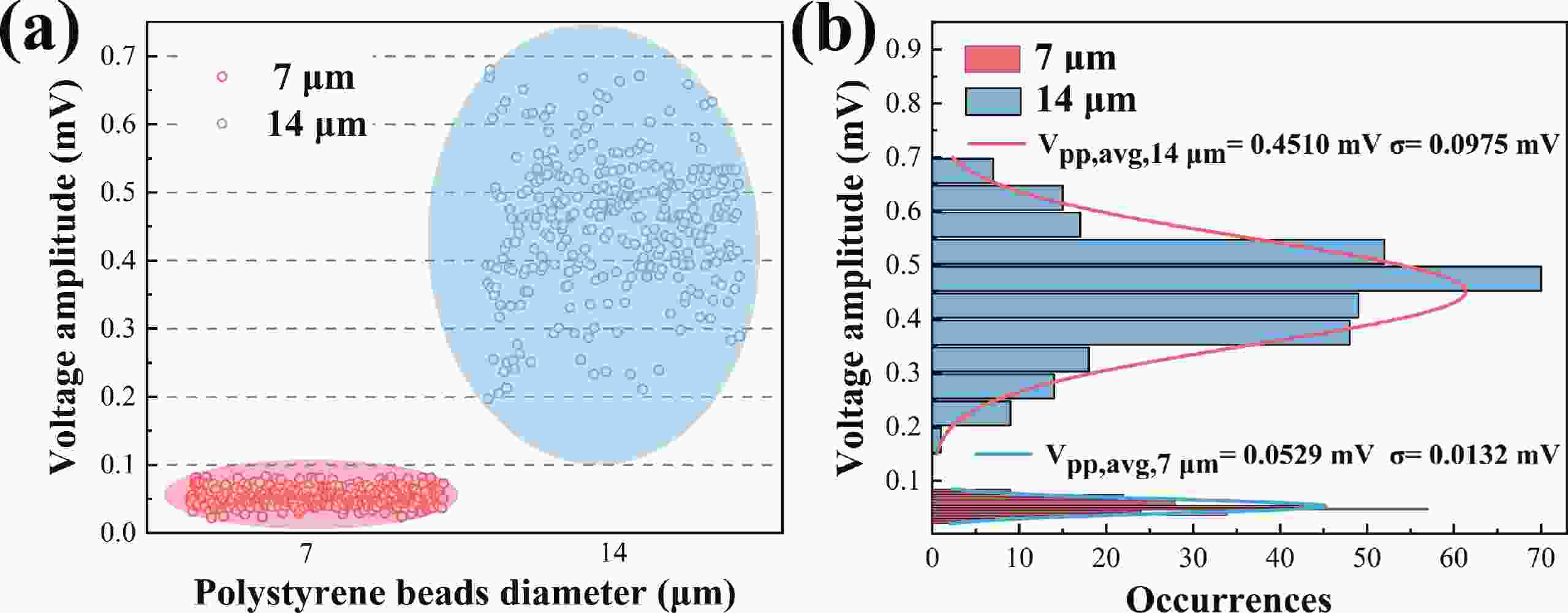
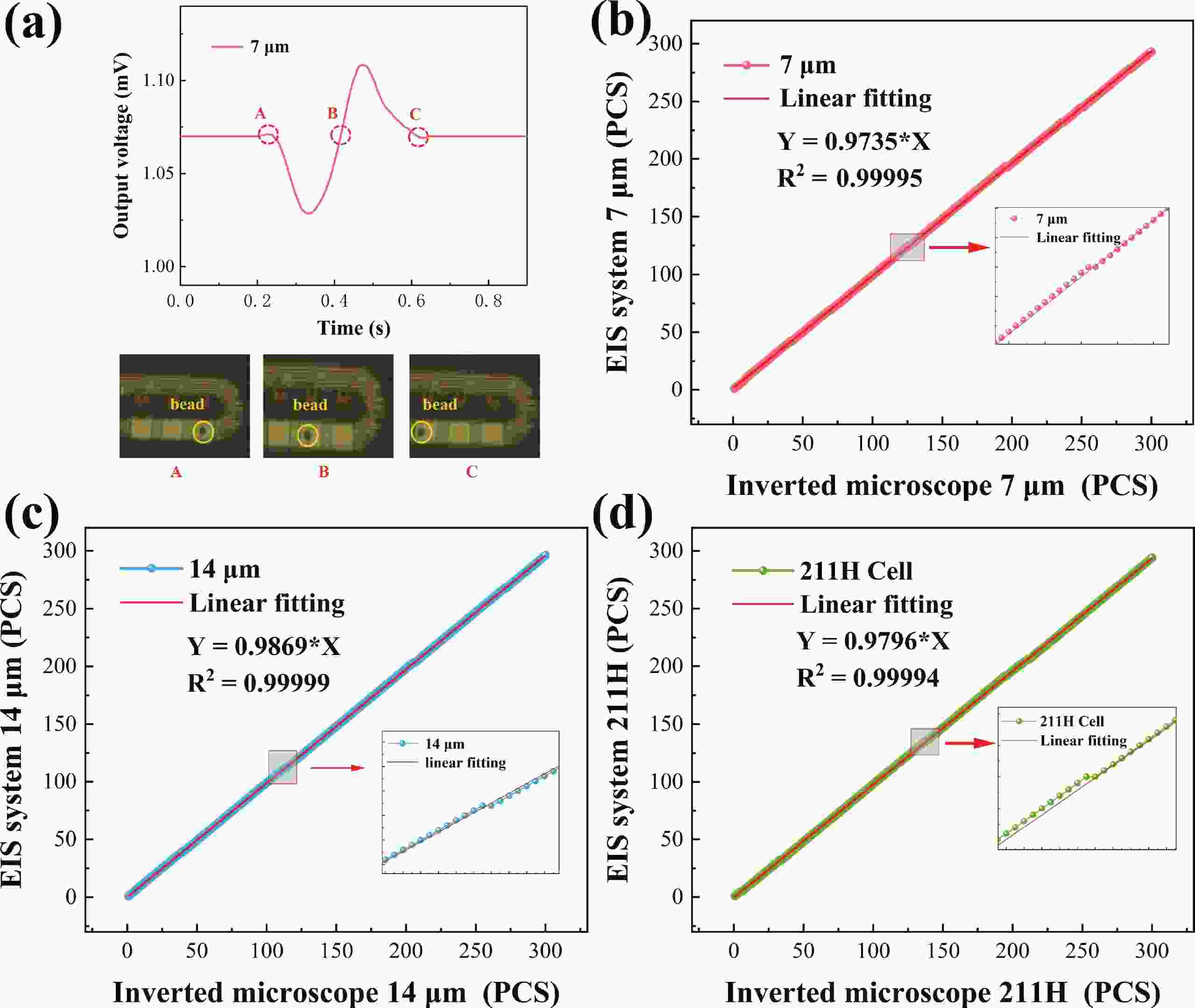
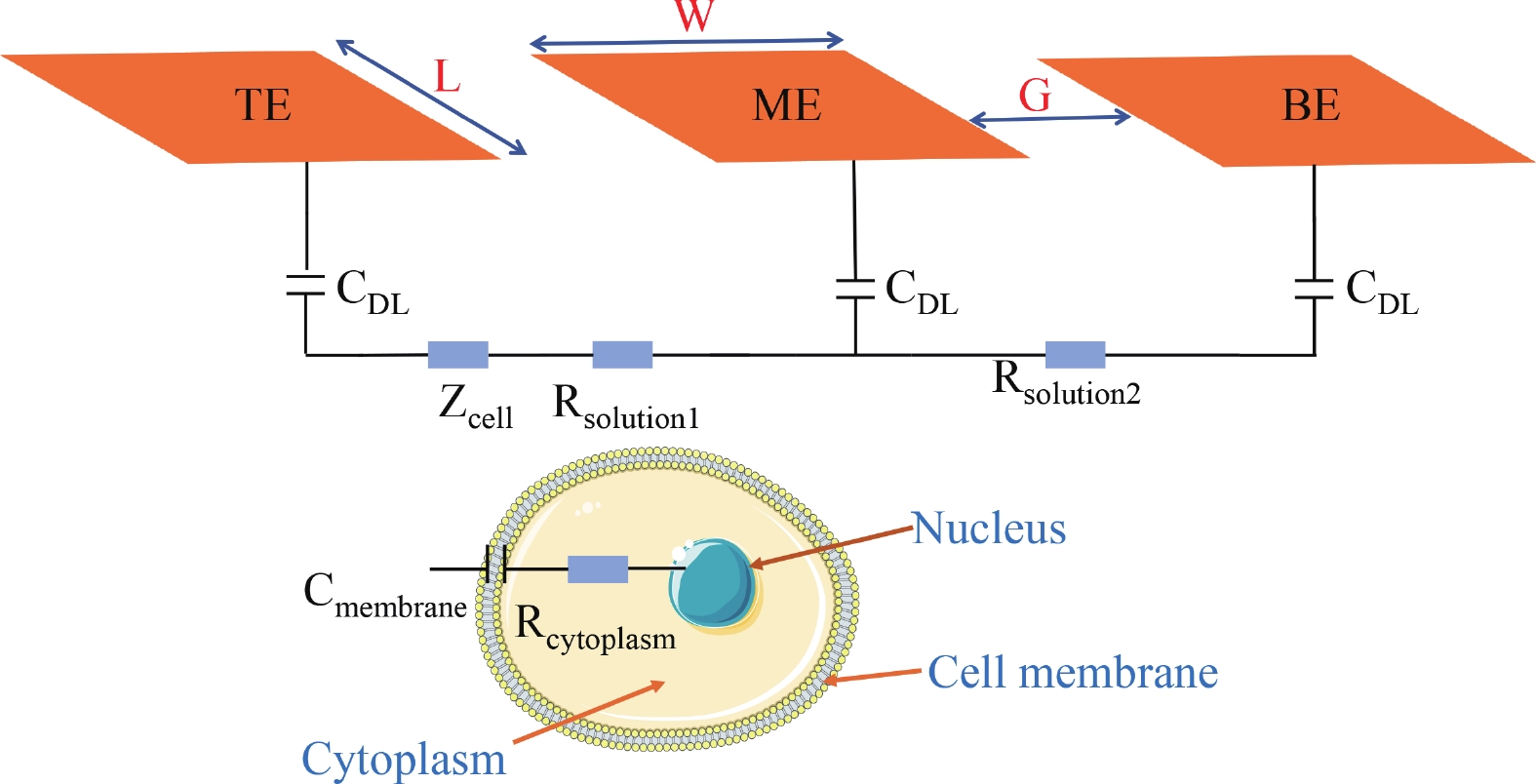
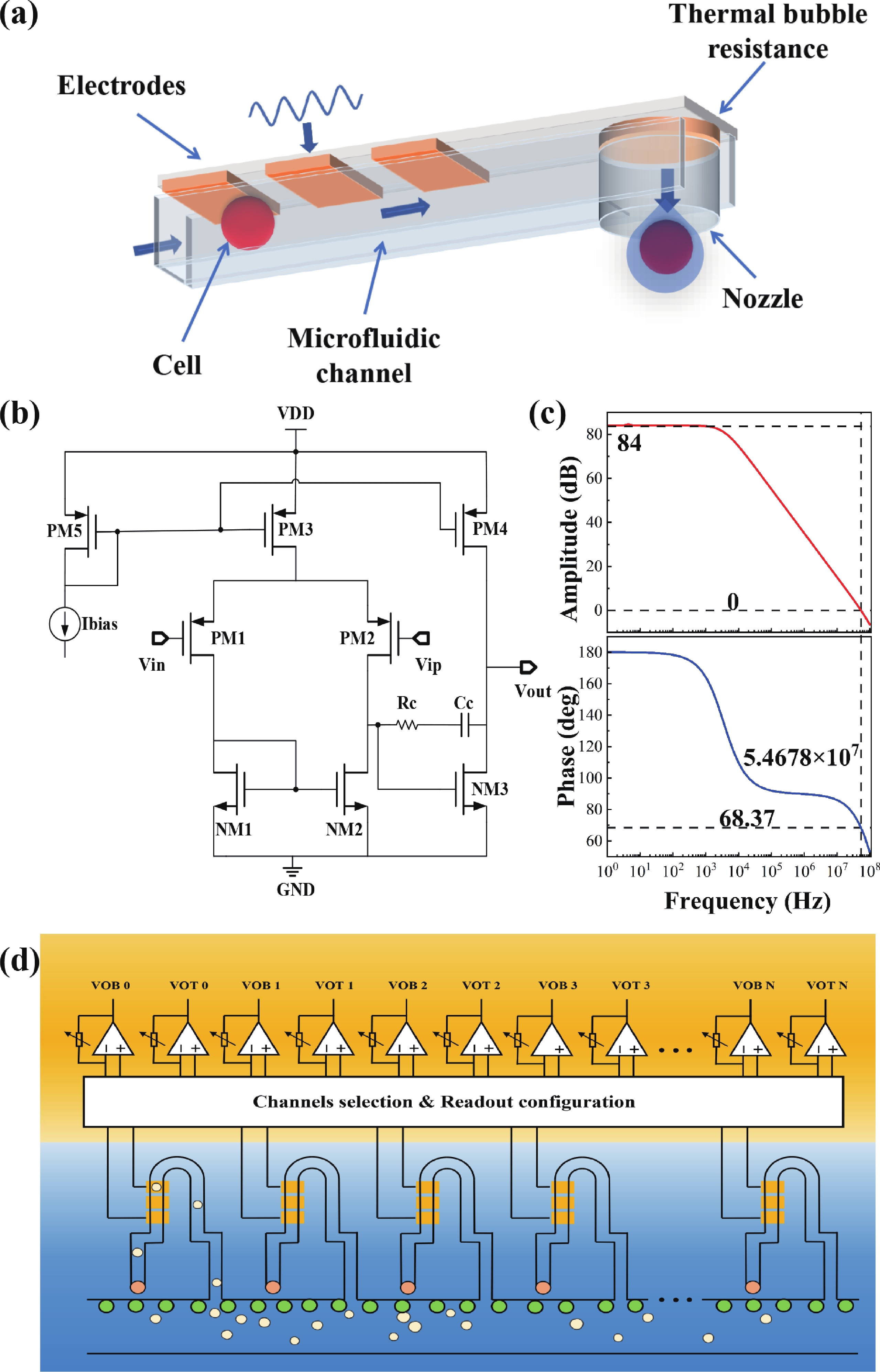
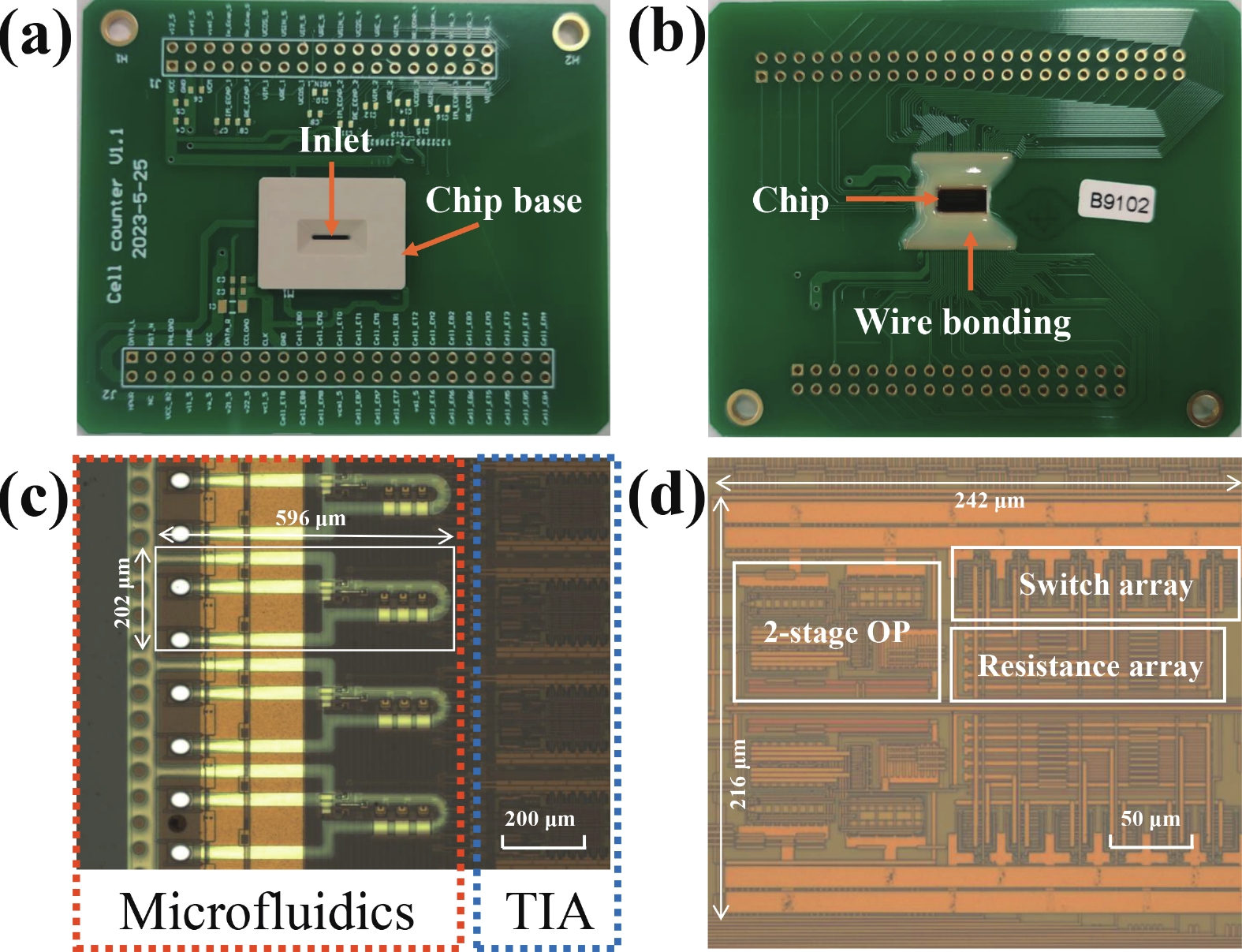
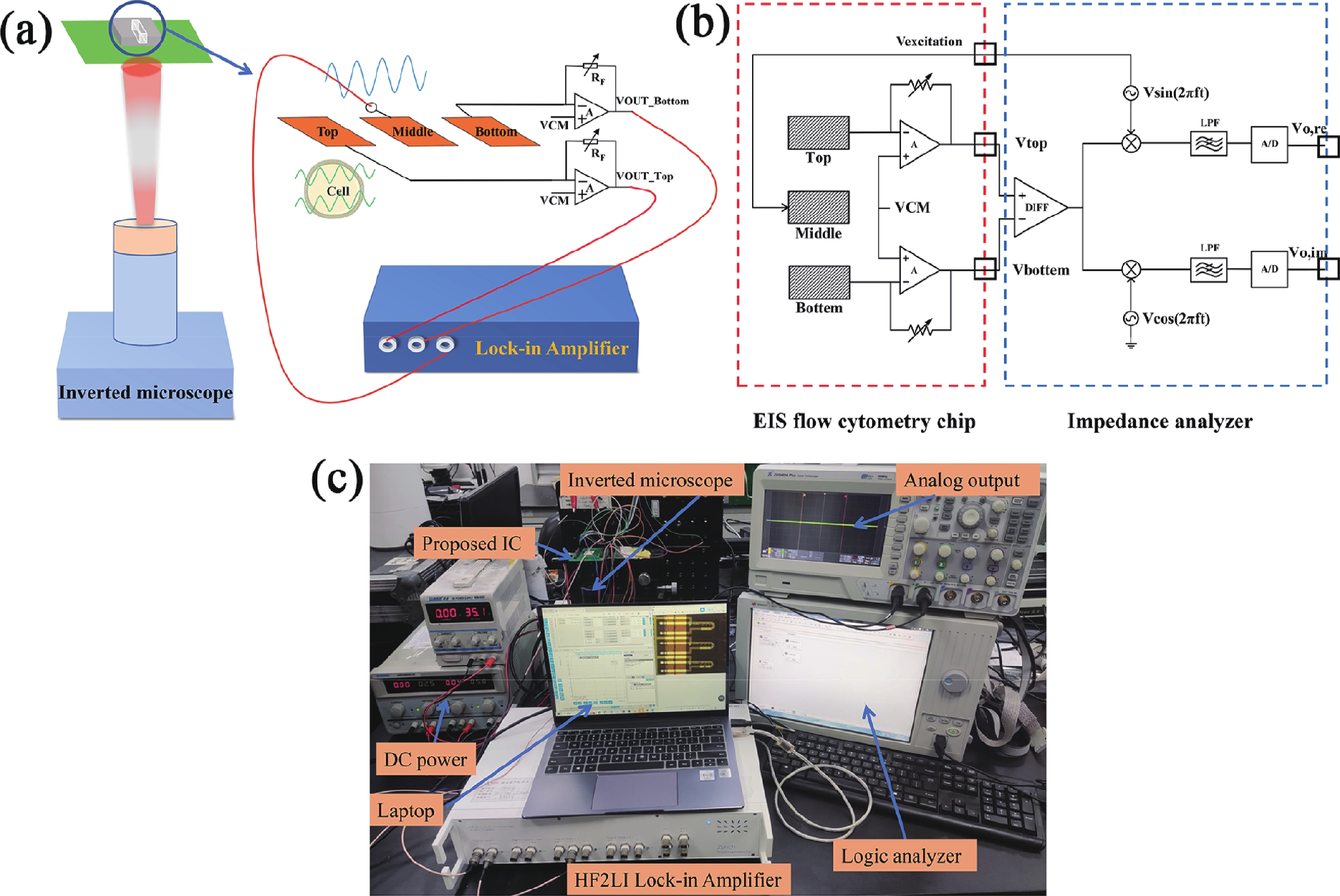
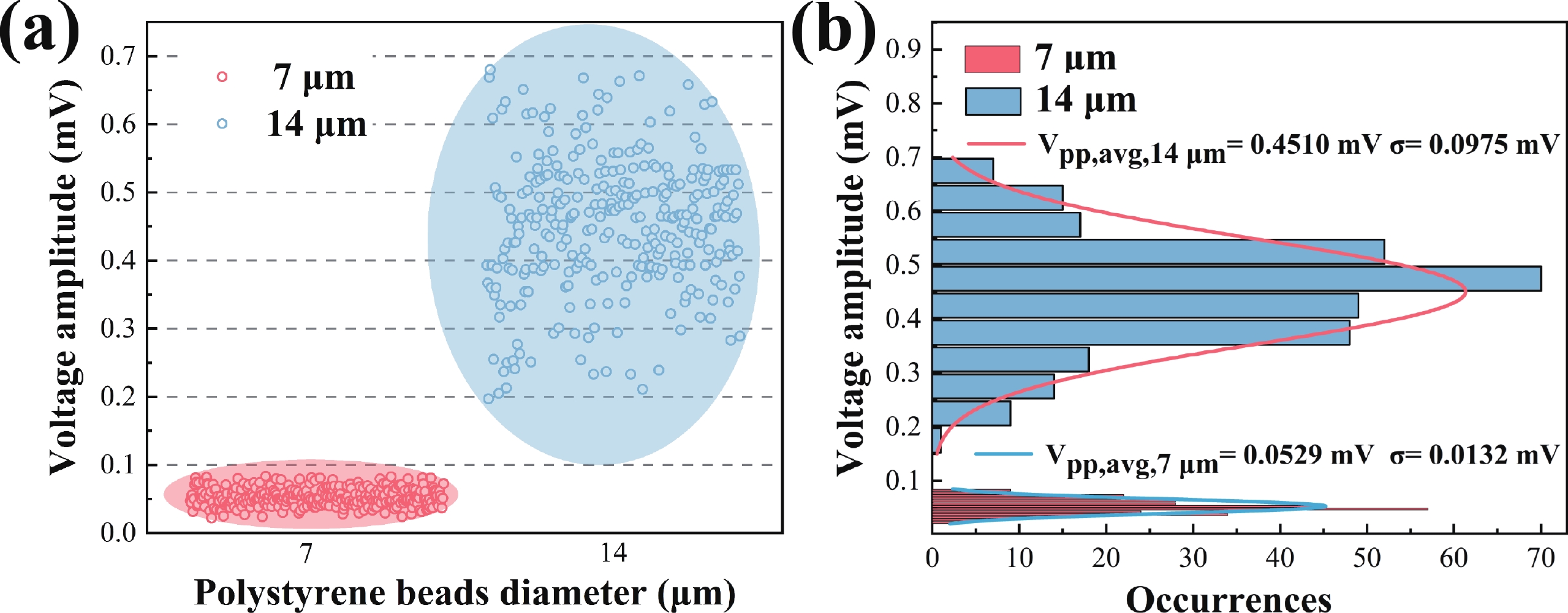
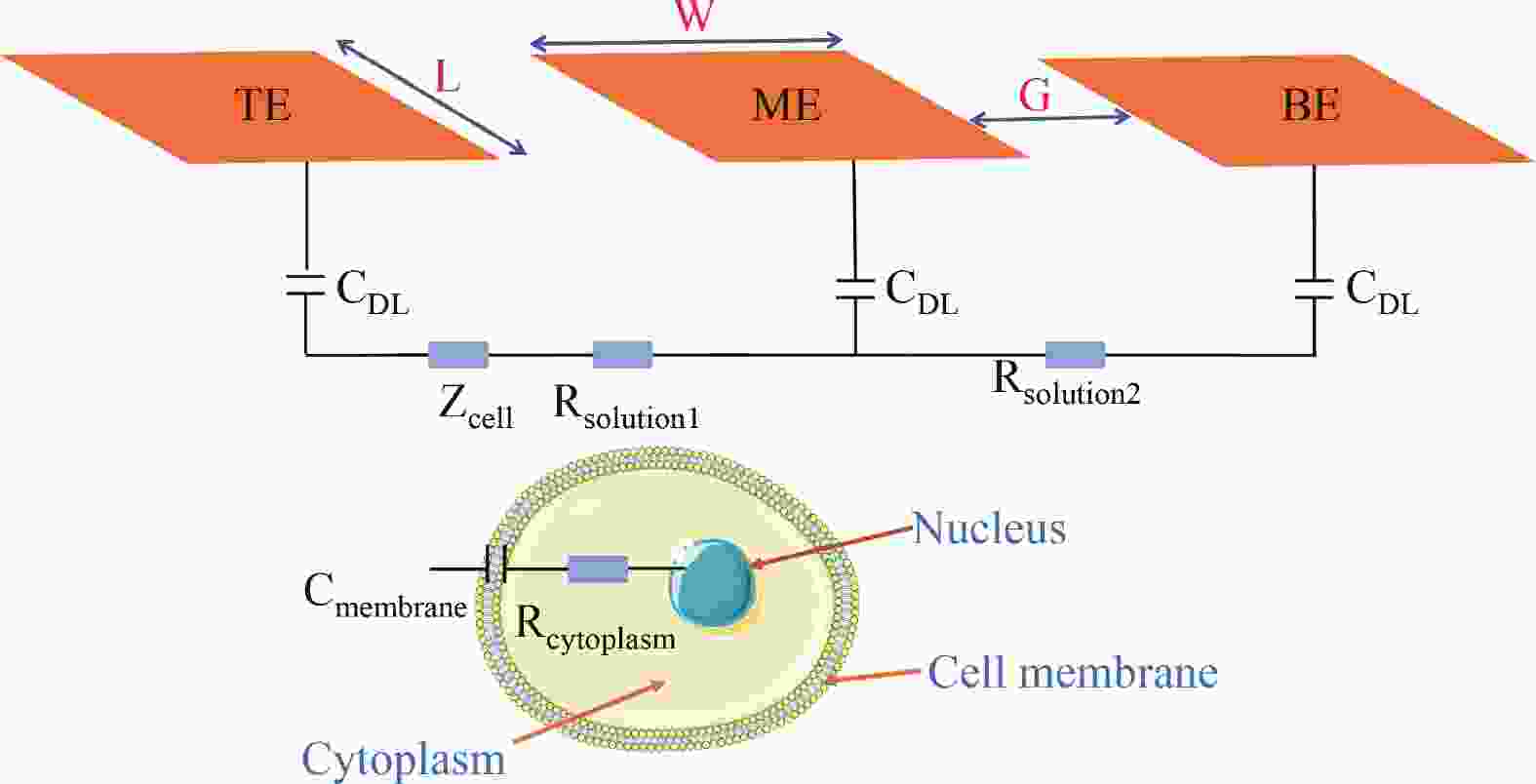
Electrochemical impedance spectroscopy (EIS) flow cytometry offers the advantages of speed, affordability, and portability in cell analysis and cytometry applications. However, the integration challenges of microfluidic and EIS read-out circuits hinder the downsizing of cytometry devices. To address this, we developed a thermal-bubble-driven impedance flow cytometric application-specific integrated circuit (ASIC). The thermal-bubble micropump avoids external piping and equipment, enabling high-throughput designs. With a total of 36 cell counting channels, each measuring 884 × 220 μm2, the chip significantly enhances the throughput of flow cytometers. Each cell counting channel incorporates a differential trans-impedance amplifier (TIA) to amplify weak biosensing signals. By eliminating the parasitic parameters created at the complementary metal-oxide-semiconductor transistor (CMOS)-micro-electromechanical systems (MEMS) interface, the counting accuracy can be increased. The on-chip TIA can adjust feedback resistance from 5 to 60 kΩ to accommodate solutions with different impedances. The chip effectively classifies particles of varying sizes, demonstrated by the average peak voltages of 0.0529 and 0.4510 mV for 7 and 14 μm polystyrene beads, respectively. Moreover, the counting accuracies of the chip for polystyrene beads and MSTO-211H cells are both greater than 97.6%. The chip exhibits potential for impedance flow cytometer at low cost, high-throughput, and miniaturization for the application of point-of-care diagnostics.-
Keywords:
- EIS,
- flow cytometry,
- CMOS-MEMS,
- thermal bubble,
- lab-on-chip
-
References
[1] Adan A, Alizada G, Kiraz Y, et al. Flow cytometry: Basic principles and applications. Crit Rev Biotechnol, 2017, 37(2), 163 doi: 10.3109/07388551.2015.1128876[2] Lu N, Tay H M, Petchakup C, et al. Label-free microfluidic cell sorting and detection for rapid blood analysis. Lab Chip, 2023, 23(5), 1226 doi: 10.1039/D2LC00904H[3] McKinnon K M. Flow Cytometry: An Overview, Curr Protoc Immunol, 2018, 120(1), 5 doi: 10.1002/cpim.40[4] Hurley J. Sizing particles with a coulter counter. Biophys J, 1970, 10(1), 74 doi: 10.1016/S0006-3495(70)86286-5[5] Don M. The coulter principle: Foundation of an industry. JALA J Assoc Lab Autom, 2003, 8(6), 72 doi: 10.1016/S1535-5535-03-00023-6[6] Rodriguez-Trujillo R, Mills C A, Samitier J, et al. Low cost micro-Coulter counter with hydrodynamic focusing. Microfluid Nanofluid, 2007, 3(2), 171 doi: 10.1007/s10404-006-0113-8[7] He Z, Mansfeld F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ Sci, 2009, 2(2), 215 doi: 10.1039/B814914C[8] Pastor-Fernández C, Dhammika W W, Marco J, et al. Identification and quantification of ageing mechanisms in lithium-ion batteries using the EIS technique. 2016 IEEE Transportation Electrification Conference and Expo (ITEC), 2016, 1 doi: 10.1109/ITEC.2016.7520198[9] Giagkoulovits C, Cheah B C, Al-Rawhani M A, et al. A 16 x 16 CMOS amperometric microelectrode array for simultaneous electrochemical measurements. IEEE Trans Circuits Syst I Regul Pap, 2018, 65(9), 2821 doi: 10.1109/TCSI.2018.2794502[10] Xu J W, Hong Z L. Low power bio-impedance sensor interfaces: Review and electronics design methodology. IEEE Rev Biomed Eng, 2022, 15, 23 doi: 10.1109/RBME.2020.3041053[11] Bounik R, Cardes F, Ulusan H, et al. Impedance imaging of cells and tissues: Design and applications. BME Front, 2022, 2022, 1 doi: 10.34133/2022/9857485[12] Park J Y, Park S M. DNA hybridization sensors based on electrochemical impedance spectroscopy as a detection tool. Sensors, 2009, 9(12), 9513 doi: 10.3390/s91209513[13] Bernabini C, Holmes D, Morgan H. Micro-impedance cytometry for detection and analysis of micron-sized particles and bacteria. Lab Chip, 2011, 11(3), 407 doi: 10.1039/C0LC00099J[14] Cheung K, Gawad S, Renaud P. Impedance spectroscopy flow cytometry: On-chip label-free cell differentiation. Cytometry Pt A, 2005, 65A(2), 124 doi: 10.1002/cyto.a.20141[15] Honrado C, Bisegna P, Swami N S, et al. Single-cell microfluidic impedance cytometry: From raw signals to cell phenotypes using data analytics. Lab Chip, 2021, 21(1), 22 doi: 10.1039/D0LC00840K[16] Haandbæk N, With O, Bürgel S C, et al. Resonance-enhanced microfluidic impedance cytometer for detection of single bacteria. Lab Chip, 2014, 14(17), 3313 doi: 10.1039/C4LC00576G[17] Nguyen T H, Nguyen H A, Tran Thi Y V, et al. Concepts, electrode configuration, characterization, and data analytics of electric and electrochemical microfluidic platforms: A review. Analyst, 2023, 148(9), 1912 doi: 10.1039/D2AN02027K[18] van Berkel C, Gwyer J D, Deane S, et al. Integrated systems for rapid point of care (PoC) blood cell analysis. Lab Chip, 2011, 11(7), 1249 doi: 10.1039/c0lc00587h[19] Sun D P, Lu J, Chen Z G. Microfluidic contactless conductivity cytometer for electrical cell sensing and counting. RSC Adv, 2015, 5(73), 59306 doi: 10.1039/C5RA08371K[20] Yang X P, Wang K, Huang P, et al. Thermal bubble-driven impedance-based high-throughput cell counting chip design. AIP Adv, 2023, 13(6), 065308. doi: 10.1063/5.0153846[21] Hassan U, Reddy B Jr, Damhorst G, et al. A microfluidic biochip for complete blood cell counts at the point-of-care. TECHNOLOGY, 2015, 3(4), 201 doi: 10.1142/S2339547815500090[22] Zhong J W, Liang M H, Ai Y. Submicron-precision particle characterization in microfluidic impedance cytometry with double differential electrodes. Lab Chip, 2021, 21(15), 2869 doi: 10.1039/D1LC00481F[23] Shen B Y, Dawes J, Johnston M L. A 10 MΩ, 50 kHz-40 MHz impedance measurement architecture for source-differential flow cytometry. IEEE Trans Biomed Circuits Syst, 2022, 16(5), 766 doi: 10.1109/TBCAS.2022.3182905[24] Iyer V, Issadore D A, Aflatouni F. The next generation of hybrid microfluidic/integrated circuit chips: Recent and upcoming advances in high-speed, high-throughput, and multifunctional lab-on-IC systems. Lab Chip, 2023, 23(11), 2553 doi: 10.1039/D2LC01163H[25] Carminati M, Gervasoni G, Sampietro M, et al. Note: Differential configurations for the mitigation of slow fluctuations limiting the resolution of digital lock-in amplifiers. Rev Sci Instrum, 2016, 87(2), 026102 doi: 10.1063/1.4941721[26] Sharma N, Kumar M, Kumari N, et al. Tantalum oxide thin films for electrochemical pH sensor. Mater Res Express, 2020, 7(3), 036405 doi: 10.1088/2053-1591/ab7ced[27] Carminati M, Ferrari G, Vahey M D, et al. Miniaturized impedance flow cytometer: Design rules and integrated readout. IEEE Trans Biomed Circuits Syst, 2017, 11, 1438 doi: 10.1109/TBCAS.2017.2748158[28] Lee K H, Nam J, Choi S, et al. A CMOS impedance cytometer for 3D flowing single-cell real-time analysis with ΔΣ error correction. 2012 IEEE International Solid-State Circuits Conference, 2012, 304 doi: 10.1109/ISSCC.2012.6177024[29] Fu Y S, Yuan Q B, Guo J H. Lab-on-PCB-based micro-cytometer for circulating tumor cells detection and enumeration. Microfluid Nanofluid, 2017, 21(2), 20 doi: 10.1007/s10404-017-1854-2[30] Chien J C, Ameri A, Yeh E C, et al. A high-throughput flow cytometry-on-a-CMOS platform for single-cell dielectric spectroscopy at microwave frequencies. Lab Chip, 2018, 18(14), 2065 doi: 10.1039/C8LC00299A[31] Feng Y X, Zhu J W, Chai H C, et al. Impedance-based multimodal electrical-mechanical intrinsic flow cytometry. Small, 2023, 19(45), 2370379 doi: 10.1002/smll.202370379 -
Proportional views




 DownLoad:
DownLoad: