| Citation: |
Xuebiao Deng, Huai Chen, Zhenyu Yang. Two-dimensional silicon nanomaterials for optoelectronics[J]. Journal of Semiconductors, 2023, 44(4): 041101. doi: 10.1088/1674-4926/44/4/041101
X B Deng, H Chen, Z Y Yang. Two-dimensional silicon nanomaterials for optoelectronics[J]. J. Semicond, 2023, 44(4): 041101. doi: 10.1088/1674-4926/44/4/041101
Export: BibTex EndNote
|
Two-dimensional silicon nanomaterials for optoelectronics
doi: 10.1088/1674-4926/44/4/041101
More Information-
Abstract
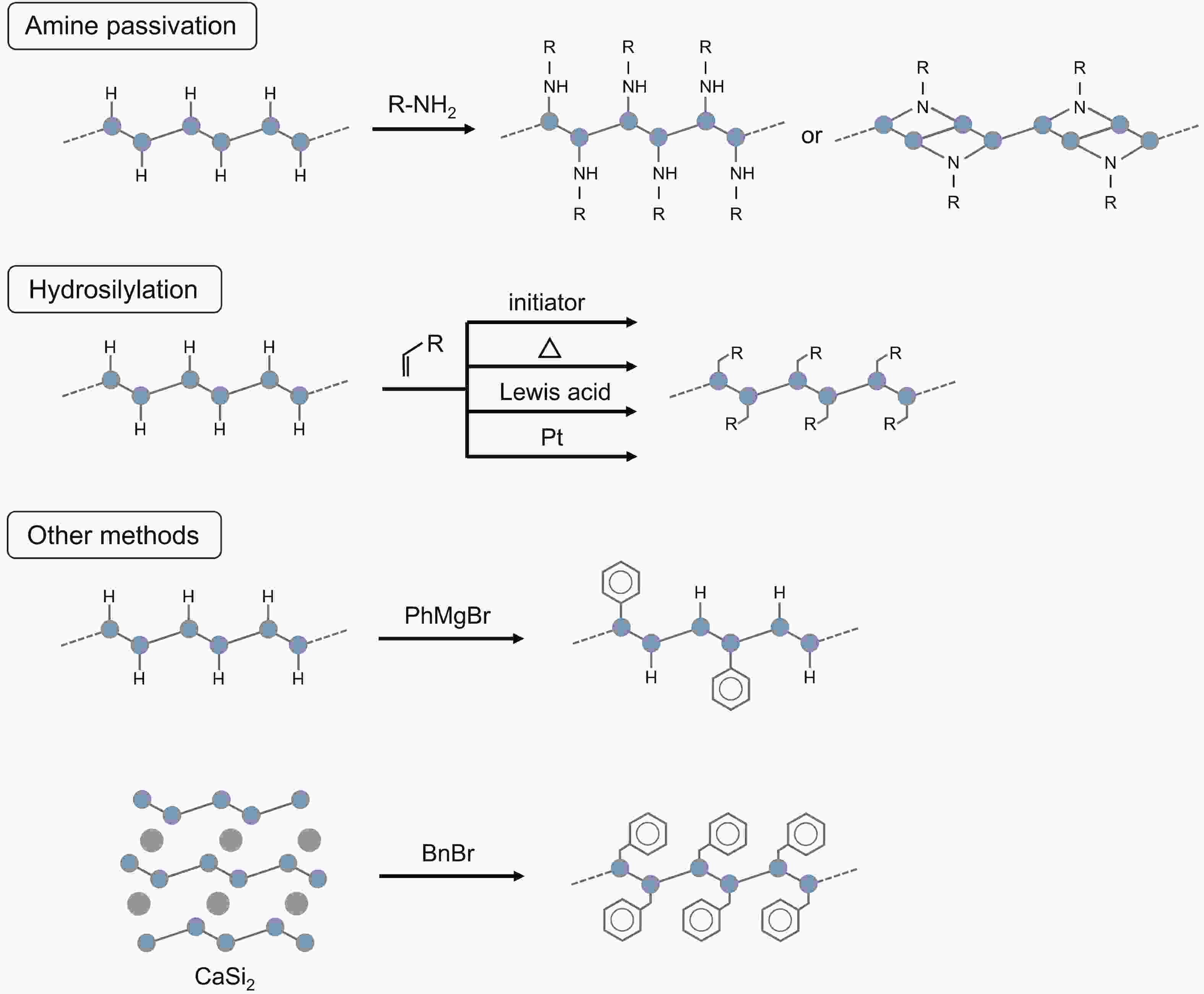
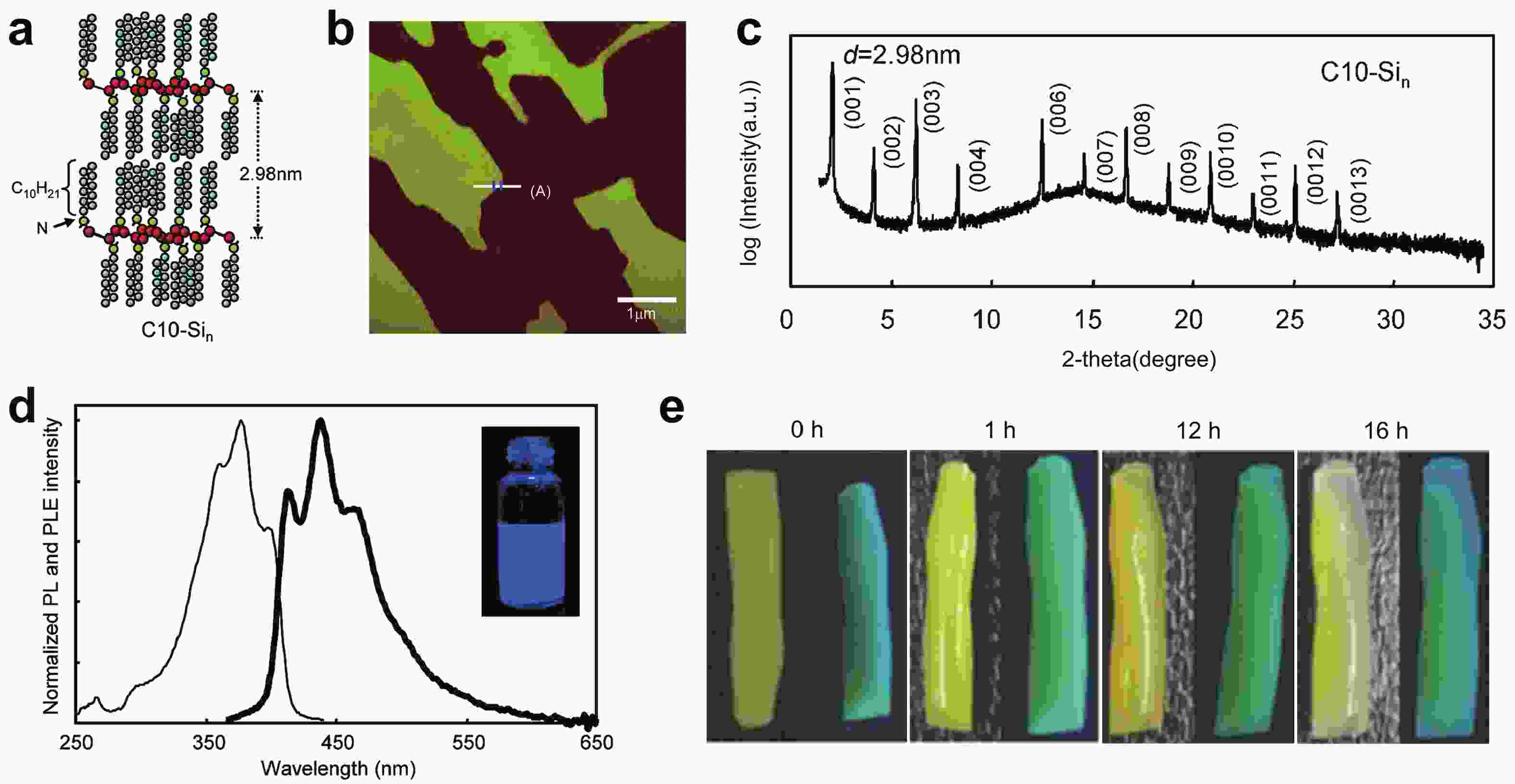
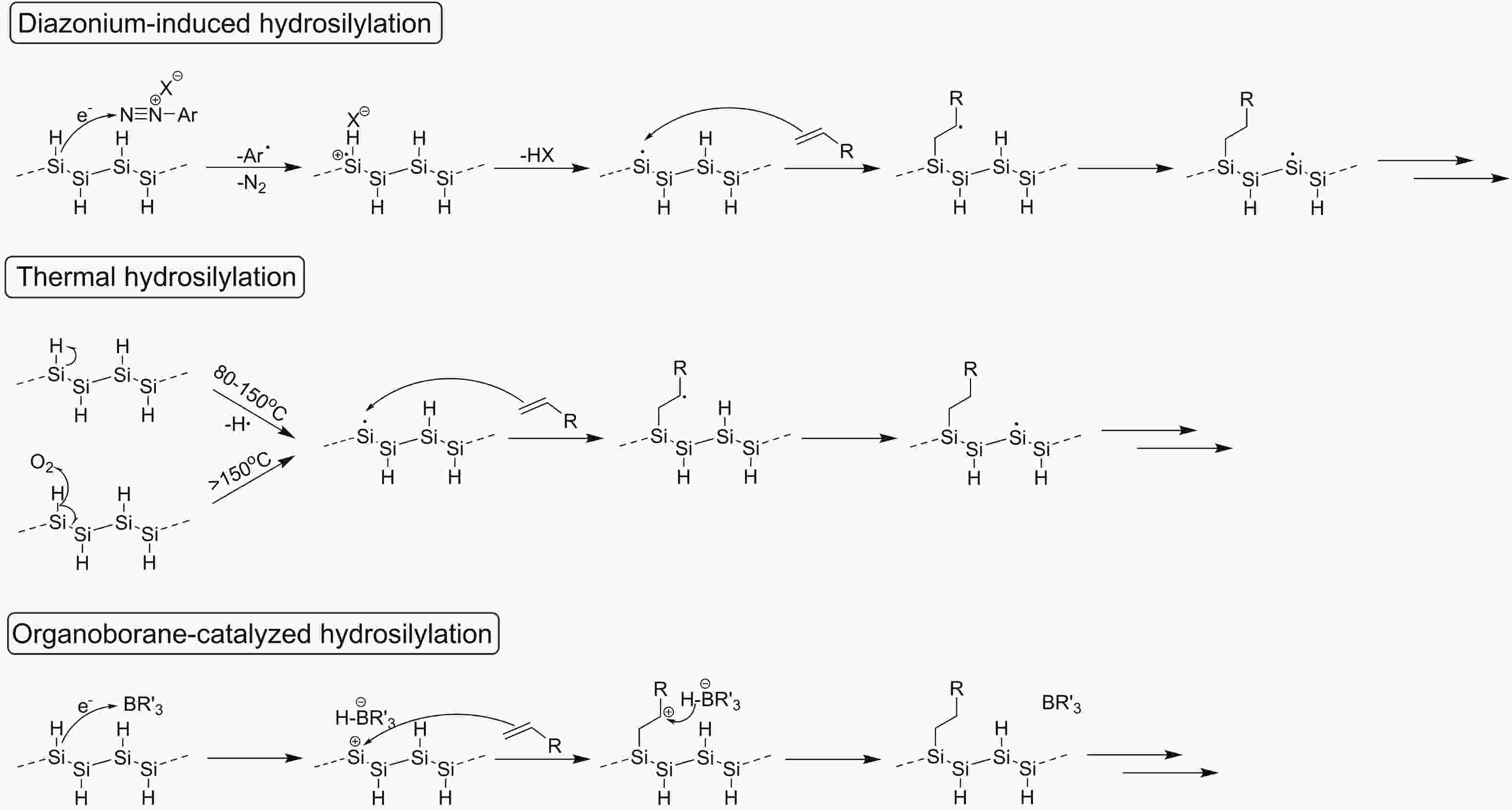
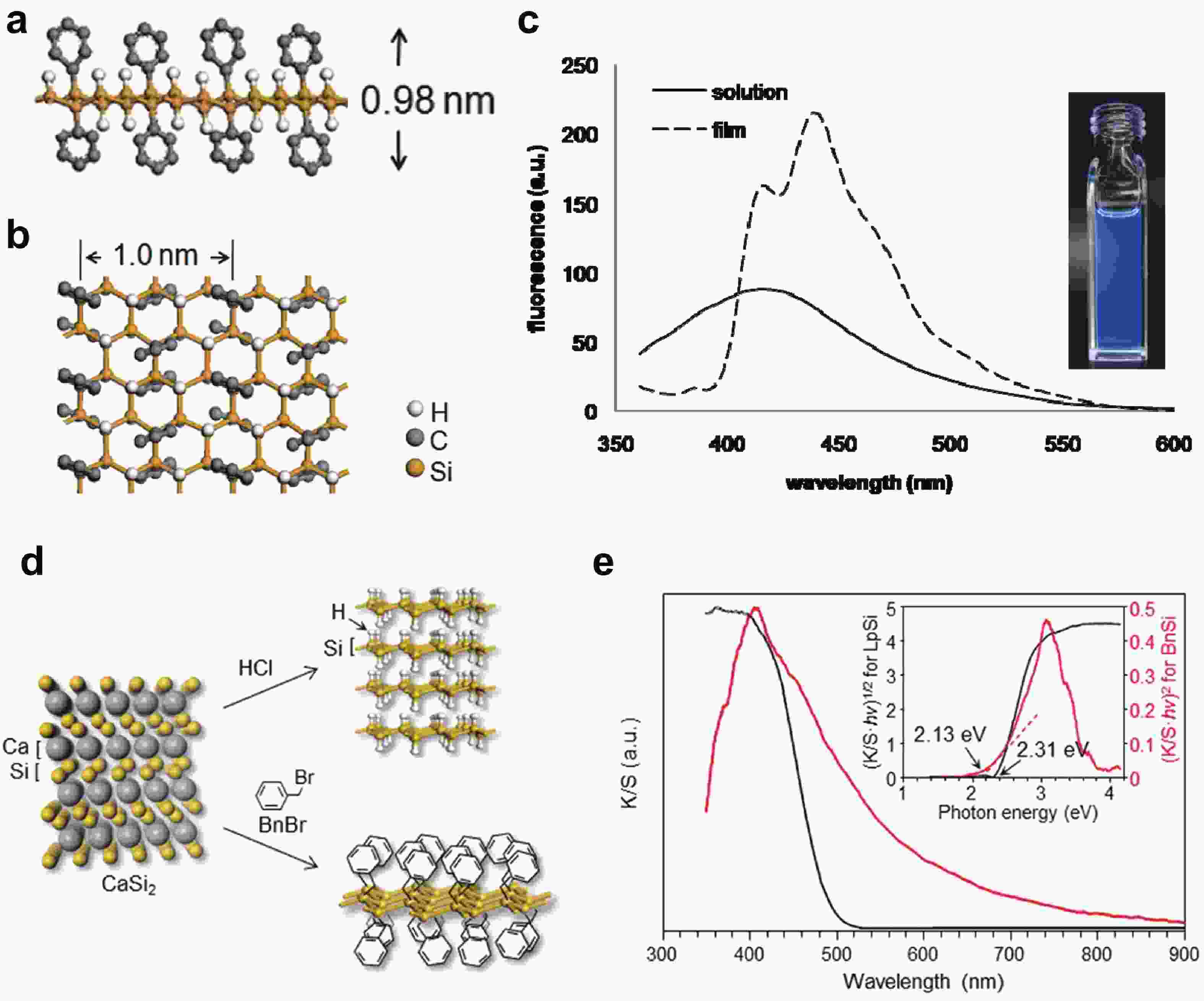
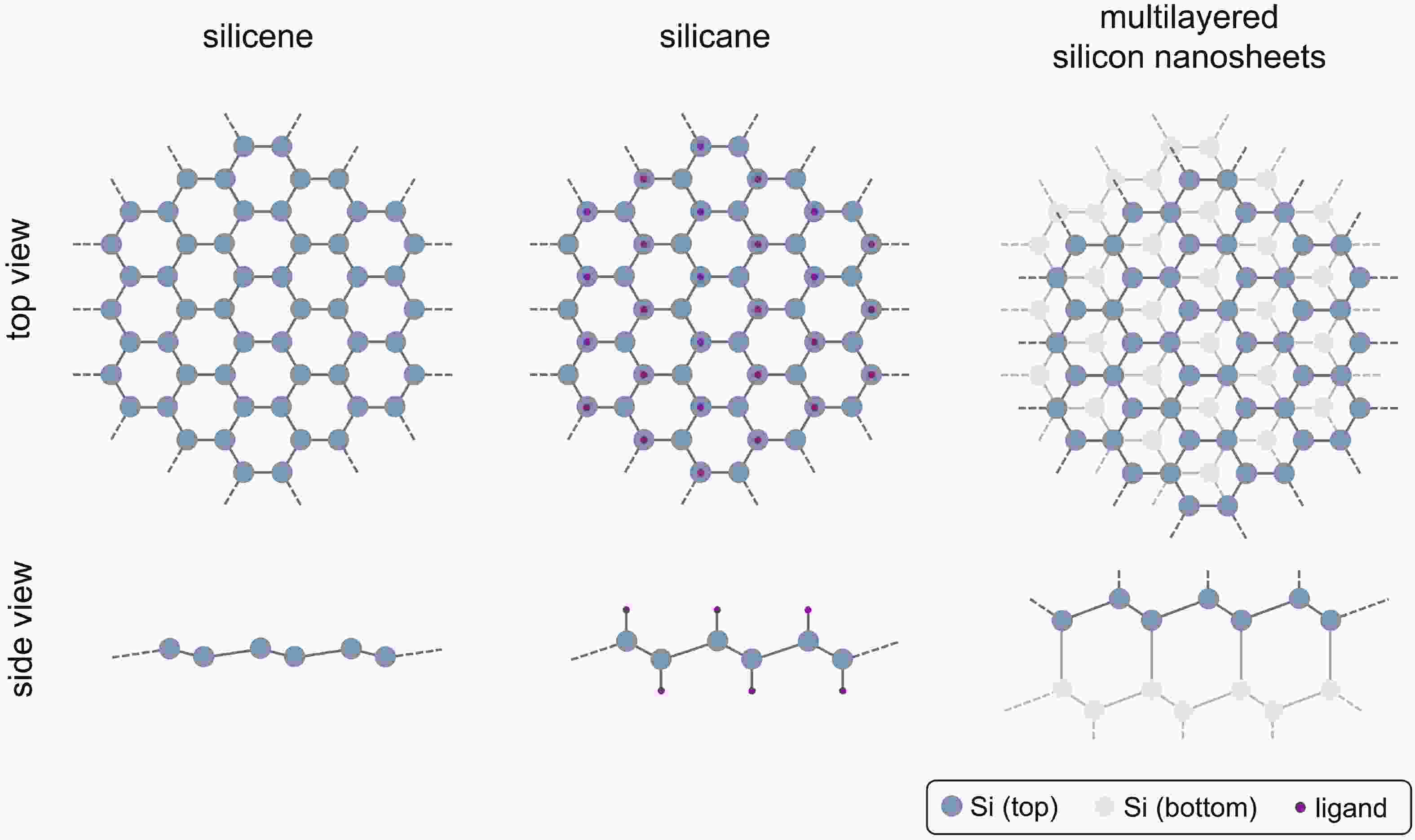
Silicon nanomaterials have been of immense interest in the last few decades due to their remarkable optoelectronic responses, elemental abundance, and higher biocompatibility. Two-dimensional silicon is one of the new allotropes of silicon and has many compelling properties such as quantum-confined photoluminescence, high charge carrier mobilities, anisotropic electronic and magnetic response, and non-linear optical properties. This review summarizes the recent advances in the synthesis of two-dimensional silicon nanomaterials with a range of structures (silicene, silicane, and multilayered silicon), surface ligand engineering, and corresponding optoelectronic applications. -
References
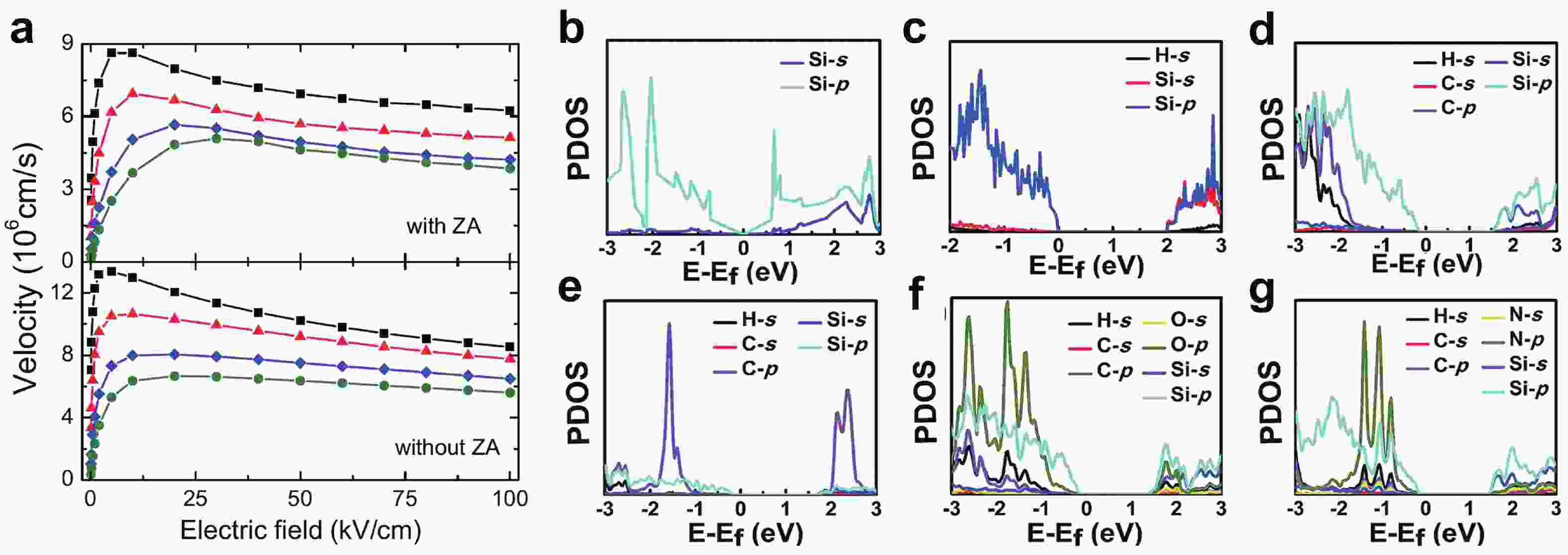
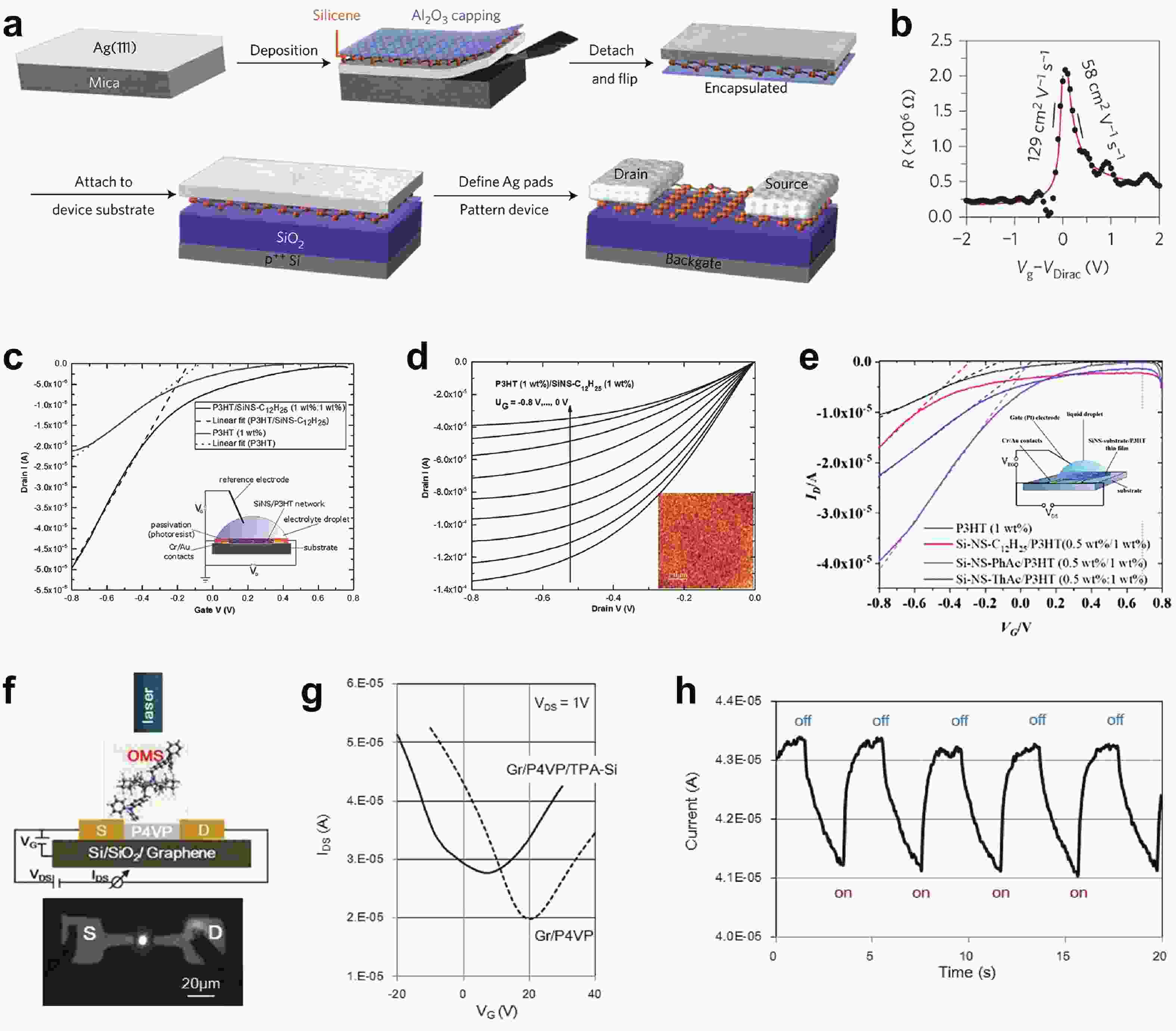
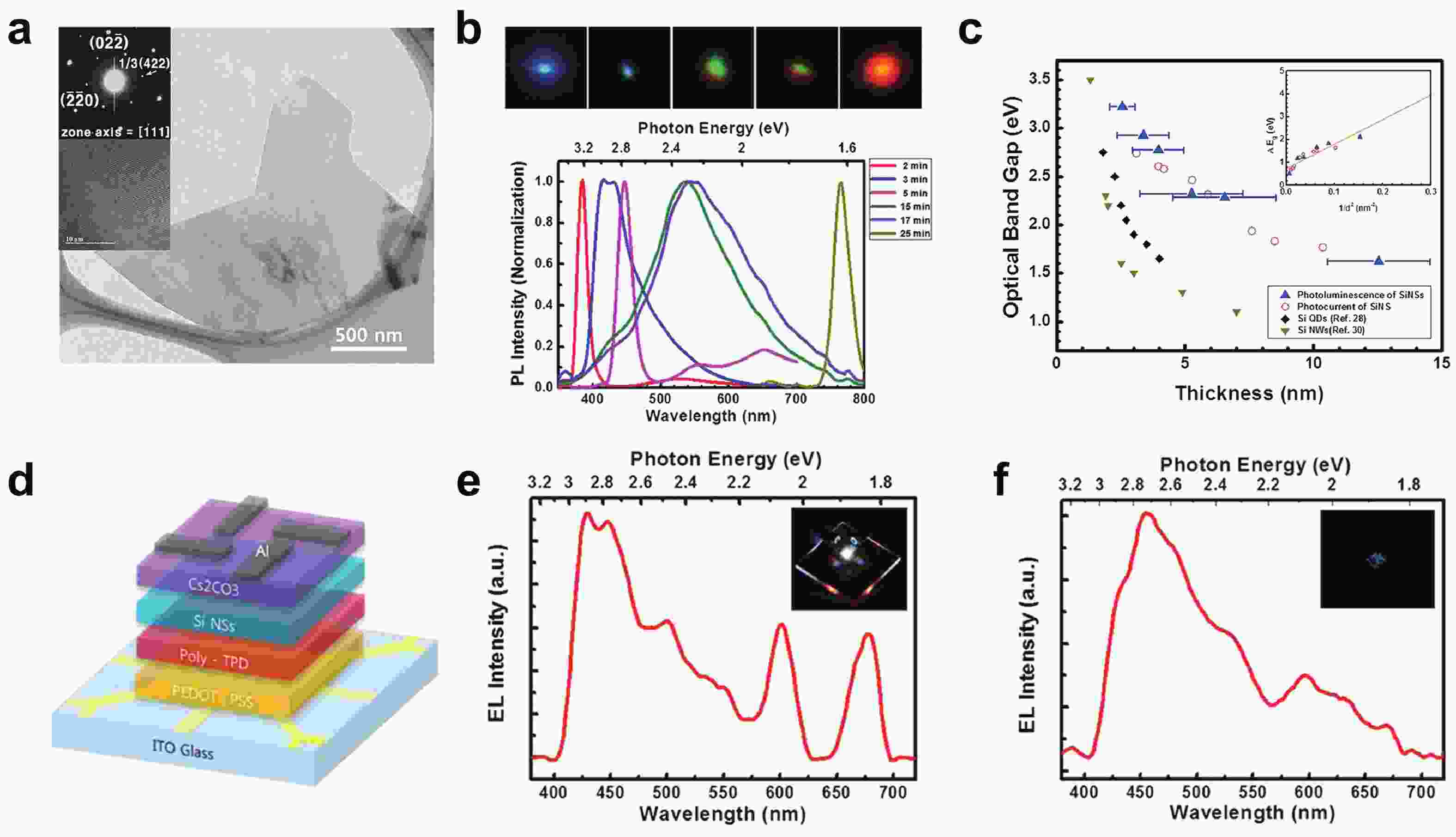
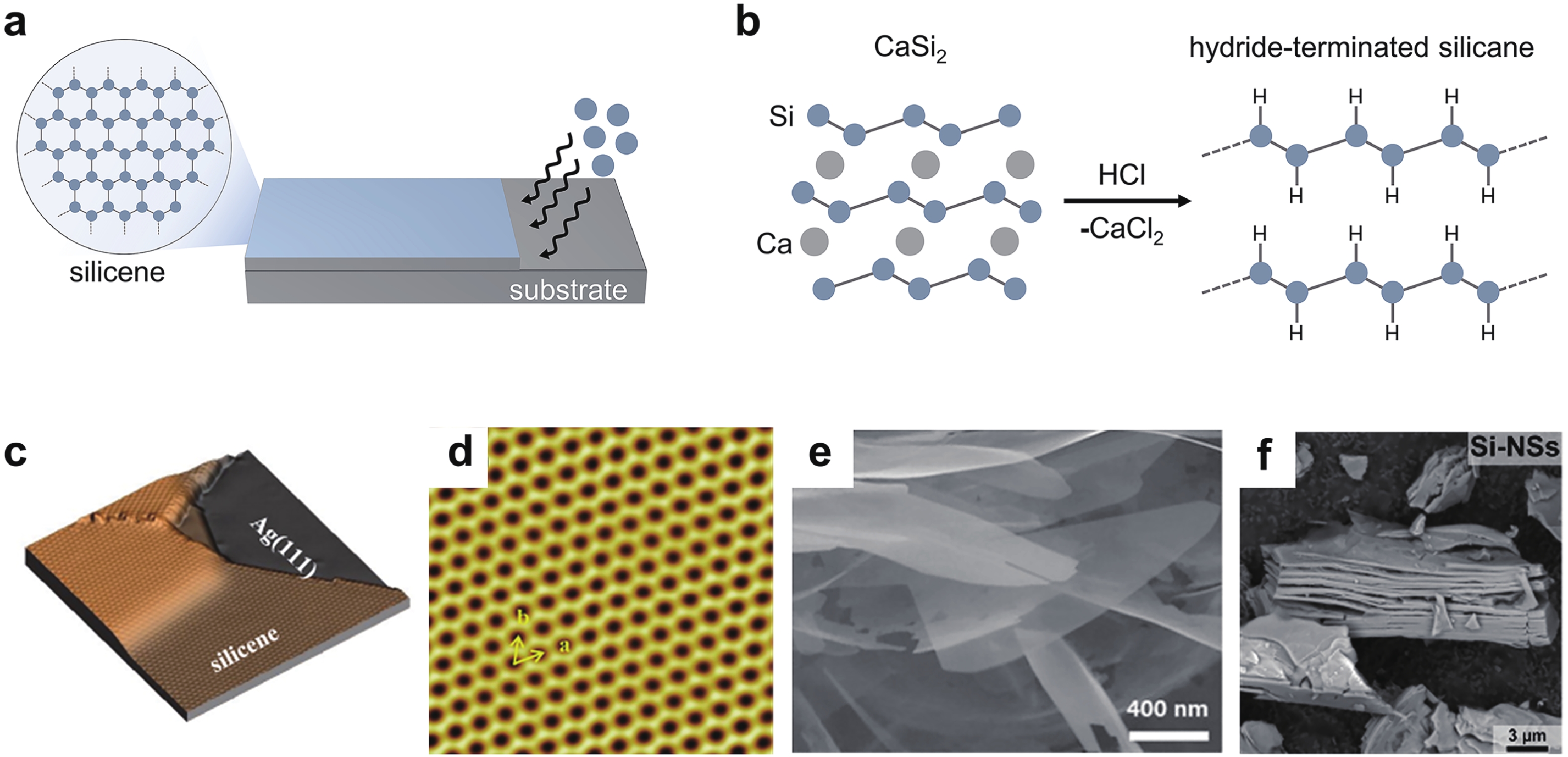
[1] Heersche H B, Jarillo-Herrero P, Oostinga J B, et al. Bipolar supercurrent in graphene. Nature, 2007, 446(7131), 56 doi: 10.1038/nature05555[2] Du X, Skachko I, Barker A, et al. Approaching ballistic transport in suspended graphene. Nat Nanotechnol, 2008, 3(8), 491 doi: 10.1038/nnano.2008.199[3] Wang F, Zhang Y, Tian C, et al. Gate-variable optical transitions in graphene. Science, 2008, 320(5873), 206 doi: 10.1126/science.1152793[4] Lee C, Wei X, Kysar J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 2008, 321(5887), 385 doi: 10.1126/science.1157996[5] Scheuermann G M, Rumi L, Steurer P, et al. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki−Miyaura Coupling reaction. J Am Chem Soc, 2009, 131(23), 8262 doi: 10.1021/ja901105a[6] Lu Y, Lu Y, Niu Z, et al. Graphene-based nanomaterials for sodium-ion batteries. Ad Energy Mater, 2018, 8(17), 1702469 doi: 10.1002/aenm.201702469[7] Lin L, Peng H, Liu Z. Synthesis challenges for graphene industry. Nat Mater, 2019, 18(6), 520 doi: 10.1038/s41563-019-0341-4[8] Deng X, Zheng X, Yuan T, et al. Ligand impact of silicanes as anode materials for lithium-ion batteries. Chem Mater, 2021, 33(23), 9357 doi: 10.1021/acs.chemmater.1c03254[9] Xu H, Chen H, Gao C. Advanced graphene materials for sodium/potassium/aluminum-ion batteries. ACS Mater Lett, 2021, 3(8), 1221 doi: 10.1021/acsmaterialslett.1c00280[10] Wang Q H, Kalantar-Zadeh K, Kis A, et al. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat Nanotechnol, 2012, 7(11), 699 doi: 10.1038/nnano.2012.193[11] Mak K F, Shan J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat Photonics, 2016, 10(4), 216 doi: 10.1038/nphoton.2015.282[12] Meng J L, Wang T Y, Chen L, et al. Energy-efficient flexible photoelectric device with 2D/0D hybrid structure for bio-inspired artificial heterosynapse application. Nano Energy, 2021, 83, 105815 doi: 10.1016/j.nanoen.2021.105815[13] Meng J L, Wang T Y, He Z Y, et al. Flexible boron nitride-based memristor for in situ digital and analogue neuromorphic computing applications. Mater Horiz, 2021, 8(2), 538 doi: 10.1039/D0MH01730B[14] Wang C, Xu X, Pi X, et al. Neuromorphic device based on silicon nanosheets. Nat Commun, 2022, 13(1), 5216 doi: 10.1038/s41467-022-32884-y[15] Meng J, Wang T, Zhu H, et al. Integrated in-sensor computing optoelectronic device for environment-adaptable artificial retina perception application. Nano Lett, 2022, 22(1), 81 doi: 10.1021/acs.nanolett.1c03240[16] Cahangirov S, Topsakal M, Aktürk E, et al. Two- and one-dimensional honeycomb structures of silicon and germanium. Phys Rev Lett, 2009, 102(23), 236804 doi: 10.1103/PhysRevLett.102.236804[17] Guzmán-Verri G G, Lew Yan Voon L C. Electronic structure of silicon-based nanostructures. Phys Rev B, 2007, 76(7), 075131 doi: 10.1103/PhysRevB.76.075131[18] Morishita T, Russo S P, Snook I K, et al. First-principles study of structural and electronic properties of ultrathin silicon nanosheets. Phys Rev B, 2010, 82(4), 045419 doi: 10.1103/PhysRevB.82.045419[19] Liu C C, Feng W, Yao Y. Quantum spin Hall effect in silicene and two-dimensional germanium. Phys Rev Lett, 2011, 107(7), 076802 doi: 10.1103/PhysRevLett.107.076802[20] Spencer M J S, Morishita T, Snook I K. Reconstruction and electronic properties of silicon nanosheets as a function of thickness. Nanoscale, 2012, 4(9), 2906 doi: 10.1039/c2nr30100h[21] Chigo Anota E, Bautista Hernández A, Castro M, et al. Investigating the electronic properties of silicon nanosheets by first-principles calculations. J Molecul Model, 2012, 18(5), 2147 doi: 10.1007/s00894-011-1235-9[22] Kamal C, Chakrabarti A, Banerjee A, et al. Silicene beyond mono-layers—different stacking configurations and their properties. J Physics: Conden Matter, 2013, 25(8), 085508 doi: 10.1088/0953-8984/25/8/085508/meta[23] Gao J, Zhang J, Liu H, et al. Structures, mobilities, electronic and magnetic properties of point defects in silicene. Nanoscale, 2013, 5(20), 9785 doi: 10.1039/C3NR02826G[24] Li S, Wu Y, Liu W, et al. Control of band structure of van der Waals heterostructures: Silicene on ultrathin silicon nanosheets. Chem Phys Lett, 2014, 609, 161 doi: 10.1016/j.cplett.2014.06.047[25] Roman R E, Cranford S W. Mechanical properties of silicene. Comput Mater Sci, 2014, 82, 50 doi: 10.1016/j.commatsci.2013.09.030[26] Quhe R, Fei R, Liu Q, et al. Tunable and sizable band gap in silicene by surface adsorption. Sci Rep, 2012, 2(1), 853 doi: 10.1038/srep00853[27] Ryan B J, Hanrahan M P, Wang Y, et al. Silicene, siloxene, or silicane? Revealing the structure and optical properties of silicon nanosheets derived from calcium disilicide. Chem Mater, 2020, 32(2), 795 doi: 10.1021/acs.chemmater.9b04180[28] De Padova P, Kubo O, Olivieri B, et al. Multilayer silicene nanoribbons. Nano Lett, 2012, 12(11), 5500 doi: 10.1021/nl302598x[29] Feng B, Ding Z, Meng S, et al. Evidence of silicene in honeycomb structures of silicon on Ag(111). Nano Lett, 2012, 12(7), 3507 doi: 10.1021/nl301047g[30] Kim S W, Lee J, Sung J H, et al. Two-dimensionally grown single-crystal silicon nanosheets with tunable visible-light emissions. ACS Nano, 2014, 8(7), 6556 doi: 10.1021/nn501683f[31] Tao L, Cinquanta E, Chiappe D, et al. Silicene field-effect transistors operating at room temperature. Nat Nanotechnol, 2015, 10(3), 227 doi: 10.1038/nnano.2014.325[32] Lyuleeva A, Helbich T, Rieger B, et al. Polymer-silicon nanosheet composites: bridging the way to optoelectronic applications. J Phys D, 2017, 50(13), 135106 doi: 10.1088/1361-6463/aa5005[33] Berger C, Song Z, Li T, et al. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J Phys Chem B, 2004, 108(52), 19912 doi: 10.1021/jp040650f[34] Sutter P W, Flege J I, Sutter E A. Epitaxial graphene on ruthenium. Nat Mater, 2008, 7(5), 406 doi: 10.1038/nmat2166[35] Hao R, Qian W, Zhang L, et al. Aqueous dispersions of TCNQ-anion-stabilized graphene sheets. Chem Commun, 2008, 48, 6576 doi: 10.1039/B816971C[36] Novoselov K S, Jiang D, Schedin F, et al. Two-dimensional atomic crystals. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(30), 10451 doi: 10.1073/pnas.0502848102[37] Wöhler F. Ueber verbindungen des siliciums mit sauerstoff und wasserstoff. Justus Liebigs Annalen der Chemie, 1863, 127(3), 257 doi: 10.1002/jlac.18631270302[38] Weiss A, Beil G, Meyer H. The topochemical reaction of CaSi2 to a two-dimensional subsiliceous acid Si6H3(OH)3 (= Kautsky' Siloxene). J Zeitschrift für Naturforschung B, 1980, 35, 25 doi: doi.org/10.1515/znb-1980-0108[39] Dahn J R, Way B M, Fuller E, et al. Structure of siloxene and layered polysilane (Si6H6). Phys Rev B, 1993, 48(24), 17872 doi: 10.1103/PhysRevB.48.17872[40] Min H, Hill J E, Sinitsyn N A, et al. Intrinsic and Rashba spin-orbit interactions in graphene sheets. Phys Rev B, 2006, 74(16), 165310 doi: 10.1103/PhysRevB.74.165310[41] Ezawa M. A topological insulator and helical zero mode in silicene under an inhomogeneous electric field. New J Phys, 2012, 14(3), 033003 doi: 10.1088/1367-2630/14/3/033003[42] Liu J, Yang Y, Lyu P, et al. Few-layer silicene nanosheets with superior lithium-storage properties. Adv Mater, 2018, 30(26), 1800838 doi: 10.1002/adma.201800838[43] Linti G. Silicon chemistry. From the Atom to Extended Systems. Edited by Peter Jutzi and Ulrich Schubert. Angew Chem Int Ed, 2004, 43(23), 135 doi: 10.1002/anie.200385120[44] Okamoto H, Kumai Y, Sugiyama Y, et al. Silicon nanosheets and their self-assembled regular stacking structure. J Am Chem Soc, 2010, 132(8), 2710 doi: 10.1021/ja908827z[45] Li F, Lu R, Yao Q, et al. Geometric and electronic structures as well as thermodynamic stability of hexyl-modified silicon nanosheet. J Phys Chem C, 2013, 117(25), 13283 doi: 10.1021/jp402875t[46] De Padova P, Generosi A, Paci B, et al. Multilayer silicene: clear evidence. 2D Mater, 2016, 3(3), 031011 doi: 10.1088/2053-1583/3/3/031011[47] Grazianetti C, Cinquanta E, Tao L, et al. Silicon nanosheets: Crossover between multilayer silicene and diamond-like growth regime. ACS Nano, 2017, 11(3), 3376 doi: 10.1021/acsnano.7b00762[48] Chen H D, Chien K H, Lin C Y, et al. Few-layer silicon films on the Ag(111) surface. J Phys Chem C, 2016, 120(5), 2698 doi: 10.1021/acs.jpcc.5b10208[49] Padova P D, Generosi A, Paci B, et al. Corrigendum: Multilayer silicene: clear evidence. 2D Mater, 2016, 3(4), 049501 doi: 10.1088/2053-1583/3/4/049501[50] Okamoto H, Sugiyama Y, Nakano H. Synthesis and modification of silicon nanosheets and other silicon nanomaterials. Chem Eur J, 2011, 17(36), 9864 doi: 10.1002/chem.201100641[51] Chen J, Du Y, Li Z, et al. Delocalized surface state in epitaxial Si(111) film with spontaneous${\sqrt{3}}$ ×${\sqrt{3}}$ superstructure. Sci Rep, 2015, 5(1), 13590 doi: 10.1038/srep13590[52] Chen L, Liu C C, Feng B, et al. Evidence for Dirac fermions in a honeycomb lattice based on silicon. Phys Rev Lett, 2012, 109(5), 056804 doi: 10.1103/PhysRevLett.109.056804[53] De Padova P, Vogt P, Resta A, et al. Evidence of Dirac fermions in multilayer silicene. Appl Phys Lett, 2013, 102(16), 163106 doi: 10.1063/1.4802782[54] Hwang G C, Blom D A, Vogt T, et al. Pressure-driven phase transitions and reduction of dimensionality in 2D silicon nanosheets. Nat Commun, 2018, 9(1), 5412 doi: 10.1038/s41467-018-07832-4[55] Nakano H. Synthesis and modification of two-dimensional crystalline silicon nanosheets. J Ceram Soc Jpn, 2014, 122(1429), 748 doi: 10.2109/jcersj2.122.748[56] Nakano H, Ikuno T. Soft chemical synthesis of silicon nanosheets and their applications. Appl Phys Rev, 2016, 3(4), 040803 doi: 10.1063/1.4952442[57] Yu X, Xue F, Huang H, et al. Synthesis and electrochemical properties of silicon nanosheets by DC arc discharge for lithium-ion batteries. Nanoscale, 2014, 6(12), 6860 doi: 10.1039/C3NR06418B[58] Ryu J, Chen T, Bok T, et al. Mechanical mismatch-driven rippling in carbon-coated silicon sheets for stress-resilient battery anodes. Nat Commun, 2018, 9(1), 2924 doi: 10.1038/s41467-018-05398-9[59] Lalmi B, Oughaddou H, Enriquez H, et al. Epitaxial growth of a silicene sheet. Appl Phys Lett, 2010, 97(22), 223109 doi: 10.1063/1.3524215[60] Meng L, Wang Y, Zhang L, et al. Buckled silicene formation on Ir(111). Nano Lett, 2013, 13(2), 685 doi: 10.1021/nl304347w[61] Fleurence A, Friedlein R, Ozaki T, et al. Experimental evidence for epitaxial silicene on diboride thin films. Phys Rev Lett, 2012, 108(24), 245501 doi: 10.1103/PhysRevLett.108.245501[62] Warner J H, Rümmeli M H, Bachmatiuk A, et al. Atomic resolution imaging and topography of boron nitride sheets produced by chemical exfoliation. ACS Nano, 2010, 4(3), 1299 doi: 10.1021/nn901648q[63] Xu J, Zhang L, Shi R, et al. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J Mater Chem A, 2013, 1(46), 14766 doi: 10.1039/c3ta13188b[64] Ding Y, Chen Y P, Zhang X, et al. Controlled intercalation and chemical exfoliation of layered metal –organic frameworks using a chemically labile intercalating agent. J Am Chem Soc, 2017, 139(27), 9136 doi: 10.1021/jacs.7b04829[65] Yamanaka S, Matsu-ura H, Ishikawa M. New deintercalation reaction of calcium from calcium disilicide. Synthesis of layered polysilane. Mater Res Bull, 1996, 31(3), 307 doi: 10.1016/0025-5408(95)00195-6[66] Nakano H, Mitsuoka T, Harada M, et al. Soft synthesis of single-crystal silicon monolayer sheets. Angew Chem Int Ed, 2006, 45(38), 6303 doi: 10.1002/anie.200600321[67] Lang J, Ding B, Zhang S, et al. Scalable synthesis of 2D Si nanosheets. Adv Mater, 2017, 29(31), 1701777 doi: 10.1002/adma.201701777[68] Qiu J, Fu H, Xu Y, et al. From silicene to half-silicane by hydrogenation. ACS Nano, 2015, 9(11), 11192 doi: 10.1021/acsnano.5b04722[69] Wang W, Olovsson W, Uhrberg R I G. Band structure of hydrogenated silicene on Ag(111): Evidence for half-silicane. Phys Rev B, 2016, 93(8), 081406 doi: 10.1103/PhysRevB.93.081406[70] Liao W S, Lee S C. Water-induced room-temperature oxidation of Si–H and –Si–Si– bonds in silicon oxide. J Appl Phys, 1996, 80(2), 1171 doi: 10.1063/1.362915[71] Pereira R N, Rowe D J, Anthony R J, et al. Oxidation of freestanding silicon nanocrystals probed with electron spin resonance of interfacial dangling bonds. Phys Rev B, 2011, 83(15), 155327 doi: 10.1103/PhysRevB.83.155327[72] Acun A, Poelsema B, Zandvliet H J W, et al. The instability of silicene on Ag(111). Appl Phys Lett, 2013, 103(26), 263119 doi: 10.1063/1.4860964[73] Cottrell T L. The strengths of chemical bonds. Butterworths Scientific, 1958[74] Song J H, Sailor M J. Dimethyl sulfoxide as a mild oxidizing agent for porous silicon and its effect on photoluminescence. Inorg Chem, 1998, 37(13), 3355 doi: 10.1021/ic971587u[75] Dasog M, Yang Z, Regli S, et al. Chemical insight into the origin of red and blue photoluminescence arising from freestanding silicon nanocrystals. ACS Nano, 2013, 7(3), 2676 doi: 10.1021/nn4000644[76] Ohshita J, Yamamoto K, Tanaka D, et al. Preparation and photocurrent generation of silicon nanosheets with aromatic substituents on the surface. J Phys Chem C, 2016, 120(20), 10991 doi: 10.1021/acs.jpcc.6b03014[77] Helbich T, Lyuleeva A, Ludwig T, et al. One-step synthesis of photoluminescent covalent polymeric nanocomposites from 2D silicon nanosheets. Adv Funct Mater, 2016, 26(37), 6711 doi: 10.1002/adfm.201602137[78] Linford M R, Chidsey C E D. Alkyl monolayers covalently bonded to silicon surfaces. J Am Chem Soc, 1993, 115(26), 12631 doi: 10.1021/ja00079a071[79] Terry J, Linford M R, Wigren C, et al. Determination of the bonding of alkyl monolayers to the Si(111) surface using chemical-shift, scanned-energy photoelectron diffraction. Appl Phys Lett, 1997, 71(8), 1056 doi: 10.1063/1.119726[80] Terry J, Linford M R, Wigren C, et al. Alkyl-terminated Si(111) surfaces: A high-resolution, core level photoelectron spectroscopy study. J Appl Phys, 1998, 85(1), 213 doi: 10.1063/1.369473[81] Effenberger F, Götz G, Bidlingmaier B, et al. Photoactivated preparation and patterning of self-assembled monolayers with 1-alkenes and aldehydes on silicon hydride surfaces. Angew Chem Int Ed, 1998, 37(18), 2462 doi: 10.1002/(SICI)1521-3773(19981002)37:18<2462::AID-ANIE2462>3.0.CO;2-R[82] Cicero R L, Linford M R, Chidsey C E D. Photoreactivity of unsaturated compounds with hydrogen-terminated silicon(111). Langmuir, 2000, 16(13), 5688 doi: 10.1021/la9911990[83] Stewart M P, Buriak J M. Exciton-mediated hydrosilylation on photoluminescent nanocrystalline silicon. J Am Chem Soc, 2001, 123(32), 7821 doi: 10.1021/ja011116d[84] Eves B J, Lopinski G P. Formation of organic monolayers on silicon via gas-phase photochemical reactions. Langmuir, 2006, 22(7), 3180 doi: 10.1021/la052960a[85] Wang X, Ruther R E, Streifer J A, et al. UV-induced grafting of alkenes to silicon surfaces: Photoemission versus excitons. J Am Chem Soc, 2010, 132(12), 4048 doi: 10.1021/ja910498z[86] Helbich T, Lyuleeva A, Höhlein I M D, et al. Radical-induced hydrosilylation reactions for the functionalization of two-dimensional hydride terminated silicon nanosheets. Chem Eur J, 2016, 22(18), 6194 doi: 10.1002/chem.201505134[87] Lyuleeva A, Holzmüller P, Helbich T, et al. Charge transfer doping in functionalized silicon nanosheets/P3HT hybrid material for applications in electrolyte-gated field-effect transistors. J Mater Chem C, 2018, 6(27), 7343 doi: 10.1039/C8TC01484A[88] Helbich T, Kloberg M J, Sinelnikov R, et al. Diaryliodonium salts as hydrosilylation initiators for the surface functionalization of silicon nanomaterials and their collaborative effect as ring opening polymerization initiators. Nanoscale, 2017, 9(23), 7739 doi: 10.1039/C7NR01559C[89] Kloberg M J, Helbich T, Rieger B. Silicon nanosheets as co-initiators for diaryliodonium induced radical and cationic polymerization. Nanotechnology, 2018, 30(7), 075602 doi: 10.1088/1361-6528/aaf3f2/meta[90] Sudo T, Asao N, Gevorgyan V, et al. Lewis acid catalyzed highly regio- and stereocontrolled trans-hydrosilylation of alkynes and allenes. J Org Chem, 1999, 64(7), 2494 doi: 10.1021/jo9824293[91] Buriak J M, Allen M J. Lewis acid mediated functionalization of porous silicon with substituted alkenes and alkynes. J Am Chem Soc, 1998, 120(6), 1339 doi: 10.1021/ja9740125[92] Purkait T K, Iqbal M, Wahl M H, et al. Borane-catalyzed room-temperature hydrosilylation of alkenes/alkynes on silicon nanocrystal surfaces. J Am Chem Soc, 2014, 136(52), 17914 doi: 10.1021/ja510120e[93] Helbich T, Lyuleeva A, Marx P, et al. Lewis acid induced functionalization of photoluminescent 2D silicon nanosheets for the fabrication of functional hybrid films. Adv Funct Mater, 2017, 27(21), 1606764 doi: 10.1002/adfm.201606764[94] Sugiyama Y, Okamoto H, Mitsuoka T, et al. Synthesis and optical properties of monolayer organosilicon nanosheets. J Am Chem Soc, 2010, 132(17), 5946 doi: 10.1021/ja100919d[95] Ohashi M, Shirai S, Nakano H. Direct chemical synthesis of benzyl-modified silicane from calcium disilicide. Chem Mater, 2019, 31(13), 4720 doi: 10.1021/acs.chemmater.9b00715[96] Kumai Y, Kadoura H, Sudo E, et al. Si–C composite anode of layered polysilane (Si6H6) and sucrose for lithium ion rechargeable batteries. J Mater Chem, 2011, 21(32), 11941 doi: 10.1039/c1jm10532a[97] Sun L, Wang B, Wang Y. A novel silicon carbide nanosheet for high-performance humidity sensor. Adv Mater Interfaces, 2018, 5(6), 1701300 doi: 10.1002/admi.201701300[98] Lyuleeva A, Helbich T, Bobinger M, et al. Functionalized and oxidized silicon nanosheets: Customized design for enhanced sensitivity towards relative humidity. Sens Actuators B, 2019, 283, 451 doi: 10.1016/j.snb.2018.11.049[99] Ryu J, Jang Y J, Choi S, et al. All-in-one synthesis of mesoporous silicon nanosheets from natural clay and their applicability to hydrogen evolution. NPG Asia Mater, 2016, 8(3), e248 doi: 10.1038/am.2016.35[100] Wang S, Wang C, Pan W, et al. Two-dimensional silicon for (photo)catalysis. Sol RRL, 2020, 2000392 doi: 10.1002/solr.202000392[101] Liu F, Liu C C, Wu K, et al. d+id′ chiral superconductivity in bilayer silicene. Phys Rev Lett, 2013, 111(6), 066804 doi: 10.1103/PhysRevLett.111.066804[102] Ezawa M. Valley-polarized metals and quantum anomalous Hall effect in silicene. Phys Rev Lett, 2012, 109(5), 055502 doi: 10.1103/PhysRevLett.109.055502[103] Li X, Mullen J T, Jin Z, et al. Intrinsic electrical transport properties of monolayer silicene and MoS2 from first principles. Phys Rev B, 2013, 87(11), 115418 doi: 10.1103/PhysRevB.87.115418[104] Ding Y, Wang Y. Electronic structures of silicene fluoride and hydride. Appl Phys Lett, 2012, 100(8), 083102 doi: 10.1063/1.3688035[105] Wang R, Pi X, Ni Z, et al. Density functional theory study on organically surface-modified silicene. RSC Adv, 2015, 5(43), 33831 doi: 10.1039/C5RA05751E[106] Ni Z, Liu Q, Tang K, et al. Tunable bandgap in silicene and germanene. Nano Lett, 2012, 12(1), 113 doi: 10.1021/nl203065e[107] Nakano H, Tanaka Y, Yamamoto K, et al. Silicanes modified by conjugated substituents for optoelectronic devices. Adv Opt Mater, 2019, 7(18), 1900696 doi: 10.1002/adom.201900696[108] Dasog M, Kehrle J, Rieger B, et al. Silicon nanocrystals and silicon-polymer hybrids: Synthesis, surface engineering, and applications. Angew Chem Int Ed, 2016, 55(7), 2322 doi: 10.1002/anie.201506065[109] Karar D, Bandyopadhyay N R, Pramanick A K, et al. Quasi-two-dimensional luminescent silicon nanosheets. J Phys Chem C, 2018, 122(33), 18912 doi: 10.1021/acs.jpcc.8b03988[110] Ezawa M. Quasi-topological insulator and trigonal warping in gated bilayer silicene. J Phys Soc Jpn, 2012, 81(10), 104713 doi: 10.1143/JPSJ.81.104713[111] Huang B, Deng H X, Lee H, et al. Exceptional optoelectronic properties of hydrogenated bilayer silicene. Phys Rev X, 2014, 4(2), 021029 doi: 10.1103/PhysRevX.4.021029[112] Hussain T, Chakraborty S, Ahuja R. Metal-functionalized silicene for efficient hydrogen storage. ChemPhysChem, 2013, 14(15), 3463 doi: 10.1002/cphc.201300548[113] Pi X, Ni Z, Liu Y, et al. Density functional theory study on boron- and phosphorus-doped hydrogen-passivated silicene. Phys Chem Chem Phys, 2015, 17(6), 4146 doi: 10.1039/C4CP05196C -
Proportional views





 DownLoad:
DownLoad: