| Citation: |
Yingqi Liang, Guobin Mao, Junbiao Dai, Yingxin Ma. Biofunctionalized semiconductor quantum dots for virus detection[J]. Journal of Semiconductors, 2023, 44(2): 023101. doi: 10.1088/1674-4926/44/2/023101
****
Y Q Liang, G B Mao, J B Dai, Y X Ma. Biofunctionalized semiconductor quantum dots for virus detection[J]. J. Semicond, 2023, 44(2): 023101. doi: 10.1088/1674-4926/44/2/023101
|
Biofunctionalized semiconductor quantum dots for virus detection
DOI: 10.1088/1674-4926/44/2/023101
More Information
-
Abstract
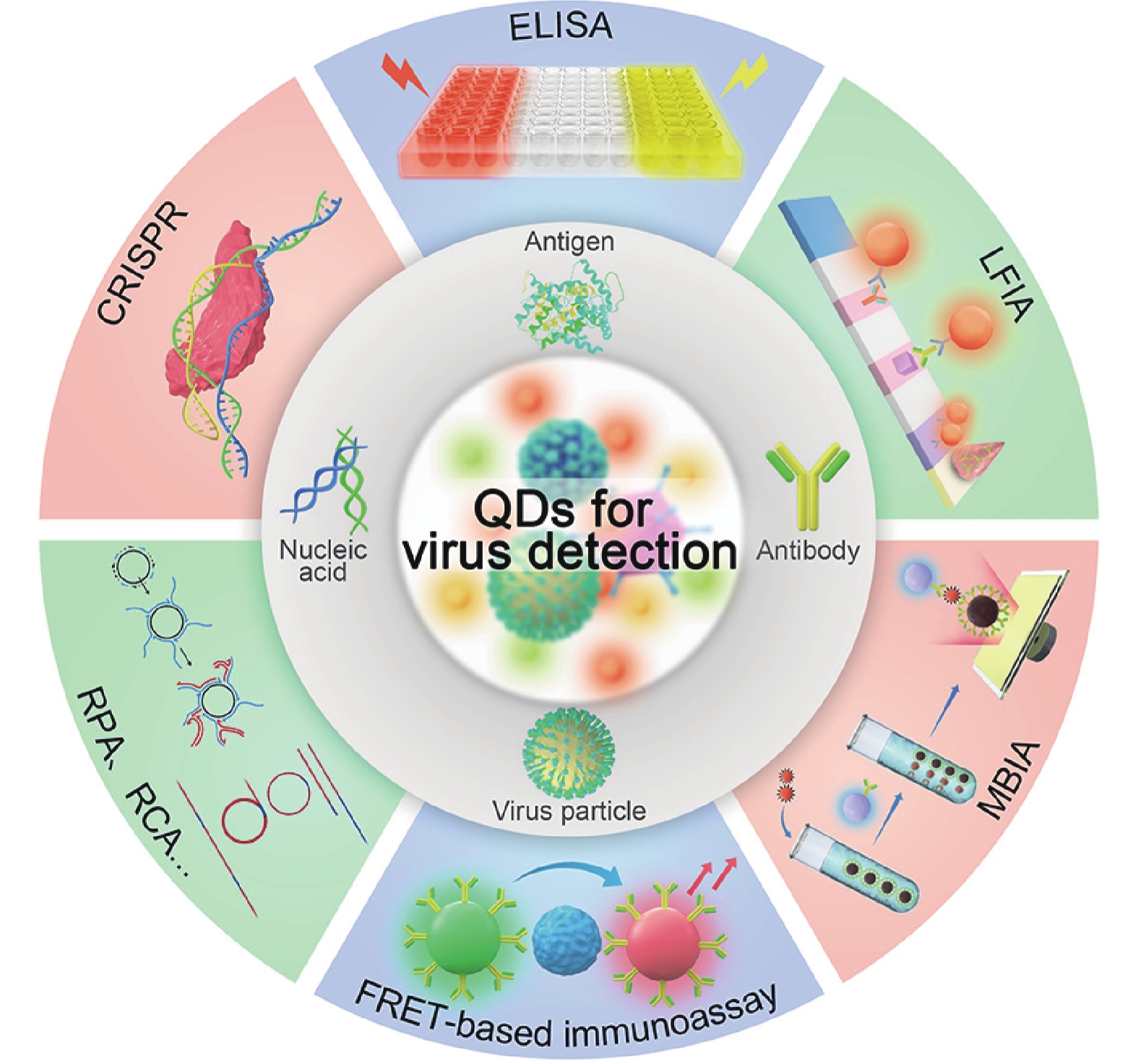
Virus is a kind of microorganism and possesses simple structure and contains one nucleic acid, which must be replicated using the host cell system. It causes large-scale infectious diseases and poses serious threats to the health, social well-being, and economic conditions of millions of people worldwide. Therefore, there is an urgent need to develop novel strategies for accurate diagnosis of virus infection to prevent disease transmission. Quantum dots (QDs) are typical fluorescence nanomaterials with high quantum yield, broad absorbance range, narrow and size-dependent emission, and good stability. QDs-based nanotechnology has been found to be effective method with rapid response, easy operation, high sensitivity, and good specificity, and has been widely applied for the detection of different viruses. However, until now, no systematic and critical review has been published on this important research area. Hence, in this review, we aim to provide a comprehensive coverage of various QDs-based virus detection methods. The fundamental investigations have been reviewed, including information related to the synthesis and biofunctionalization of QDs, QDs-based viral nucleic acid detection strategies, and QDs-based immunoassays. The challenges and perspectives regarding the potential application of QDs for virus detection is also discussed. -
References
[1] Kim Y A, Przytycka T M. The language of a virus. Science, 2021, 371, 233 doi: 10.1126/science.abf6894[2] Mukherjee S, et al. Before virus, after virus: A reckoning. Cell, 2020, 183, 308 doi: 10.1016/j.cell.2020.09.042[3] Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med, 2005, 11, 982 doi: 10.1038/nm1286[4] Kukura P, Ewers H, Müller C, et al. High-speed nanoscopic tracking of the position and orientation of a single virus. Nat Methods, 2009, 6, 923 doi: 10.1038/nmeth.1395[5] Xiao M, Tian F, Liu X, et al. Virus detection: From state-of-the-art laboratories to smartphone-based point-of-care testing. Adv Sci, 2022, 9, e2105904 doi: 10.1002/advs.202105904[6] Hassanpour S, et al. Recent trends in rapid detection of influenza infections by bio and nanobiosensor. Trac Trends Anal Chem, 2018, 98, 201 doi: 10.1016/j.trac.2017.11.012[7] Wiersinga W J, Prescott H C. What is COVID-19? JAMA, 2020, 324, 816 doi: 10.1001/jama.2020.12984[8] Matheson N J, Lehner P J. How does SARS-CoV-2 cause COVID-19?. Science, 2020, 369, 510 doi: 10.1126/science.abc6156[9] Deng J Q, Zhao S, Liu Y, et al. Nanosensors for diagnosis of infectious diseases. ACS Appl Bio Mater, 2021, 4, 3863 doi: 10.1021/acsabm.0c01247[10] Abbasi J. Combining rapid PCR and antibody tests improved COVID-19 diagnosis. JAMA, 2020, 324, 1386 doi: 10.1001/jama.2020.19129[11] Deshpande K, Pt U, Kaduskar O, et al. Performance assessment of seven SARS-CoV-2 IgG enzyme-linked immunosorbent assays. J Med Virol, 2021, 93, 6696 doi: 10.1002/jmv.27251[12] Krajewski R, et al. Update on serologic testing in COVID-19. Clin Chimica Acta, 2020, 510, 746 doi: 10.1016/j.cca.2020.09.015[13] Song M L, et al. Pathogenic virus detection by optical nanobiosensors. Cell Rep Phys Sci, 2021, 2, 100288 doi: 10.1016/j.xcrp.2020.100288[14] Abdolhosseini M, et al. A review on colorimetric assays for DNA virus detection. J Virol Methods, 2022, 301, 114461 doi: 10.1016/j.jviromet.2022.114461[15] Nasrollahzadeh M, Sajjadi M, Soufi G J, et al. Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials, 2020, 10, 1072 doi: 10.3390/nano10061072[16] Lou B B, Liu Y F, Shi M L, et al. Aptamer-based biosensors for virus protein detection. Trac Trends Anal Chem, 2022, 157, 116738 doi: 10.1016/j.trac.2022.116738[17] Jelen Ž, Majerič P, Zadravec M, et al. Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests. Nanotechnol Rev, 2021, 10, 1978 doi: 10.1515/ntrev-2021-0120[18] Tian J P, Zhao H M, Liu M, et al. Detection of influenza A virus based on fluorescence resonance energy transfer from quantum dots to carbon nanotubes. Anal Chimica Acta, 2012, 723, 83 doi: 10.1016/j.aca.2012.02.030[19] Wang C W, Wang C G, Wang X L, et al. Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses. ACS Appl Mater Interfaces, 2019, 11, 19495 doi: 10.1021/acsami.9b03920[20] Lee J, Ahmed S R, Oh S, et al. A plasmon-assisted fluoro-immunoassay using gold nanoparticle-decorated carbon nanotubes for monitoring the influenza virus. Biosens Bioelectron, 2015, 64, 311 doi: 10.1016/j.bios.2014.09.021[21] Zhou W D, Coleman J J. Semiconductor quantum dots. Curr Opin Solid State Mater Sci, 2016, 20, 352 doi: 10.1016/j.cossms.2016.06.006[22] García de Arquer F P, Talapin D V, Klimov V I, et al. Semiconductor quantum dots: Technological progress and future challenges. Science, 2021, 373, eaaz8541 doi: 10.1126/science.aaz8541[23] Zhang L J, Xia L, Xie H Y, et al. Quantum dot based biotracking and biodetection. Anal Chem, 2019, 91, 532 doi: 10.1021/acs.analchem.8b04721[24] Lisichkin G V, Olenin A Y. Synthesis of surface-modified quantum dots. Russ Chem Bull, 2020, 69, 1819 doi: 10.1007/s11172-020-2968-3[25] Chang X H, Zhang J, Wu L H, et al. Research progress of near-infrared fluorescence immunoassay. Micromachines, 2019, 10, 422 doi: 10.3390/mi10060422[26] Pastucha M, Farka Z, Lacina K, et al. Magnetic nanoparticles for smart electrochemical immunoassays: A review on recent developments. Mikrochim Acta, 2019, 186, 312 doi: 10.1007/s00604-019-3410-0[27] Stanisavljevic M, Krizkova S, Vaculovicova M, et al. Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens Bioelectron, 2015, 74, 562 doi: 10.1016/j.bios.2015.06.076[28] Zhao Q, Lu D, Zhang G Y, et al. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta, 2021, 223, 121722 doi: 10.1016/j.talanta.2020.121722[29] Wagner A M, Knipe J M, Orive G, et al. Quantum dots in biomedical applications. Acta Biomater, 2019, 94, 44 doi: 10.1016/j.actbio.2019.05.022[30] Zhou J, Yang Y, Zhang C Y. Toward biocompatible semiconductor quantum dots: From biosynthesis and bioconjugation to biomedical application. Chem Rev, 2015, 115, 11669 doi: 10.1021/acs.chemrev.5b00049[31] Gill R, Zayats M, Willner I. Semiconductor quantum dots for bioanalysis. Angew Chem Int Ed, 2008, 47, 7602 doi: 10.1002/anie.200800169[32] Reiss P, Carrière M, Lincheneau C, et al. Synthesis of semiconductor nanocrystals, focusing on nontoxic and earth-abundant materials. Chem Rev, 2016, 116, 10731 doi: 10.1021/acs.chemrev.6b00116[33] Zhao D, He Z K, Chan W H, et al. Synthesis and characterization of high-quality water-soluble near-infrared-emitting CdTe/CdS quantum dots capped by N-acetyl-l-cysteine via hydrothermal method. J Phys Chem C, 2009, 113, 1293 doi: 10.1021/jp808465s[34] Blanco-Canosa J B, Wu M, Susumu K, et al. Recent progress in the bioconjugation of quantum dots. Coord Chem Rev, 2014, 263/264, 101 doi: 10.1016/j.ccr.2013.08.030[35] Salaheldin A M, Walter J, Herre P, et al. Automated synthesis of quantum dot nanocrystals by hot injection: Mixing induced self-focusing. Chem Eng J, 2017, 320, 232 doi: 10.1016/j.cej.2017.02.154[36] Park J Y, Jeong D W, Lim K M, et al. Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu. Appl Surf Sci, 2017, 415, 8 doi: 10.1016/j.apsusc.2017.02.026[37] Wang W T, Kapur A, Ji X, et al. Photoligation of an amphiphilic polymer with mixed coordination provides compact and reactive quantum dots. J Am Chem Soc, 2015, 137, 5438 doi: 10.1021/jacs.5b00671[38] Jiang Z X, Matras-Postolek K, Yang P. Hydrophobic CdSe and CdTe quantum dots: Shell coating, shape control, and self-assembly. RSC Adv, 2016, 6, 25656 doi: 10.1039/C6RA03408J[39] Adegoke O, Seo M W, Kato T, et al. An ultrasensitive SiO2-encapsulated alloyed CdZnSeS quantum dot-molecular beacon nanobiosensor for norovirus. Biosens Bioelectron, 2016, 86, 135 doi: 10.1016/j.bios.2016.06.027[40] Zhan N Q, Palui G, Merkl J P, et al. Bio-orthogonal coupling as a means of quantifying the ligand density on hydrophilic quantum dots. J Am Chem Soc, 2016, 138, 3190 doi: 10.1021/jacs.5b13574[41] Yang P, Ando M, Murase N. Controlled self-assembly of hydrophobic quantum dots through silanization. J Colloid Interface Sci, 2011, 361, 9 doi: 10.1016/j.jcis.2011.05.056[42] He Y, Lu H T, Sai L M, et al. Microwave synthesis of water-dispersed CdTe/CdS/ZnS core-shell-shell quantum dots with excellent photostability and biocompatibility. Adv Mater, 2008, 20, 3416 doi: 10.1002/adma.200701166[43] Gaponik N, Talapin D V, Rogach A L, et al. Thiol-capping of CdTe nanocrystals: An alternative to organometallic synthetic routes. J Phys Chem B, 2002, 106, 7177 doi: 10.1021/jp025541k[44] Mou M Y, Wu Y, Niu Q Q, et al. Aggregation-induced emission properties of hydrothermally synthesized Cu-In-S quantum dots. Chem Commun, 2017, 53, 3357 doi: 10.1039/C7CC00170C[45] Zhao D, Fang Y, Wang H Y, et al. Synthesis and characterization of high-quality water-soluble CdTe: Zn2+ quantum dots capped by N-acetyl-l-cysteineviahydrothermal method. J Mater Chem, 2011, 21, 13365 doi: 10.1039/c1jm11861g[46] Zhang C L, Yan J, Liu C, et al. One-pot synthesis of DNA-CdTe: Zn2+ nanocrystals using Na2TeO3 as the Te source. ACS Appl Mater Interfaces, 2014, 6, 3189 doi: 10.1021/am405864z[47] Nekolla K, Kick K, Sellner S, et al. Influence of surface modifications on the spatiotemporal microdistribution of quantum dots In vivo. Small, 2016, 12, 2641 doi: 10.1002/smll.201600071[48] Mao G B, Peng W Q, Tian S B, et al. Dual-protein visual detection using ratiometric fluorescent probe based on Rox-DNA functionalized CdZnTeS QDs. Sens Actuat B, 2019, 283, 755 doi: 10.1016/j.snb.2018.12.065[49] Ma Y X, Mao G B, Wu G Q, et al. A novel nano-beacon based on DNA functionalized QDs for intracellular telomerase activity monitoring. Sens Actuat B, 2020, 304, 127385 doi: 10.1016/j.snb.2019.127385[50] Mao G B, Liu C, Du M Y, et al. One-pot synthesis of the stable CdZnTeS quantum dots for the rapid and sensitive detection of copper-activated enzyme. Talanta, 2018, 185, 123 doi: 10.1016/j.talanta.2018.03.054[51] Mao G B, Cai Q, Wang F B, et al. One-step synthesis of rox-DNA functionalized CdZnTeS quantum dots for the visual detection of hydrogen peroxide and blood glucose. Anal Chem, 2017, 89, 11628 doi: 10.1021/acs.analchem.7b03053[52] Mao G B, Du M Y, Wang X X, et al. Simple construction of ratiometric fluorescent probe for the detection of dopamine and tyrosinase by the naked eye. Analyst, 2018, 143, 5295 doi: 10.1039/C8AN01640B[53] Zhan N Q, Palui G, Safi M, et al. Multidentate zwitterionic ligands provide compact and highly biocompatible quantum dots. J Am Chem Soc, 2013, 135, 13786 doi: 10.1021/ja405010v[54] Bhang S H, Won N, Lee T J, et al. Hyaluronic acid-quantum dot conjugates for in vivo lymphatic vessel imaging. ACS Nano, 2009, 3, 1389 doi: 10.1021/nn900138d[55] Jennings T L, Becker-Catania S G, Triulzi R C, et al. Reactive semiconductor nanocrystals for chemoselective biolabeling and multiplexed analysis. ACS Nano, 2011, 5, 5579 doi: 10.1021/nn201050g[56] Wang M, Xie J L, Li J, et al. 3-aminophenyl boronic acid functionalized quantum-dot-based ratiometric fluorescence sensor for the highly sensitive detection of tyrosinase activity. ACS Sens, 2020, 5, 1634 doi: 10.1021/acssensors.0c00122[57] Zhou M, Nakatani E, Gronenberg L S, et al. Peptide-labeled quantum dots for imaging GPCRs in whole cells and as single molecules. Bioconjug Chem, 2007, 18, 323 doi: 10.1021/bc0601929[58] Feng Z C, Ma R N, Du A, et al. Enhanced performance of near-infrared-absorption CdSeTe quantum dot-sensitized solar cells via octa-aminopropyl polyhedral oligomeric silsesquioxane modification. Nano, 2019, 14, 1950087 doi: 10.1142/S1793292019500875[59] Song F Y, Chan W C W. Principles of conjugating quantum dots to proteins via carbodiimide chemistry. Nanotechnology, 2011, 22, 494006 doi: 10.1088/0957-4484/22/49/494006[60] Chi C W, Lao Y H, Li Y S, et al. A quantum dot-aptamer beacon using a DNA intercalating dye as the FRET reporter: Application to label-free thrombin detection. Biosens Bioelectron, 2011, 26, 3346 doi: 10.1016/j.bios.2011.01.015[61] Schieber C, Bestetti A, Lim J P, et al. Conjugation of transferrin to azide-modified CdSe/ZnS core-shell quantum dots using cyclooctyne click chemistry. Angew Chem Int Ed, 2012, 51, 10523 doi: 10.1002/anie.201202876[62] Mao G B, Ma Y X, Wu G Q, et al. Novel method of clickable quantum dot construction for bioorthogonal labeling. Anal Chem, 2021, 93, 777 doi: 10.1021/acs.analchem.0c03078[63] Clapp A R, Medintz I L, Mauro J M, et al. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J Am Chem Soc, 2004, 126, 301 doi: 10.1021/ja037088b[64] Zhang C L, Ji X H, Zhang Y, et al. One-pot synthesized aptamer-functionalized CdTe: Zn2+ quantum dots for tumor-targeted fluorescence imaging in vitro and in vivo. Anal Chem, 2013, 85, 5843 doi: 10.1021/ac400606e[65] Wu D, Song G F, Li Z, et al. A two-dimensional molecular beacon for mRNA-activated intelligent cancer theranostics. Chem Sci, 2015, 6, 3839 doi: 10.1039/C4SC03894K[66] Ma Y X, Mao G B, Huang W R, et al. Quantum dot nanobeacons for single RNA labeling and imaging. J Am Chem Soc, 2019, 141, 13454 doi: 10.1021/jacs.9b04659[67] Shen M Z, et al. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal, 2020, 10, 97 doi: 10.1016/j.jpha.2020.02.010[68] Bustamante-Jaramillo L F, Fingal J, Blondot M L, et al. Imaging of hepatitis B virus nucleic acids: Current advances and challenges. Viruses, 2022, 14, 557 doi: 10.3390/v14030557[69] Castillo-Henríquez L, Brenes-Acuña M, Castro-Rojas A, et al. Biosensors for the detection of bacterial and viral clinical pathogens. Sensors, 2020, 20, 6926 doi: 10.3390/s20236926[70] Lesiak A, Drzozga K, Cabaj J, et al. Optical sensors based on II-VI quantum dots. Nanomaterials, 2019, 9, 192 doi: 10.3390/nano9020192[71] Jiang X X, Liu X J, Wu M, et al. Facile off-on fluorescence biosensing of human papillomavirus using DNA probe coupled with sunflower seed shells carbon dots. Microchem J, 2022, 181, 107742 doi: 10.1016/j.microc.2022.107742[72] Shamsipur M, Nasirian V, Mansouri K, et al. A highly sensitive quantum dots-DNA nanobiosensor based on fluorescence resonance energy transfer for rapid detection of nanomolar amounts of human papillomavirus 18. J Pharm Biomed Anal, 2017, 136, 140 doi: 10.1016/j.jpba.2017.01.002[73] Kim J H, Chaudhary S, Ozkan M. Multicolour hybrid nanoprobes of molecular beacon conjugated quantum dots: FRET and gel electrophoresis assisted target DNA detection. Nanotechnology, 2007, 18, 195105 doi: 10.1088/0957-4484/18/19/195105[74] Samanta A, Zhou Y D, Zou S L, et al. Fluorescence quenching of quantum dots by gold nanoparticles: A potential long range spectroscopic ruler. Nano Lett, 2014, 14, 5052 doi: 10.1021/nl501709s[75] Adegoke O, Morita M, Kato T, et al. Localized surface plasmon resonance-mediated fluorescence signals in plasmonic nanoparticle-quantum dot hybrids for ultrasensitive Zika virus RNA detection via hairpin hybridization assays. Biosens Bioelectron, 2017, 94, 513 doi: 10.1016/j.bios.2017.03.046[76] Dove A. Technology Feature| PCR: Thirty-five years and counting. Science, 2018, 360, 673 doi: 10.1126/science.360.6389.673-[77] Wang Y X, Chen H, Wei H J, et al. Tetra-primer ARMS-PCR combined with dual-color fluorescent lateral flow assay for the discrimination of SARS-CoV-2 and its mutations with a handheld wireless reader. Lab Chip, 2022, 22, 1531 doi: 10.1039/D1LC01167G[78] Kumar P, Pandya D, Singh N, et al. Loop-mediated isothermal amplification assay for rapid and sensitive diagnosis of tuberculosis. J Infect, 2014, 69, 607 doi: 10.1016/j.jinf.2014.08.017[79] Fowler V L, Armson D, Gonzales J L, et al. A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2 infection. J Infect, 2021, 82, 117 doi: 10.1016/j.jinf.2020.10.039[80] Wang S Y, Qin A L, Chau L Y, et al. Amine-functionalized quantum dots as a universal fluorescent nanoprobe for a one-step loop-mediated isothermal amplification assay with single-copy sensitivity. ACS Appl Mater Interfaces, 2022, 14, 35299 doi: 10.1021/acsami.2c02508[81] Dai J Y, He H F, Duan Z J, et al. Self-replicating catalyzed hairpin assembly for rapid signal amplification. Anal Chem, 2017, 89, 11971 doi: 10.1021/acs.analchem.7b01946[82] Li Y F, Li J W, Cao Y, et al. A visual method for determination of hepatitis C virus RNAs based on a 3D nanocomposite prepared from graphene quantum dots. Anal Chimica Acta, 2022, 1203, 339693 doi: 10.1016/j.aca.2022.339693[83] Zhou J, Wang Q X, Zhang C Y. Liposome-quantum dot complexes enable multiplexed detection of attomolar DNAs without target amplification. J Am Chem Soc, 2013, 135, 2056 doi: 10.1021/ja3110329[84] Cui H Y, Song W Q, Cao Z J, et al. Simultaneous and sensitive detection of dual DNA targets via quantum dot-assembled amplification labels. Luminescence, 2016, 31, 281 doi: 10.1002/bio.2959[85] Wang J J, Liu Y, Ding Z, et al. The exploration of quantum dot-molecular beacon based MoS2 fluorescence probing for myeloma-related Mirnas detection. Bioact Mater, 2022, 17, 360 doi: 10.1016/j.bioactmat.2021.12.036[86] Chen J S, Ma E B, Harrington L B, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science, 2018, 360, 436 doi: 10.1126/science.aar6245[87] Zhou W H, Hu L, Ying L M, et al. A CRISPR–Cas9-triggered strand displacement amplification method for ultrasensitive DNA detection. Nat Commun, 2018, 9, 5012 doi: 10.1038/s41467-018-07324-5[88] Wang J J, Zheng C S, Jiang Y Z, et al. One-step monitoring of multiple enterovirus 71 infection-related microRNAs using core-satellite structure of magnetic nanobeads and multicolor quantum dots. Anal Chem, 2020, 92, 830 doi: 10.1021/acs.analchem.9b03317[89] Kocak D D, Gersbach C A. From CRISPR scissors to virus sensors. Nature, 2018, 557, 168 doi: 10.1038/d41586-018-04975-8[90] Bao M D, Jensen E, Chang Y, et al. Magnetic bead-quantum dot (MB-qdot) clustered regularly interspaced short palindromic repeat assay for simple viral DNA detection. ACS Appl Mater Interfaces, 2020, 12, 43435 doi: 10.1021/acsami.0c12482[91] Zhang Q, Li J H, Li Y, et al. SARS-CoV-2 detection using quantum dot fluorescence immunochromatography combined with isothermal amplification and CRISPR/Cas13a. Biosens Bioelectron, 2022, 202, 113978 doi: 10.1016/j.bios.2022.113978[92] Gao L, Yang Q F, Wu P, et al. Recent advances in nanomaterial-enhanced enzyme-linked immunosorbent assays. Analyst, 2020, 145, 4069 doi: 10.1039/D0AN00597E[93] Liang Y, Huang X L, Yu R J, et al. Fluorescence ELISA for sensitive detection of ochratoxin A based on glucose oxidase-mediated fluorescence quenching of CdTe QDs. Anal Chimica Acta, 2016, 936, 195 doi: 10.1016/j.aca.2016.06.018[94] Wu Y Q, Zeng L F, Xiong Y, et al. Fluorescence ELISA based on glucose oxidase-mediated fluorescence quenching of quantum dots for highly sensitive detection of Hepatitis B. Talanta, 2018, 181, 258 doi: 10.1016/j.talanta.2018.01.026[95] Zhou J J, Ren M S, Wang W J, et al. Pomegranate-inspired silica nanotags enable sensitive dual-modal detection of rabies virus nucleoprotein. Anal Chem, 2020, 92, 8802 doi: 10.1021/acs.analchem.0c00200[96] Zhao W W, Han Y M, Zhu Y C, et al. DNA labeling generates a unique amplification probe for sensitive photoelectrochemical immunoassay of HIV-1 p24 antigen. Anal Chem, 2015, 87, 5496 doi: 10.1021/acs.analchem.5b01360[97] Jo A, Kim T H, Kim D M, et al. Sensitive detection of virus with broad dynamic range based on highly bright quantum dot-embedded nanoprobe and magnetic beads. J Ind Eng Chem, 2020, 90, 319 doi: 10.1016/j.jiec.2020.07.030[98] Nasrin F, Chowdhury A D, Takemura K, et al. Single-step detection of norovirus tuning localized surface plasmon resonance-induced optical signal between gold nanoparticles and quantum dots. Biosens Bioelectron, 2018, 122, 16 doi: 10.1016/j.bios.2018.09.024[99] Byrnes S A, Huynh T, Chang T C, et al. Wash-free, digital immunoassay in polydisperse droplets. Anal Chem, 2020, 92, 3535 doi: 10.1021/acs.analchem.9b02526[100] Wu Z, Zeng T, Guo W J, et al. Digital single virus immunoassay for ultrasensitive multiplex avian influenza virus detection based on fluorescent magnetic multifunctional nanospheres. ACS Appl Mater Interfaces, 2019, 11, 5762 doi: 10.1021/acsami.8b18898[101] Soleimani R, Deckers C, Huang T D, et al. Rapid COVID-19 antigenic tests: Usefulness of a modified method for diagnosis. J Med Virol, 2021, 93, 5655 doi: 10.1002/jmv.27094[102] Wang C W, Yang X S, Zheng S, et al. Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza A virus. Sens Actuat B, 2021, 345, 130372 doi: 10.1016/j.snb.2021.130372[103] Han H, Wang C W, Yang X S, et al. Rapid field determination of SARS-CoV-2 by a colorimetric and fluorescent dual-functional lateral flow immunoassay biosensor. Sens Actuat B, 2022, 351, 130897 doi: 10.1016/j.snb.2021.130897[104] Chen L, Zhang X W, Zhang C L, et al. Dual-color fluorescence and homogeneous immunoassay for the determination of human enterovirus 71. Anal Chem, 2011, 83, 7316 doi: 10.1021/ac201129d[105] Chen L, Zhang X W, Zhou G H, et al. Simultaneous determination of human Enterovirus 71 and Coxsackievirus B3 by dual-color quantum dots and homogeneous immunoassay. Anal Chem, 2012, 84, 3200 doi: 10.1021/ac203172x[106] Zhang X Y, Zhou Q, Shen Z F, et al. Quantum dot incorporated Bacillus spore as nanosensor for viral infection. Biosens Bioelectron, 2015, 74, 575 doi: 10.1016/j.bios.2015.07.011[107] Yao Z, Drecun L, Aboualizadeh F, et al. A homogeneous split-luciferase assay for rapid and sensitive detection of anti-SARS CoV-2 antibodies. Nat Commun, 2021, 12, 1806 doi: 10.1038/s41467-021-22102-6[108] Tang Y N, Song T R, Gao L, et al. A CRISPR-based ultrasensitive assay detects attomolar concentrations of SARS-CoV-2 antibodies in clinical samples. Nat Commun, 2022, 13, 4667 doi: 10.1038/s41467-022-32371-4 -
Proportional views





 DownLoad:
DownLoad:











 Yingqi Liang:got her BS degree from University of Science and Technology Beijing in 2019 and got her MS from Beijing University of Technology in 2022. Now she is a research assistant at Shenzhen Institute of Advanced Technology of Prof. Yingxin Ma. Her research focuses on development of virus detection technology in vitro
Yingqi Liang:got her BS degree from University of Science and Technology Beijing in 2019 and got her MS from Beijing University of Technology in 2022. Now she is a research assistant at Shenzhen Institute of Advanced Technology of Prof. Yingxin Ma. Her research focuses on development of virus detection technology in vitro Guobin Mao:is an associate professor of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. He received his Ph.D. degree in analytical chemistry from Wuhan University in 2019. He was a post-doctoral fellow at Shenzhen Institutes of Advanced Sciences, Chinese Academy of Sciences from 2019 to 2021. His research interests are focused on nano-biosensors, in vitro diagnosis, labeling and imaging of virus
Guobin Mao:is an associate professor of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. He received his Ph.D. degree in analytical chemistry from Wuhan University in 2019. He was a post-doctoral fellow at Shenzhen Institutes of Advanced Sciences, Chinese Academy of Sciences from 2019 to 2021. His research interests are focused on nano-biosensors, in vitro diagnosis, labeling and imaging of virus Junbiao Dai:is currently the deputy director of Institute of Synthetic Biology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. He received his Master of Science in Biology from Tsinghua University and Ph.D. in Molecular, Cellular and Developmental Biology from Iowa State University. He was a post-doctoral fellow at the Johns Hopkins University School of Medicine. His research interests lie in synthetic biology using different model organisms, focusing on development of new technologies for genes synthesis, assembly and synthetic genomics
Junbiao Dai:is currently the deputy director of Institute of Synthetic Biology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. He received his Master of Science in Biology from Tsinghua University and Ph.D. in Molecular, Cellular and Developmental Biology from Iowa State University. He was a post-doctoral fellow at the Johns Hopkins University School of Medicine. His research interests lie in synthetic biology using different model organisms, focusing on development of new technologies for genes synthesis, assembly and synthetic genomics Yingxin Ma:received her Ph.D. degree in Biochemistry from Beijing University of Chemical Technology in 2016. She is currently a professor in Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. Her research interests are focused on fluorescence nanosensors, diagnosis and treatment of tumor, labeling and imaging of virus
Yingxin Ma:received her Ph.D. degree in Biochemistry from Beijing University of Chemical Technology in 2016. She is currently a professor in Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. Her research interests are focused on fluorescence nanosensors, diagnosis and treatment of tumor, labeling and imaging of virus



