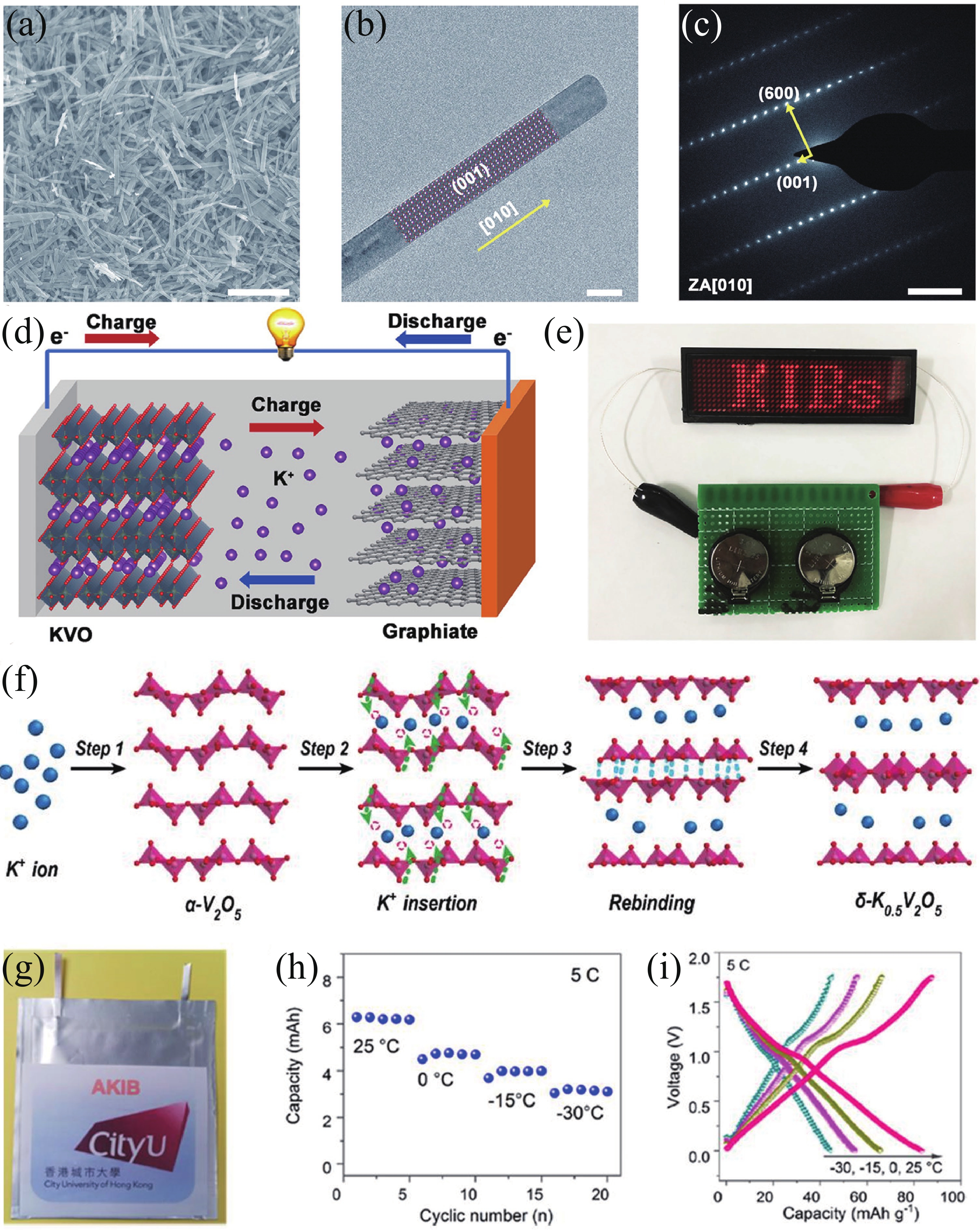
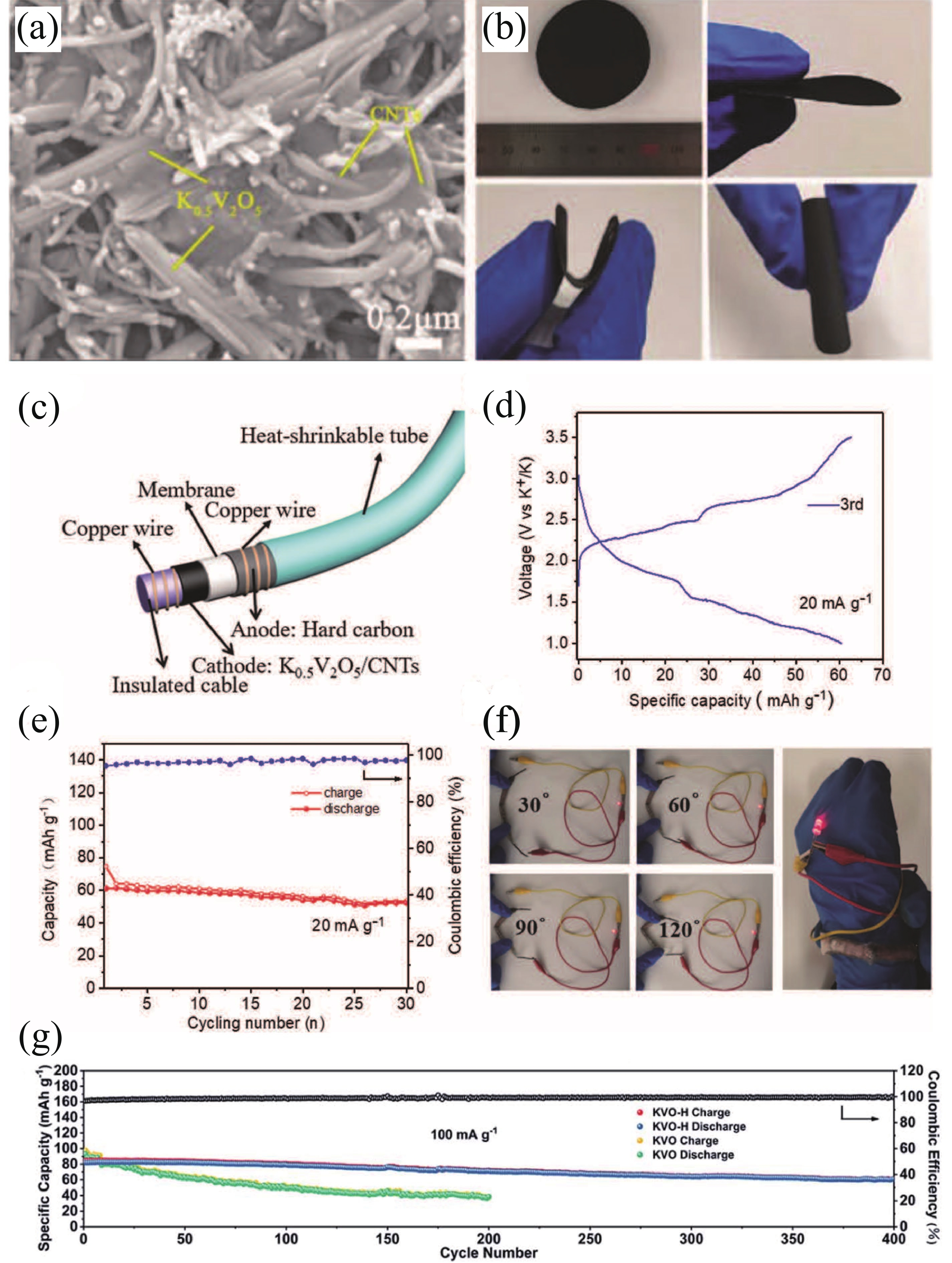
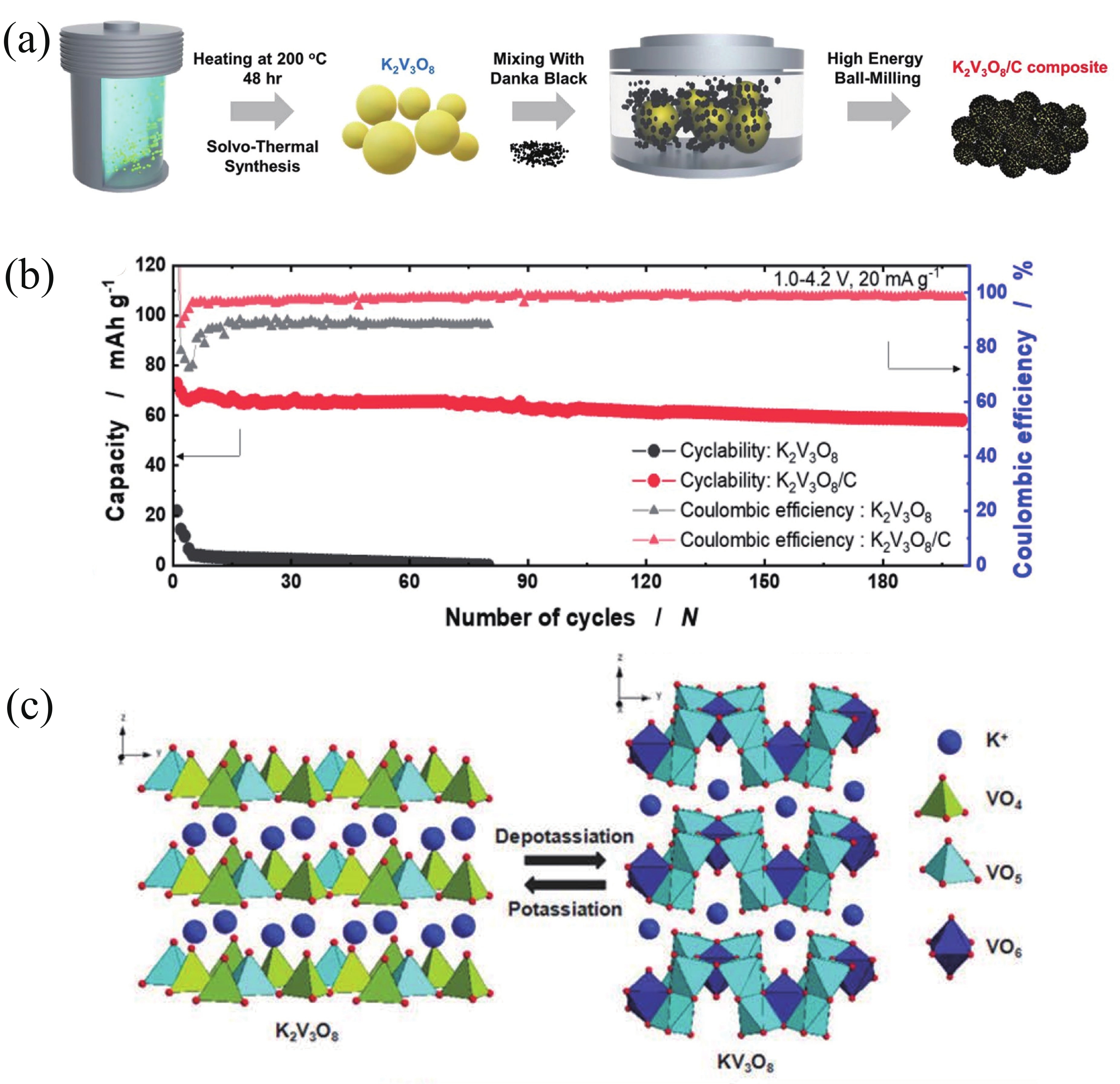
| Citation: |
Yuhan Wu, Guangbo Chen, Xiaonan Wu, Lin Li, Jinyu Yue, Yinyan Guan, Juan Hou, Fanian Shi, Jiyan Liang. Research progress on vanadium oxides for potassium-ion batteries[J]. Journal of Semiconductors, 2023, 44(4): 041701. doi: 10.1088/1674-4926/44/4/041701
Y H Wu, G B Chen, X N Wu, L Li, J Y Yue, Y Y Guan, J Hou, F N Shi, J Y Liang. Research progress on vanadium oxides for potassium-ion batteries[J]. J. Semicond, 2023, 44(4): 041701. doi: 10.1088/1674-4926/44/4/041701
Export: BibTex EndNote
|
Research progress on vanadium oxides for potassium-ion batteries
doi: 10.1088/1674-4926/44/4/041701
More Information-
Abstract
Potassium-ion batteries (PIBs) have been considered as promising candidates in the post-lithium-ion battery era. Till now, a large number of materials have been used as electrode materials for PIBs, among which vanadium oxides exhibit great potentiality. Vanadium oxides can provide multiple electron transfers during electrochemical reactions because vanadium possesses a variety of oxidation states. Meanwhile, their relatively low cost and superior material, structural, and physicochemical properties endow them with strong competitiveness. Although some inspiring research results have been achieved, many issues and challenges remain to be further addressed. Herein, we systematically summarize the research progress of vanadium oxides for PIBs. Then, feasible improvement strategies for the material properties and electrochemical performance are introduced. Finally, the existing challenges and perspectives are discussed with a view to promoting the development of vanadium oxides and accelerating their practical applications. -
References
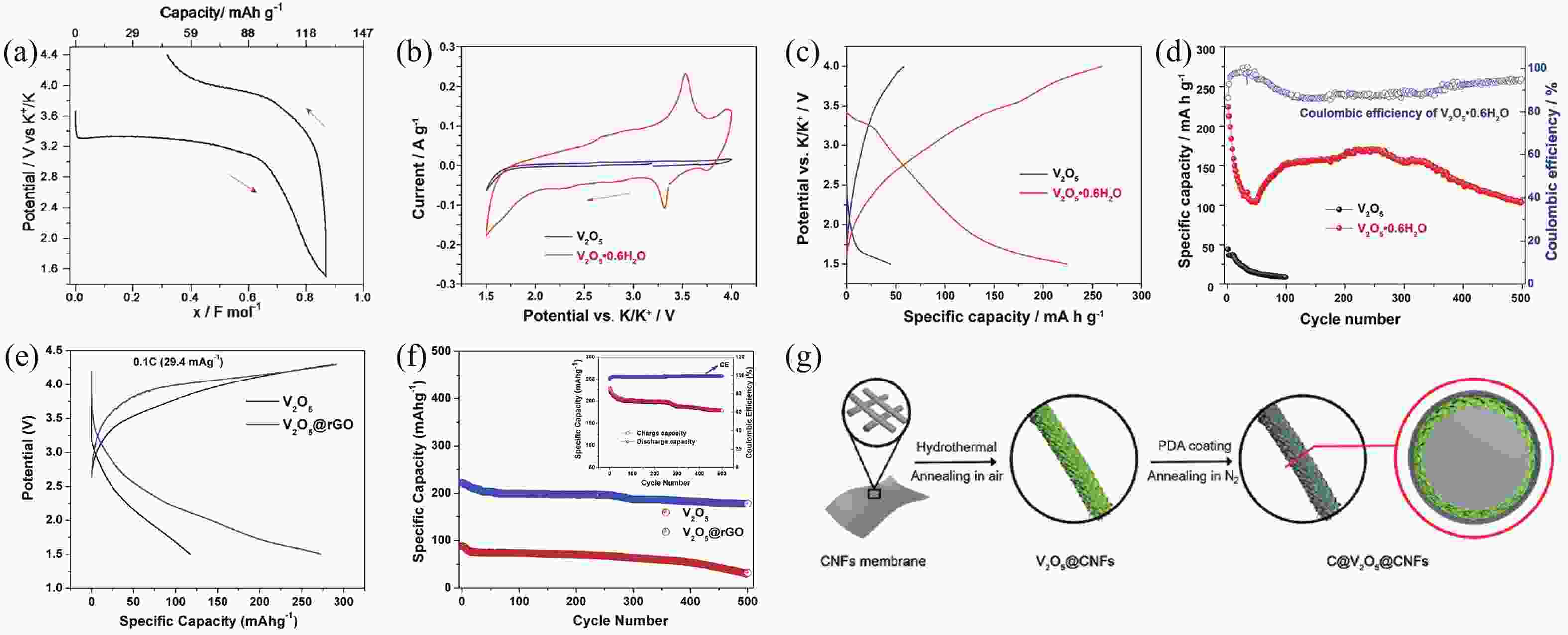
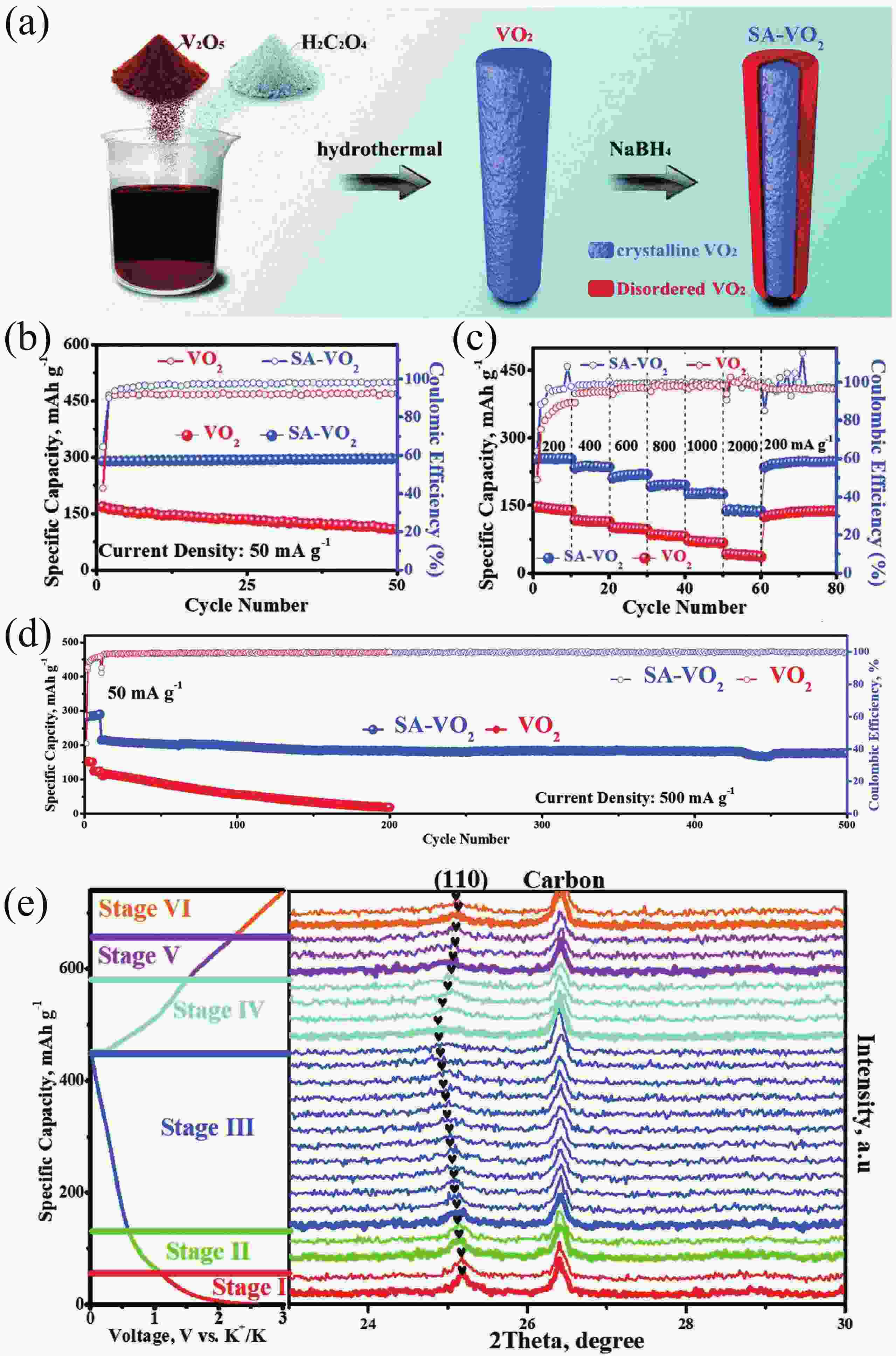
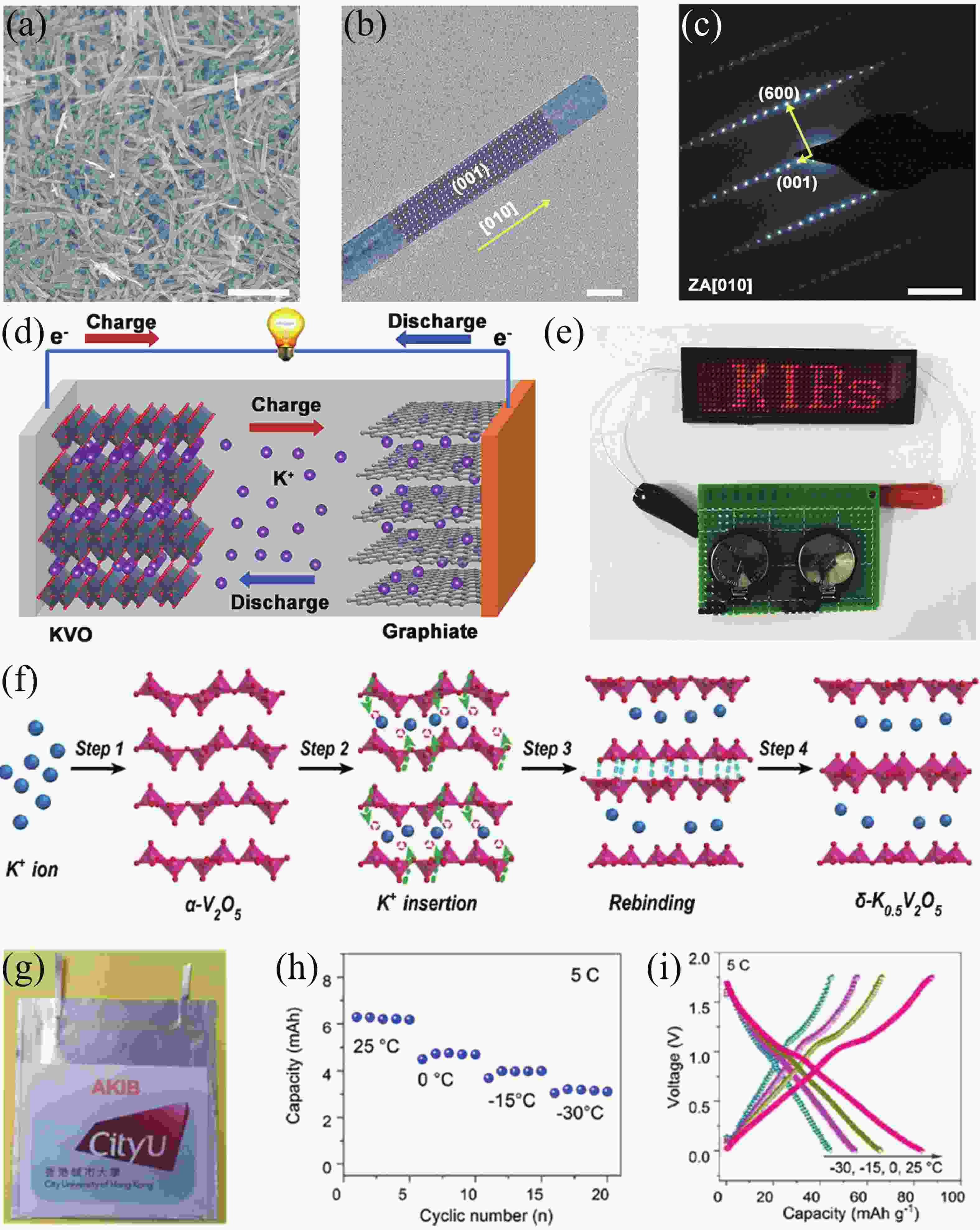
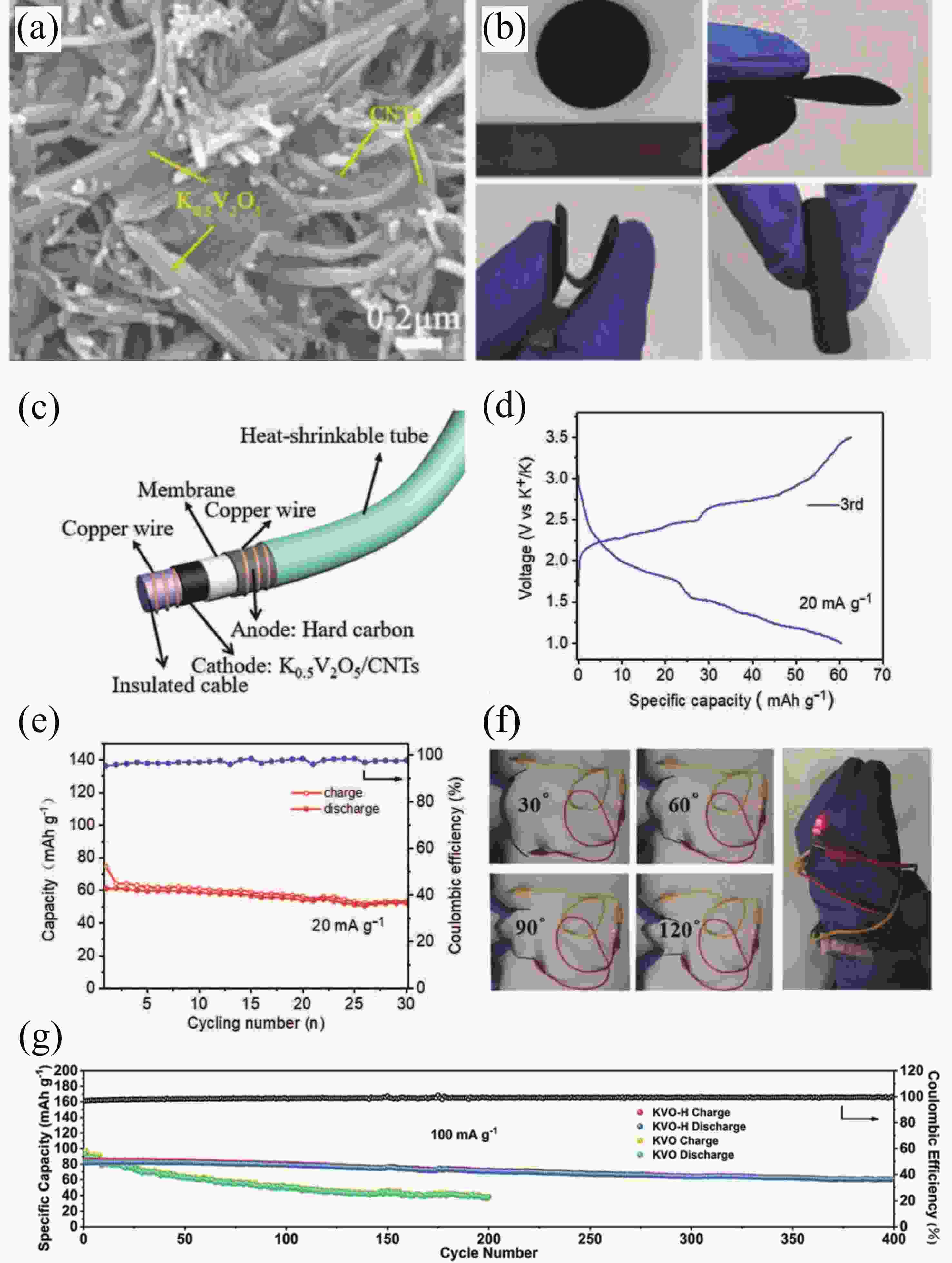
[1] Zhou M, Xu Y, Lei Y. Heterogeneous nanostructure array for electrochemical energy conversion and storage. Nano Today, 2018, 20, 33 doi: 10.1016/j.nantod.2018.04.002[2] Xu R, Du L, Adekoya D, et al. Well-defined nanostructures for electrochemical energy conversion and storage. Adv Energy Mater, 2020, 11, 2001537 doi: 10.1002/aenm.202001537[3] Xu Y, Zhou M, Lei Y. Nanoarchitectured array electrodes for rechargeable lithium- and sodium-ion batteries. Adv Energy Mater, 2016, 6, 1502514 doi: 10.1002/aenm.201502514[4] Wu J, Hong Y and Wang B. The applications of carbon nanomaterials in fiber-shaped energy storage devices. J Semicond, 2018, 39, 011004 doi: 10.1088/1674-4926/39/1/011004[5] Guo Y, Zhang Y, Lu H. Manganese-based materials as cathode for rechargeable aqueous zinc-ion batteries. Battery Energy, 2022, 1, 20210014 doi: 10.1002/bte2.20210014[6] Zhu Y, Xiao Y, Dou S, et al. Spinel/Post-spinel engineering on layered oxide cathodes for sodium-ion batteries. eScience, 2021, 1, 13 doi: 10.1016/j.esci.2021.10.003[7] Hang W, Qi Y, Nan Z, et al. Roadmap of amorphous metal-organic framework for electrochemical energy conversion and storage. Nano Res, 2022 doi: 10.1007/s12274-022-5114-8[8] Zhu H, Sha M, Zhao H, et al. Highly-rough surface carbon nanofibers film as an effective interlayer for lithium–sulfur batteries. J Semicond, 2020, 41, 092701 doi: 10.1088/1674-4926/41/9/092701[9] Nasori N, Dai T, Jia X, et al. Realizing super-long Cu2O nanowires arrays for high-efficient water splitting applications with a convenient approach. J Semicond, 2019, 40, 052701 doi: 10.1088/1674-4926/40/5/052701[10] Liu J, Wang Z, Lei Y. A close step towards industrialized application of solar water splitting. J Semicond, 2020, 41, 090401 doi: 10.1088/1674-4926/41/9/090401[11] Zhao C, Wang Z, Shu D, et al. Preface to the special issue on challenges and possibilities of energy storage. J Semicond, 2020, 41, 090101 doi: 10.1088/1674-4926/42/9/090101[12] Yang S, Zhang F, Ding H, et al. Lithium metal extraction from seawater. Joule, 2018, 2, 1648 doi: 10.1016/j.joule.2018.07.006[13] Wu Y, Zhang C, Zhao H, et al. Recent advances in ferromagnetic metal sulfides and selenides as anodes for sodium- and potassium-ion batteries. J Mater Chem A, 2021, 9, 9506 doi: 10.1039/D1TA00831E[14] Sigma-Aldrich. https://www.sigmaaldrich.com[15] Xu Y S, Duan S Y, Sun Y G, et al. Recent developments in electrode materials for potassium-ion batteries. J Mater Chem A, 2019, 7, 4334 doi: 10.1039/C8TA10953B[16] Hosaka T, Kubota K, Hameed A S, et al. Research development on K-ion batteries. Chem Rev, 2020, 120, 6358 doi: 10.1021/acs.chemrev.9b00463[17] Kim H, Kim J C, Bianchini M, et al. Recent progress and perspective in electrode materials for K-ion batteries. Adv Energy Mater, 2017, 8, 1702384 doi: 10.1002/aenm.201702384[18] Pramudita J C, Sehrawat D, Goonetilleke D, et al. An initial review of the status of electrode materials for potassium-ion batteries. Adv Energy Mater, 2017, 7, 1602911 doi: 10.1002/aenm.201602911[19] Kubota K, Dahbi M, Hosaka T, et al. Towards K-ion and Na-ion batteries as "beyond Li-ion". Chem Rec, 2018, 18, 459 doi: 10.1002/tcr.201700057[20] Yabuuchi N, Kubota K, Dahbi M, et al. Research development on sodium-ion batteries. Chem Rev, 2014, 114, 11636 doi: 10.1021/cr500192f[21] Wu Y, Wu X, Guan Y, et al. Carbon-based flexible electrodes for electrochemical potassium storage devices. New Carbon Mater, 2022, 37, 852 doi: 10.1016/S1872-5805(22)60631-0[22] Wu X, Leonard D P, Ji X. Emerging non-aqueous potassium-ion batteries: Challenges and opportunities. Chem Mater, 2017, 29, 5031 doi: 10.1021/acs.chemmater.7b01764[23] Xu Y, Zhang C, Zhou M, et al. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat Commun, 2018, 9, 1720 doi: 10.1038/s41467-018-04190-z[24] Jian Z, Luo W, Ji X. Carbon electrodes for K-ion batteries. J Am Chem Soc, 2015, 137, 11566 doi: 10.1021/jacs.5b06809[25] Wu Y, Zhang Q, Xu Y, et al. Enhanced potassium storage capability of two-dimensional transition-metal chalcogenides enabled by a collective strategy. ACS Appl Mater Interfaces, 2021, 13, 18838 doi: 10.1021/acsami.1c01891[26] Wu Y, Xu R, Wang Z, et al. Carbon-free crystal-like Fe1– xS as an anode for potassium-ion batteries. ACS Appl Mater Interfaces, 2021, 13, 55218 doi: 10.1021/acsami.1c17799[27] Wu Y, Xu Y, Li Y, et al. Unexpected intercalation-dominated potassium storage in WS2 as a potassium-ion battery anode. Nano Res, 2019, 12, 2997 doi: 10.1007/s12274-019-2543-0[28] Lei K, Wang C, Liu L, et al. A porous network of bismuth used as the anode material for high-energy-density potassium-ion batteries. Angew Chem Int Ed, 2018, 57, 4687 doi: 10.1002/anie.201801389[29] Liu Q, Fan L, Ma R, et al. Super long-life potassium-ion batteries based on an antimony@carbon composite anode. Chem Commun, 2018, 54, 11773 doi: 10.1039/C8CC05257C[30] Liang Y, Luo C, Wang F, et al. An organic anode for high temperature potassium-ion batteries. Adv Energy Mater, 2018, 9, 1802986 doi: 10.1002/aenm.201802986[31] Ma J, Zhou E, Fan C, et al. Endowing CuTCNQ with a new role: a high-capacity cathode for K-ion batteries. Chem Commun, 2018, 54, 5578 doi: 10.1039/C8CC00802G[32] Chen M, Liu Q, Hu Z, et al. Designing advanced vanadium‐based materials to achieve electrochemically active multielectron reactions in sodium/potassium-ion batteries. Adv Energy Mater, 2020, 10, 2002244 doi: 10.1002/aenm.202002244[33] Wang Q, Xu J, Zhang W, et al. Research progress on vanadium-based cathode materials for sodium ion batteries. J Mater Chem A, 2018, 6, 8815 doi: 10.1039/C8TA01627E[34] Esparcia E, Joo J, Lee J. Vanadium oxide bronzes as cathode active materials for non-lithium-based batteries. CrystEngComm, 2021, 23, 5267 doi: 10.1039/D1CE00339A[35] Luo W, Gaumet J J, Mai L. Nanostructured layered vanadium oxide as cathode for high-performance sodium-ion batteries: A perspective. MRS Commun, 2017, 7, 152 doi: 10.1557/mrc.2017.25[36] Koch D, Kulish V V, Manzhos S. A first-principles study of potassium insertion in crystalline vanadium oxide phases as possible potassium-ion battery cathode materials. MRS Commun, 2017, 7, 819 doi: 10.1557/mrc.2017.107[37] Baddour-Hadjean R, Safrany Renard M, Pereira-Ramos J P. Unraveling the structural mechanism of Li insertion in γ′-V2O5 and its effect on cycling properties. Acta Mater, 2019, 165, 183 doi: 10.1016/j.actamat.2018.11.043[38] Safrany Renard M, Emery N, Baddour-Hadjean R, et al. γ'-V2O5: A new high voltage cathode material for sodium-ion battery. Electrochim Acta, 2017, 252, 4 doi: 10.1016/j.electacta.2017.08.175[39] Muller-Bouvet D, Baddour-Hadjean R, Tanabe M, et al. Electrochemically formed α’-NaV2O5: A new sodium intercalation compound. Electrochim Acta, 2015, 176, 586 doi: 10.1016/j.electacta.2015.07.030[40] Baddour-Hadjean R, Safrany Renard M, Emery N, et al. The richness of V2O5 polymorphs as superior cathode materials for sodium insertion. Electrochim Acta, 2018, 270, 129 doi: 10.1016/j.electacta.2018.03.062[41] Bhatia A, Pereira-Ramos J P, Emery N, et al. γ′-V2O5 Polymorph as a promising host structure for potassium storage: an electrochemical and structural study. Chem Mater, 2021, 33, 5276 doi: 10.1021/acs.chemmater.1c01390[42] Tian B, Tang W, Su C, et al. Reticular V2O5·0.6H2O xerogel as cathode for rechargeable potassium ion batteries. ACS Appl Mater Interfaces, 2018, 10, 642 doi: 10.1021/acsami.7b15407[43] Ye F, Lu D, Gui X, et al. Atomic layer deposition of core-shell structured V2O5@CNT sponge as cathode for potassium ion batteries. J Materiomics, 2019, 5, 344 doi: 10.1016/j.jmat.2018.05.009[44] Vishnuprakash P, Nithya C, Premalatha M. Exploration of V2O5 nanorod@rGO heterostructure as potential cathode material for potassium-ion batteries. Electrochim Acta, 2019, 309, 234 doi: 10.1016/j.electacta.2019.04.092[45] Tian Y, An Y, Wei H, et al. Micron-sized nanoporous vanadium pentoxide arrays for high-performance gel zinc-ion batteries and potassium batteries. Chem Mater, 2020, 32, 4054 doi: 10.1021/acs.chemmater.0c00787[46] Jiang X, Tian S, Jiang Q, et al. A V2O5-based freestanding anode with high rate and superior cycle life for potassium storage. Comp Comm, 2022, 32, 101172 doi: 10.1016/j.coco.2022.101172[47] Wei M D, Sugihara H, Honma I, et al. A new metastable phase of crystallized V2O4·0.25H2O nanowires: Synthesis and electrochemical measurements. Adv Mater, 2005, 17, 2964 doi: 10.1002/adma.200501608[48] Xu X, Xiong F, Meng J, et al. Vanadium-based nanomaterials: A promising family for emerging metal-ion batteries. Adv Funct Mater, 2020, 30, 1904398 doi: 10.1002/adfm.201904398[49] Chernova N A, Roppolo M, Dillon A C, et al. Layered vanadium and molybdenum oxides: batteries and electrochromics. J Mater Chem, 2009, 19, 2526 doi: 10.1039/b819629j[50] Wu C, Xie Y. Promising vanadium oxide and hydroxide nanostructures: from energy storage to energy saving. Energy Environ Sci, 2010, 3, 1191 doi: 10.1039/c0ee00026d[51] Li Y, Zhang Q, Yuan Y, et al. Surface amorphization of vanadium dioxide (B) for K-ion battery. Adv Energy Mater, 2020, 10, 2000717 doi: 10.1002/aenm.202000717[52] Jin D, Gao Y, Zhang D, et al. VO2@Carbon foam as a freestanding anode material for potassium-ion batteries: First principles and experimental study. J Alloys Compd, 2020, 845, 156232 doi: 10.1016/j.jallcom.2020.156232[53] Chen F, Wang S, He X D, et al. Hollow sphere structured V2O3@C as an anode material for high capacity potassium-ion batteries. J Mater Chem A, 2020, 8, 13261 doi: 10.1039/D0TA01057J[54] Chen J, Wang T, Chen C, et al. Heteroatom doping hollow vanadium oxide/carbon composites as universal anode materials for efficient alkali-metal ion storage. Carbon, 2022, 192, 30 doi: 10.1016/j.carbon.2022.02.022[55] Tong Z, Yang R, Wu S, et al. Defect-engineered vanadium trioxide nanofiber bundle@graphene hybrids for high-performance all-vanadate Na-ion and K-ion full batteries. J Mater Chem A, 2019, 7, 19581 doi: 10.1039/C9TA06538E[56] Zhao Y, Han C, Yang J, et al. Stable alkali metal ion intercalation compounds as optimized metal oxide nanowire cathodes for lithium batteries. Nano Lett, 2015, 15, 2180 doi: 10.1021/acs.nanolett.5b00284[57] Clites M, Hart J L, Taheri M L, et al. Chemically preintercalated bilayered K xV2O5·nH2O nanobelts as a high-performing cathode material for K-ion batteries. ACS Energy Lett, 2018, 3, 562 doi: 10.1021/acsenergylett.7b01278[58] Zhu Y H, Zhang Q, Yang X, et al. Reconstructed orthorhombic V2O5 polyhedra for fast ion diffusion in K-ion batteries. Chem, 2019, 5, 168 doi: 10.1016/j.chempr.2018.10.004[59] Liang G, Gan Z, Wang X, et al. Reconstructing vanadium oxide with anisotropic pathways for a durable and fast aqueous K-ion battery. ACS Nano, 2021, 15, 17717 doi: 10.1021/acsnano.1c05678[60] Zhang Y, Niu X, Tan L, et al. K0.83V2O5: A new layered compound as a stable cathode material for potassium-ion batteries. ACS Appl Mater Interfaces, 2020, 12, 9332 doi: 10.1021/acsami.9b22087[61] Li X, Zhuang C, Xu J, et al. Rational construction of K0.5V2O5 nanobelts/CNTs flexible cathode for multi-functional potassium-ion batteries. Nanoscale, 2021, 13, 8199 doi: 10.1039/D1NR00993A[62] Deng Q, Zhao Z, Wang Y, et al. A stabilized polyacrylonitrile-encapsulated matrix on a nanolayered vanadium-based cathode material facilitating the K-storage performance. ACS Appl Mater Interfaces, 2022, 14, 14243 doi: 10.1021/acsami.2c00548[63] Niu X, Qu J, Hong Y, et al. High-performance layered potassium vanadium oxide for K-ion batteries enabled by reduced long-range structural order. J Mater Chem A, 2021, 9, 13125 doi: 10.1039/D1TA01807H[64] Liu C, Luo S, Huang H, et al. Potassium vanadate K0.23V2O5 as anode materials for lithium-ion and potassium-ion batteries. J Power Sources, 2018, 389, 77 doi: 10.1016/j.jpowsour.2018.04.014[65] Lu M, Wang K F, Ke H D, et al. Potassium vanadate K2V3O8 as a superior anode material for potassium-ion batteries. Mater Lett, 2018, 232, 224 doi: 10.1016/j.matlet.2018.08.126[66] Jo J H, Hwang J Y, Choi J U, et al. Potassium vanadate as a new cathode material for potassium-ion batteries. J Power Sources, 2019, 432, 24 doi: 10.1016/j.jpowsour.2019.05.064[67] Yang Y, Liu Z, Deng L, et al. A non-topotactic redox reaction enabled K2V3O8 as a high voltage cathode material for potassium-ion batteries. Chem Commun, 2019, 55, 14988 doi: 10.1039/C9CC07996C[68] Charles D S, Feygenson M, Page K, et al. Structural water engaged disordered vanadium oxide nanosheets for high capacity aqueous potassium-ion storage. Nat Commun, 2017, 8, 15520 doi: 10.1038/ncomms15520[69] Xu Y, Dong H, Zhou M, et al. Ammonium vanadium bronze as a potassium-ion battery cathode with high rate capability and cyclability. Small Methods, 2019, 3, 1800349 doi: 10.1002/smtd.201800349[70] Rastgoo-Deylami M, Heo J W, Hong S T. High potassium storage capability of H2V3O8 in a non-aqueous electrolyte. ChemistrySelect, 2019, 4, 11711 doi: 10.1002/slct.201900618[71] Lu J, Wang C, Xia G, et al. A robust spring-like lamellar VO/C nanostructure for high-rate and long-life potassium-ion batteries. J Mater Chem A, 2020, 8, 23939 doi: 10.1039/D0TA08845E[72] Liu T, Li L, Yao T, et al. Integrating amorphous vanadium oxide into carbon nanofibers via electrospinning as high-performance anodes for alkaline ion (Li+/Na+/K+) batteries. Electrochim Acta, 2021, 369, 137711 doi: 10.1016/j.electacta.2020.137711[73] Kuai X, Li K, Chen J, et al. Interfacial engineered vanadium oxide nanoheterostructures synchronizing high-energy and long-term potassium-ion storage. ACS Nano, 2022, 16, 1502 doi: 10.1021/acsnano.1c09935[74] Ouyang D, Wang C, Yang L, et al. Enhancing potassium storage performance in VO2/V2O3@C nanosheets by synergistic effect of oxygen vacancy and C-O-V bond. ChemElectroChem, 2022, 9, 202200639[75] Ren X, Ai D, Zhan C, et al. NaCl-template-assisted freeze-drying synthesis of 3D porous carbon-encapsulated V2O3 for lithium-ion battery anode. Electrochim Acta, 2019, 318, 730 doi: 10.1016/j.electacta.2019.06.138[76] Yang G, Wu Y, Fu Q, et al. Nanostructured metal selenides as anodes for potassium-ion batteries. Sustain Energy Fuels, 2022, 6, 2087 doi: 10.1039/D2SE00067A[77] Hu Z, Liu Q, Chou S L, et al. Advances and challenges in metal sulfides/selenides for next-generation rechargeable sodium-ion batteries. Adv Mater, 2017, 29, 1700606 doi: 10.1002/adma.201700606[78] Zhu K, Wei S, Shou H, et al. Defect engineering on V2O3 cathode for long-cycling aqueous zinc metal batteries. Nat Common, 2021, 12, 6878 doi: 10.1038/s41467-021-27203-w[79] Ren Q, Qin N, Liu B, et al. An oxygen-deficient vanadium oxide@N-doped carbon heterostructure for sodium-ion batteries: insights into the charge storage mechanism and enhanced reaction kinetics. J Mater Chem A, 2020, 8, 3450 doi: 10.1039/C9TA11965E[80] Hu B, Li L, Xiong X, et al. High-performance of copper-doped vanadium pentoxide porous thin films cathode for lithium-ion batteries. J Solid State Electrochem, 2019, 23, 1315 doi: 10.1007/s10008-019-04220-w[81] Wu F, Wang Y, Ruan P, et al. Fe-doping enabled a stable vanadium oxide cathode with rapid Zn diffusion channel for aqueous zinc-ion batteries. Mater Today Energy, 2021, 21, 100842 doi: 10.1016/j.mtener.2021.100842[82] Ghosh M, Dilwale S, Vijayakumar V, et al. Scalable synthesis of manganese-doped hydrated vanadium oxide as a cathode material for aqueous zinc-metal battery. ACS Appl Mater Interfaces, 2020, 12, 48542 doi: 10.1021/acsami.0c13221[83] Xiong P, Wu Y, Liu Y, et al. Two-dimensional organic–inorganic superlattice-like heterostructures for energy storage applications. Energy Environ Sci, 2020, 13, 4834 doi: 10.1039/D0EE03206A[84] Bin D, Huo W, Yuan Y, et al. Organic-inorganic-induced polymer intercalation into layered composites for aqueous zinc-ion battery. Chem, 2020, 6, 968 doi: 10.1016/j.chempr.2020.02.001[85] Lin H, Li M, Yang X, et al. Nanosheets-assembled CuSe crystal pillar as a stable and high-power anode for sodium-ion and potassium-ion batteries. Adv Energy Mater, 2019, 9, 1900323 doi: 10.1002/aenm.201900323[86] Liu D, Shadike Z, Lin R, et al. Review of recent development of in situ/operando characterization techniques for lithium battery research. Adv Mater, 2019, 31, 1806620 doi: 10.1002/adma.201806620 -
Proportional views





 DownLoad:
DownLoad: