| Citation: |
Bowen Zhong, Xiaokun Qin, Zhexin Li, Yiqiang Zheng, Lingchen Liu, Zheng Lou, Lili Wang. Electropolymerized dopamine-based memristors using threshold switching behaviors for artificial current-activated spiking neurons[J]. Journal of Semiconductors, 2025, 46(2): 022402. doi: 10.1088/1674-4926/24070007
****
B W Zhong, X K Qin, Z X Li, Y Q Zheng, L C Liu, Z Lou, and L L Wang, Electropolymerized dopamine-based memristors using threshold switching behaviors for artificial current-activated spiking neurons[J]. J. Semicond., 2025, 46(2), 022402 doi: 10.1088/1674-4926/24070007
|
Electropolymerized dopamine-based memristors using threshold switching behaviors for artificial current-activated spiking neurons
DOI: 10.1088/1674-4926/24070007
CSTR: 32376.14.1674-4926.24070007
More Information-
Abstract
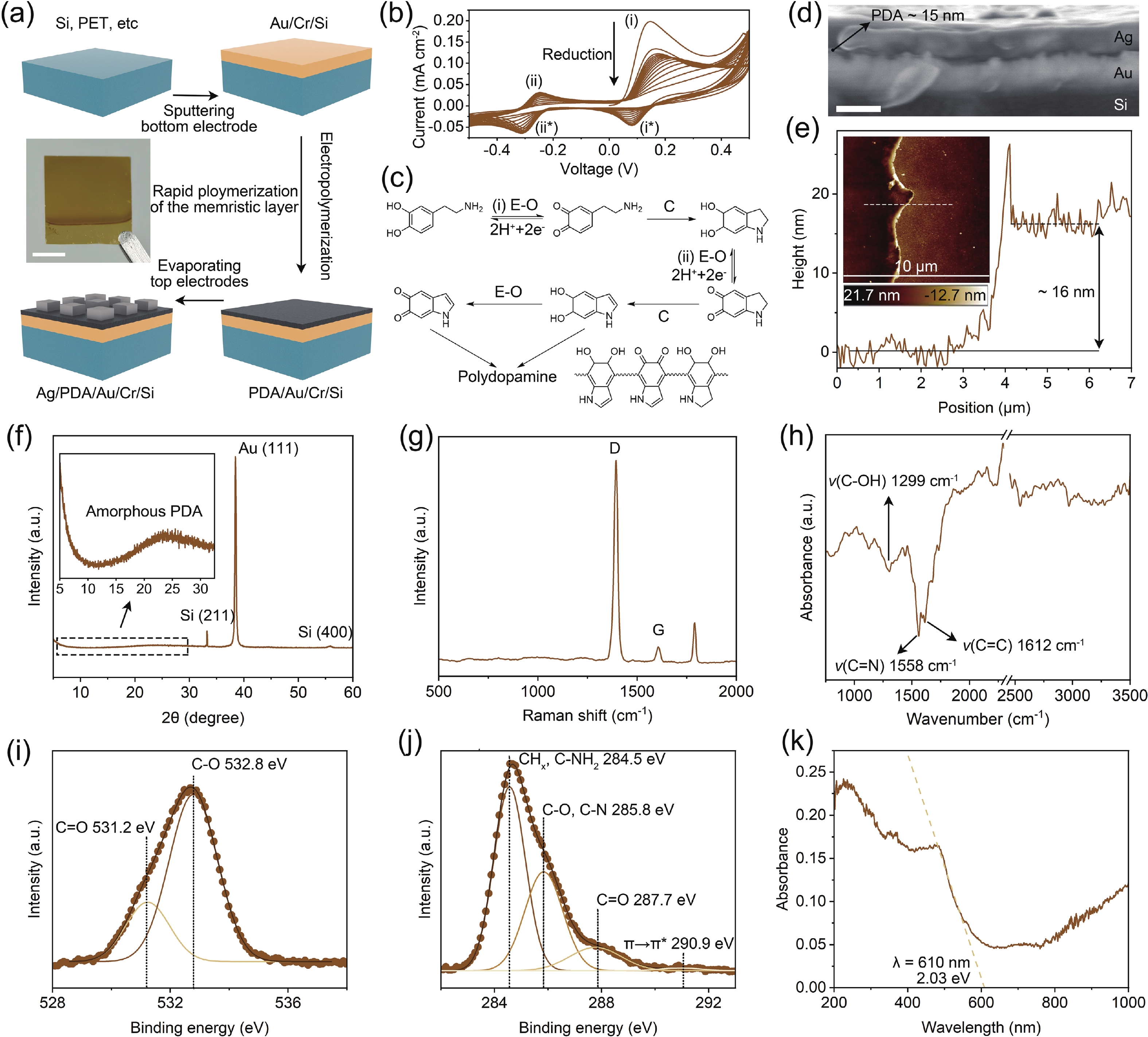
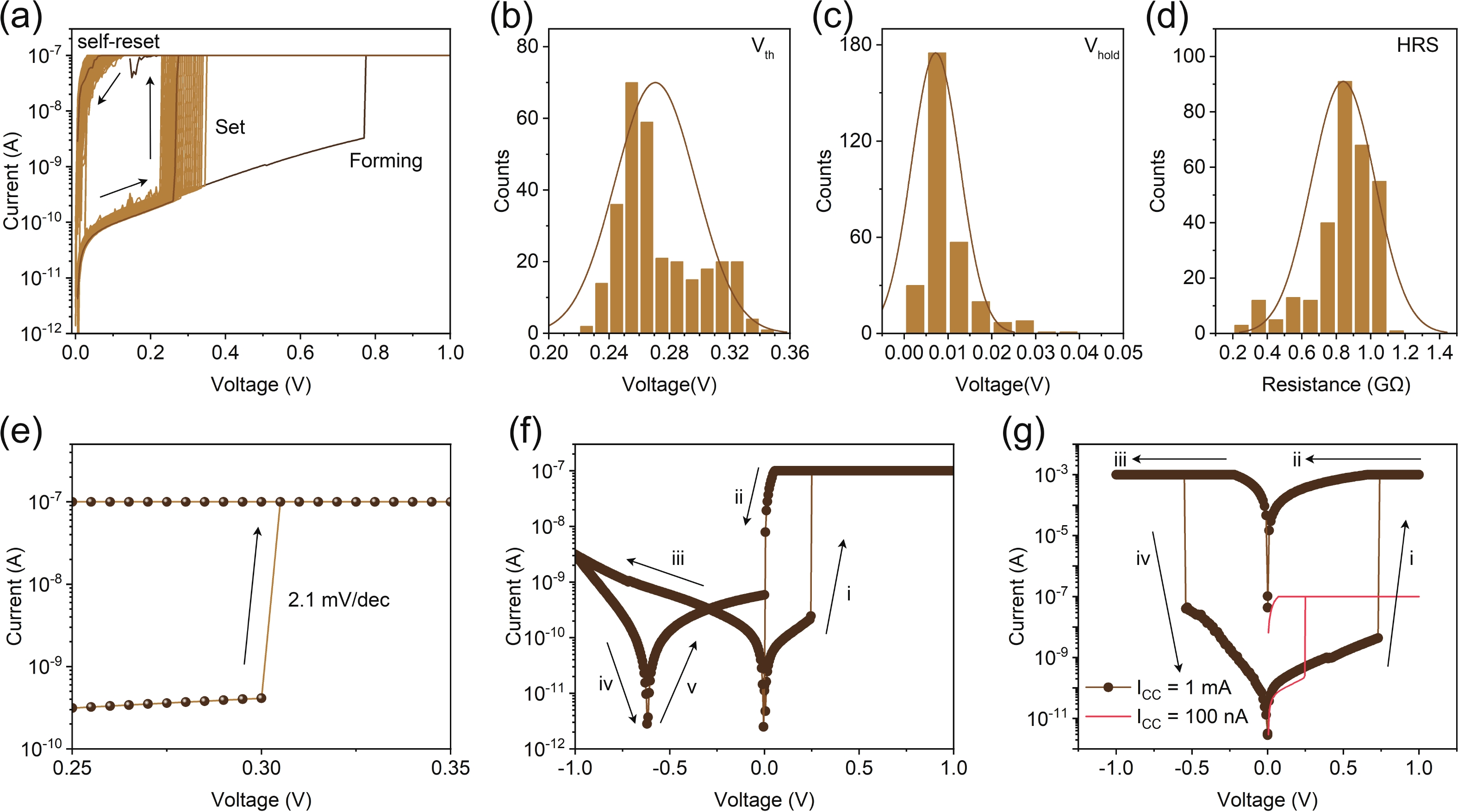
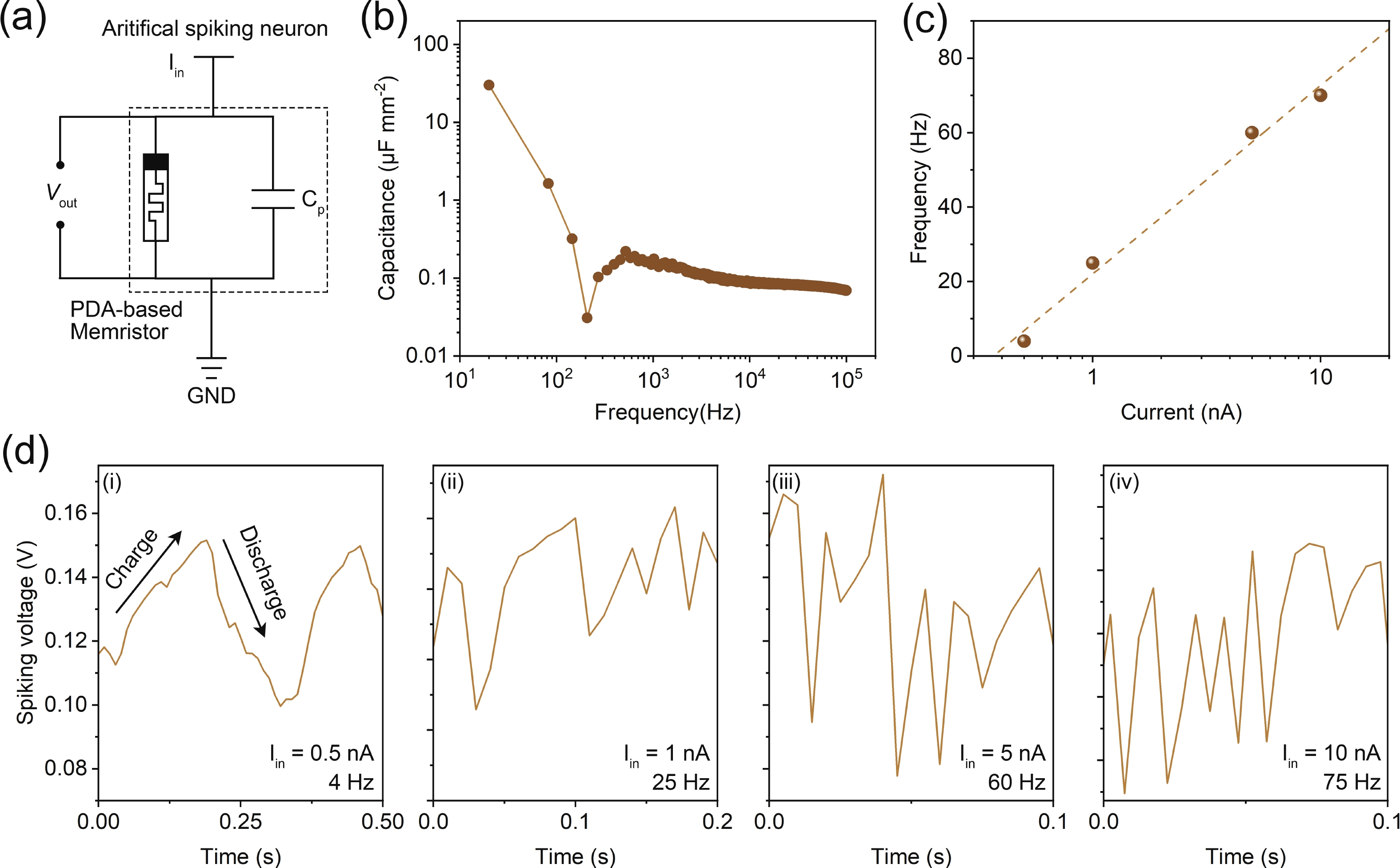
Memristors have a synapse-like two-terminal structure and electrical properties, which are widely used in the construction of artificial synapses. However, compared to inorganic materials, organic materials are rarely used for artificial spiking synapses due to their relatively poor memrisitve performance. Here, for the first time, we present an organic memristor based on an electropolymerized dopamine-based memristive layer. This polydopamine-based memristor demonstrates the improvements in key performance, including a low threshold voltage of 0.3 V, a thin thickness of 16 nm, and a high parasitic capacitance of about 1 μF∙mm−2. By leveraging these properties in combination with its stable threshold switching behavior, we construct a capacitor-free and low-power artificial spiking neuron capable of outputting the oscillation voltage, whose spiking frequency increases with the increase of current stimulation analogous to a biological neuron. The experimental results indicate that our artificial spiking neuron holds potential for applications in neuromorphic computing and systems. -
References
[1] Hu W D, Ye Z H, Liao L, et al. 128 × 128 long-wavelength/mid-wavelength two-color HgCdTe infrared focal plane array detector with ultralow spectral cross talk. Opt Lett, 2014, 39, 5184 doi: 10.1364/OL.39.005184[2] Poo M M, Pignatelli M, Ryan T J, et al. What is memory? The present state of the engram. BMC Biol, 2016, 14, 40 doi: 10.1186/s12915-016-0261-6[3] Hu W D, Chen X S, Ye Z H, et al. A hybrid surface passivation on HgCdTe long wave infrared detector with in-situ CdTe deposition and high-density hydrogen plasma modification. Appl Phys Lett, 2011, 99, 091101 doi: 10.1063/1.3633103[4] Wang Y, Yin L, Huang W, et al. Optoelectronic synaptic devices for neuromorphic computing. Adv Intell Syst, 2021, 3, 2000099 doi: 10.1002/aisy.202000099[5] Roy K, Jaiswal A, Panda P. Towards spike-based machine intelligence with neuromorphic computing. Nature, 2019, 575, 607 doi: 10.1038/s41586-019-1677-2[6] Choi S, Yang J, Wang G. Emerging memristive artificial synapses and neurons for energy-efficient neuromorphic computing. Adv Mater, 2020, 32, 2004659 doi: 10.1002/adma.202004659[7] Li Z X, Zheng Y Q, Li L L, et al. Parallel photoelectron storage and visual preprocessing based on nanowire defect engineering for image degradation. Adv Funct Mater, 2024, 34, 2304119 doi: 10.1002/adfm.202304119[8] Li N, Jiang D W, Wang G W, et al. The measurement of responsivity of infrared photodetectors using a cavity blackbody. J Semicond, 2023, 44, 102301 doi: 10.1088/1674-4926/44/10/102301[9] Wang T, Wang M, Wang J W, et al. A chemically mediated artificial neuron. Nat Electron, 2022, 5, 586 doi: 10.1038/s41928-022-00803-0[10] Qin X K, Zhong B W, Lv S X, et al. A zero-voltage-writing artificial nervous system based on biosensor integrated on ferroelectric tunnel junction. Adv Mater, 2024, 36, 2404026 doi: 10.1002/adma.202404026[11] Kim Y, Chortos A, Xu W T, et al. A bioinspired flexible organic artificial afferent nerve. Science, 2018, 360, 998 doi: 10.1126/science.aao0098[12] Lee Y, Oh J Y, Xu W T, et al. Stretchable organic optoelectronic sensorimotor synapse. Sci Adv, 2018, 4, eaat7387 doi: 10.1126/sciadv.aat7387[13] Wan C J, Pei M J, Shi K L, et al. Toward a brain–neuromorphics interface. Adv Mater, 2024, 2311288 doi: 10.1002/adma.202311288[14] Dai S L, Liu X, Liu Y D, et al. Emerging iontronic neural devices for neuromorphic sensory computing. Adv Mater, 2023, 35, 2300329 doi: 10.1002/adma.202300329[15] Li L L, Xu H, Zhang W X, et al. Multi-color detection of single sensor based on tellurium relaxation characteristics. IEEE Electron Device Lett, 2024, 45, 629 doi: 10.1109/LED.2024.3359688[16] Zhong S, Zhang Y S, Zheng H, et al. Spike-based spatiotemporal processing enabled by oscillation neuron for energy-efficient artificial sensory systems. Adv Intell Syst, 2022, 4, 2200076 doi: 10.1002/aisy.202200076[17] Chen L B, Karilanova S, Chaki S, et al. Spike timing-based coding in neuromimetic tactile system enables dynamic object classification. Science, 2024, 384, 660 doi: 10.1126/science.adf3708[18] Raspopovic S, Valle G, Petrini F M. Sensory feedback for limb prostheses in amputees. Nat Mater, 2021, 20, 925 doi: 10.1038/s41563-021-00966-9[19] Wang Y, Tao L, Guzman R, et al. A stable rhombohedral phase in ferroelectric Hf(Zr)1+xO2 capacitor with ultralow coercive field. Science, 2023, 381, 558 doi: 10.1126/science.adf6137[20] Xia Q F, Yang J J. Memristive crossbar arrays for brain-inspired computing. Nat Mater, 2019, 18, 309 doi: 10.1038/s41563-019-0291-x[21] Yang R, Huang H M, Guo X. Memristive synapses and neurons for bioinspired computing. Adv Electron Mater, 2019, 5, 1900287 doi: 10.1002/aelm.201900287[22] Zhao M R, Gao B, Tang J S, et al. Reliability of analog resistive switching memory for neuromorphic computing. Appl Phys Rev, 2020, 7, 011312 doi: 10.1063/1.5118217[23] Han J K, Yun S Y, Lee S W, et al. A review of artificial spiking neuron devices for neural processing and sensing. Adv Funct Mater, 2022, 32, 2204102 doi: 10.1002/adfm.202204102[24] Zuo W B, Zhu Q H, Fu Y Y, et al. Volatile threshold switching memristor: An emerging enabler in the AIoT era. J Semicond, 2023, 44, 053102 doi: 10.1088/1674-4926/44/5/053102[25] Wang X J, Chen C S, Zhu L, et al. Vertically integrated spiking cone photoreceptor arrays for color perception. Nat Commun, 2023, 14, 3444 doi: 10.1038/s41467-023-39143-8[26] Chen C S, He Y L, Mao H W, et al. A photoelectric spiking neuron for visual depth perception. Adv Mater, 2022, 34, 2201895 doi: 10.1002/adma.202201895[27] Wang W, Pedretti G, Milo V, et al. Learning of spatiotemporal patterns in a spiking neural network with resistive switching synapses. Sci Adv, 2018, 4, eaat4752 doi: 10.1126/sciadv.aat4752[28] Zhang X M, Zhuo Y, Luo Q, et al. An artificial spiking afferent nerve based on Mott memristors for neurorobotics. Nat Commun, 2020, 11, 51 doi: 10.1038/s41467-019-13827-6[29] Yuan R, Tiw P J, Cai L, et al. A neuromorphic physiological signal processing system based on VO2 memristor for next-generation human-machine interface. Nat Commun, 2023, 14, 3695 doi: 10.1038/s41467-023-39430-4[30] Lee D, Kwak M, Moon K, et al. Various threshold switching devices for integrate and fire neuron applications. Adv Electron Mater, 2019, 5, 1800866 doi: 10.1002/aelm.201800866[31] Sokolov A S, Ali M, Riaz R, et al. Silver-adapted diffusive memristor based on organic nitrogen-doped graphene oxide quantum dots (N-GOQDs) for artificial biosynapse applications. Adv Funct Mater, 2019, 29, 1807504 doi: 10.1002/adfm.201807504[32] Zhang W N, Li X Y, Zhang Z, et al. Tea helps neuromorphic computing: Flexible memristors from tea polyphenols. J Mater Chem C, 2024, 12, 5748 doi: 10.1039/D4TC00440J[33] Sueoka B, Zhao F. Memristive synaptic device based on a natural organic material—Honey for spiking neural network in biodegradable neuromorphic systems. J Phys D: Appl Phys, 2022, 55, 225105 doi: 10.1088/1361-6463/ac585b[34] Zhang B, Chen W L, Zeng J M, et al. 90% yield production of polymer nano-memristor for in-memory computing. Nat Commun, 2021, 12, 1984 doi: 10.1038/s41467-021-22243-8[35] Lee H A, Park E, Lee H. Polydopamine and its derivative surface chemistry in material science: A focused review for studies at KAIST. Adv Mater, 2020, 32, 1907505 doi: 10.1002/adma.201907505[36] Liu Y, Ai K and Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev, 2014, 114, 5057 doi: 10.1021/cr400407a[37] Zhao Y Y, Cheng X F, Qian W H, et al. Mussel-inspired polydopamine coating for flexible ternary resistive memory. Chem Asian J, 2018, 13, 1744 doi: 10.1002/asia.201800634[38] Zhou P K, Lin X L, Yang H L, et al. Natural biomaterial-based memristor bearing protonated polydopamine with enhanced bipolar resistive switching performance and environmental robustness. J Alloys Compd, 2022, 925, 166783 doi: 10.1016/j.jallcom.2022.166783[39] Wang H P, Jiang Q Q, Yang J, et al. Polydopamine film self-assembled at air/water interface for organic electronic memory devices. Adv Mater Interfaces, 2020, 7, 2000979 doi: 10.1002/admi.202000979[40] Gong M L, Li W, Fan F, et al. In-situ surface modification of ITO substrate via bio-inspired mussel chemistry for organic memory devices. Biomimetics, 2022, 7, 237 doi: 10.3390/biomimetics7040237[41] Wang J L, Li B C, Li Z J, et al. Electropolymerization of dopamine for surface modification of complex-shaped cardiovascular stents. Biomaterials, 2014, 35, 7679 doi: 10.1016/j.biomaterials.2014.05.047[42] Zhong B W, Qin X K, Xu H, et al. Interindividual- and blood-correlated sweat phenylalanine multimodal analytical biochips for tracking exercise metabolism. Nat Commun, 2024, 15, 624 doi: 10.1038/s41467-024-44751-z[43] Lee J H, Rim Y S, Min W K, et al. Biocompatible and biodegradable neuromorphic device based on hyaluronic acid for implantable bioelectronics. Adv Funct Mater, 2021, 31, 2107074 doi: 10.1002/adfm.202107074[44] Huang Y Y, Yuan H Y, Nie B W, et al. Deep insights into the mechanism of nitrogen on the endurance enhancement in ferroelectric field effect transistors: Trap behavior during memory window degradation. Appl Phys Lett, 2024, 124, 133504 doi: 10.1063/5.0196442[45] Li Y L, Liu M L, Xiang C H, et al. Electrochemical quartz crystal microbalance study on growth and property of the polymer deposit at gold electrodes during oxidation of dopamine in aqueous solutions. Thin Solid Films, 2006, 497, 270 doi: 10.1016/j.tsf.2005.10.048[46] Marchesi D’Alvise T, Harvey S, Hueske L, et al. Ultrathin polydopamine films with phospholipid nanodiscs containing a glycophorin A domain. Adv Funct Mater, 2020, 30, 2000378 doi: 10.1002/adfm.202000378[47] Szewczyk J, Pochylski M, Szutkowski K, et al. In-situ thickness control of centimetre-scale 2D-Like polydopamine films with large scalability. Mater Today Chem, 2022, 24, 100935 doi: 10.1016/j.mtchem.2022.100935[48] Ku S H, Lee J S, Park C B. Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine. Langmuir, 2010, 26, 15104 doi: 10.1021/la102825p[49] Zangmeister R A, Morris T A, Tarlov M J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir, 2013, 29, 8619 doi: 10.1021/la400587j[50] Jiang P F, Jiang H J, Yang Y, et al. A 256 Kbit Hf0.5Zr0.5O2-based FeRAM chip with scaled film thickness (sub-8 nm), low thermal budget (350 oC), 100% initial chip yield, low power consumption (0.7 pJ/bit at 2 V write voltage), and prominent endurance (>1012). 2023 International Electron Devices Meeting (IEDM), 2023, 1, 1 doi: 10.1109/IEDM45741.2023.10413844[51] Ge J, Zhang S, Liu Z Y, et al. Flexible artificial nociceptor using a biopolymer-based forming-free memristor. Nanoscale, 2019, 11, 6591 doi: 10.1039/C8NR08721K -
Proportional views





 Bowen Zhong is a PhD candidate at the Institute of Semiconductors, Chinese Academy of Sciences. He earned his B.S. degree (2020) in Materials Physics from the Xiangtan University. His current research interest focuses on novel biosensors for personalized healthcare and advanced memristors for neuromorphic computing.
Bowen Zhong is a PhD candidate at the Institute of Semiconductors, Chinese Academy of Sciences. He earned his B.S. degree (2020) in Materials Physics from the Xiangtan University. His current research interest focuses on novel biosensors for personalized healthcare and advanced memristors for neuromorphic computing. Lili Wang is a professor in the Institute of Semiconductors, Chinese Academy of Sciences, China. She earned her B.S. degree in Chemistry and Ph.D. degree in Microelectronics and Solid State Electronics from Jilin University in 2014. Her current research interests focus on the semiconductor multimode intelligent sensing integrated system.
Lili Wang is a professor in the Institute of Semiconductors, Chinese Academy of Sciences, China. She earned her B.S. degree in Chemistry and Ph.D. degree in Microelectronics and Solid State Electronics from Jilin University in 2014. Her current research interests focus on the semiconductor multimode intelligent sensing integrated system.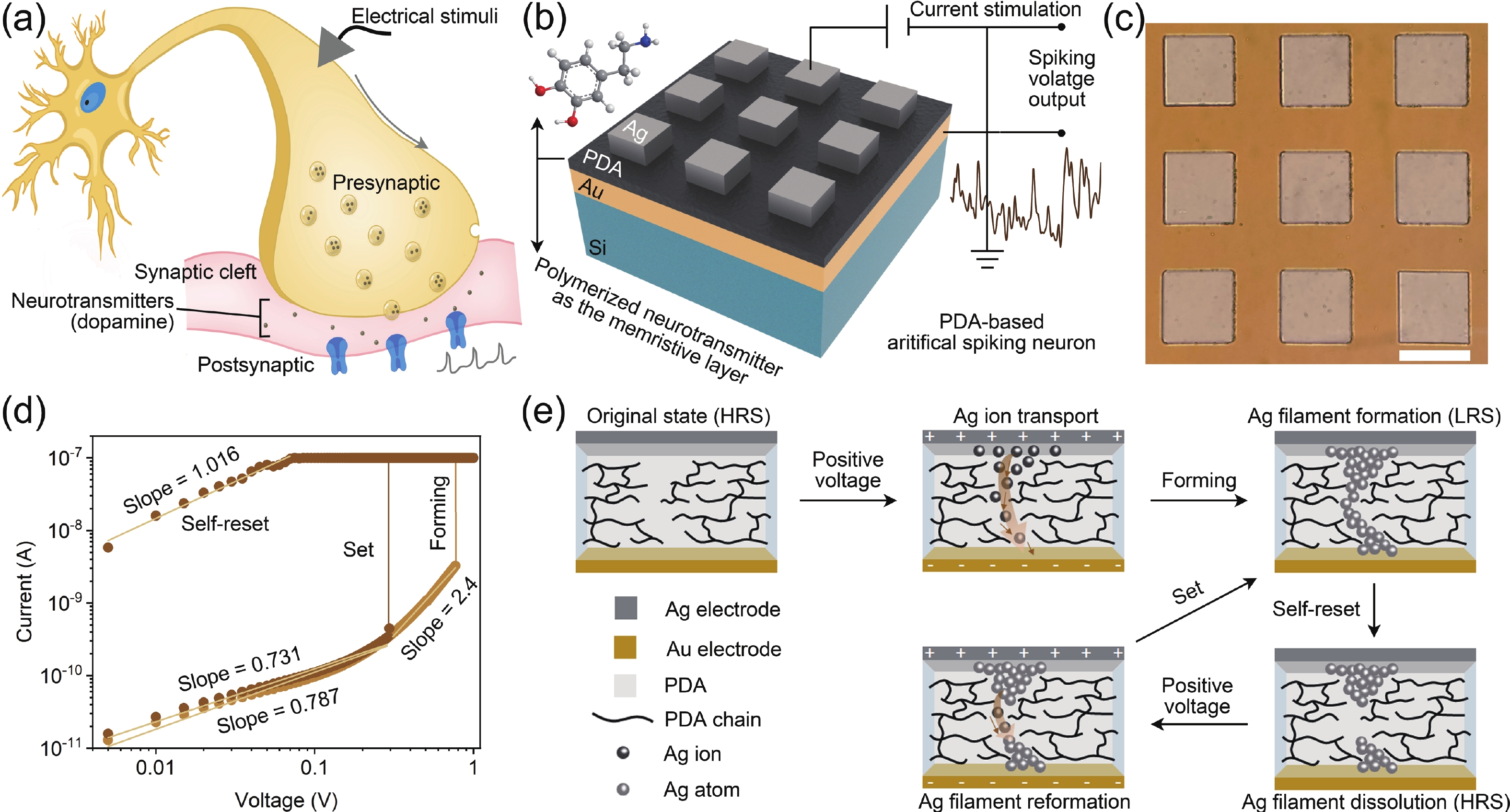
 DownLoad:
DownLoad: