| Citation: |
L. Bouhdjer, S. Addala, A. Chala, O. Halimi, B. Boudine, M. Sebais. Elaboration and characterization of a KCl single crystal doped with nanocrystalsof a Sb2O3 semiconductor[J]. Journal of Semiconductors, 2013, 34(4): 043001. doi: 10.1088/1674-4926/34/4/043001
****
L. Bouhdjer, S. Addala, A. Chala, O. Halimi, B. Boudine, M. Sebais. Elaboration and characterization of a KCl single crystal doped with nanocrystalsof a Sb2O3 semiconductor[J]. J. Semicond., 2013, 34(4): 043001. doi: 10.1088/1674-4926/34/4/043001.
|
Elaboration and characterization of a KCl single crystal doped with nanocrystalsof a Sb2O3 semiconductor
DOI: 10.1088/1674-4926/34/4/043001
More Information
-
Abstract
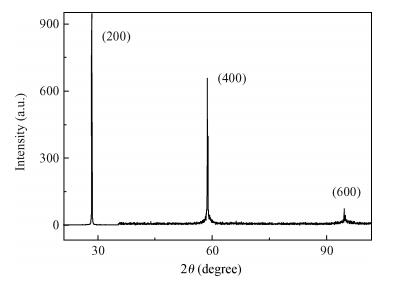
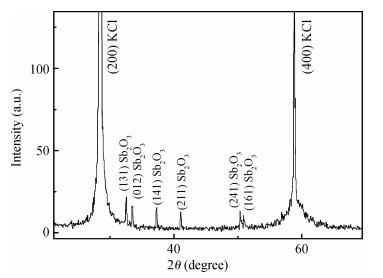
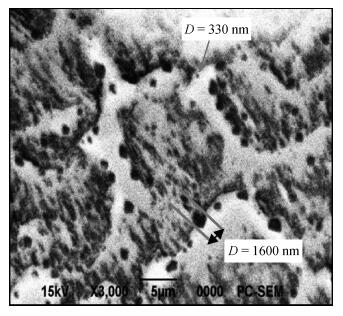
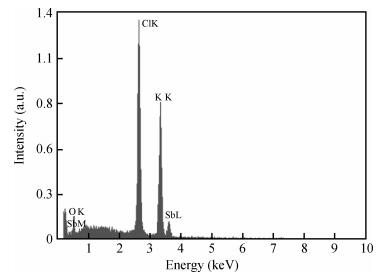
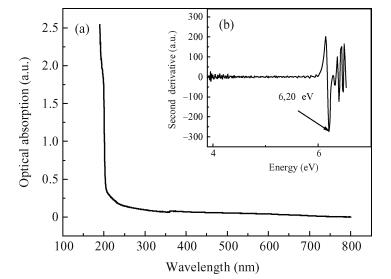
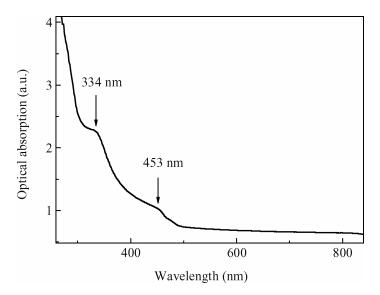
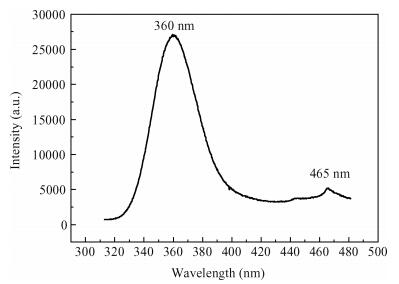
Undoped and doped KCl single crystals have been successfully elaborated via the Czochralski (Cz) method. The effects of dopant Sb2O3 nanocrystals on structural and optical properties were investigated by a number of techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray (EDAX) analysis, UV-visible and photoluminescence (PL) spectrophotometers. An XRD pattern of KCl:Sb2O3 reveals that the Sb2O3 nanocrystals are in the well-crystalline orthorhombic phase. The broadening of diffraction peaks indicated the presence of a Sb2O3 semiconductor in the nanometer size regime. The shift of absorption and PL peaks is observed near 334 nm and 360 nm respectively due to the quantum confinement effect in Sb2O3 nanocrystals. Particle sizes calculated from XRD studies agree fairly well with those estimated from optical studies. An SEM image of the surface KCl:Sb2O3 single crystal shows large quasi-spherical of Sb2O3 crystallites scattered on the surface. The elemental analysis from EDAX demonstrates that the KCl:Sb2O3 single crystal is slightly rich in oxygen and a source of excessive quantities of oxygen is discussed.-
Keywords:
- Sb2O3 semiconductor,
- KCl single crystal,
- Cz method,
- XRD,
- SEM,
- UV-vis absorption
-
References
[1] Brus L E. Electronic wave functions in semiconductor clusters:experiment and theory. Chem Phys, 1986, 90:2555 doi: 10.1021/j100403a003[2] Lieber C M. One-dimensional nanostructures:chemistry, physics and application. Solid State Commun, 1998, 107:607 doi: 10.1016/S0038-1098(98)00209-9[3] Smalley R E, Yakobson B I. The future of the fullerenes. Solid State Commun, 1998, 107:597 doi: 10.1016/S0038-1098(98)00210-5[4] Schneider J J. Nanomaterials:synthesis, properties and applications. Adv Mater, 2004, 9:997[5] O'Regan B, Gratzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Letters to Nature, 1991, 353:737 doi: 10.1038/353737a0[6] Colvin V L, Schlamp M C, Aliviastos A P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Letters to Nature, 1994, 370:354 doi: 10.1038/370354a0[7] Wyckoff R W G. Crystal structure. 2nd ed. New York:Wiley, 1994[8] Orosel D, Balog P, Liu H, et al. Sb2O4 at high pressures and high temperatures. Solid State Chem, 2005, 178:2602 doi: 10.1016/j.jssc.2005.05.037[9] Nanda K K, Sahu S N, Behera S N. Liquid-drop model for size-dependent melting of low-dimensional systems. Phys Rev, 2002, A66:013208[10] Mahdavian A R, Morshedian J, Rezaie M. The comparison between synergistic effect of Sb2O3 and Al(OH)3 on the flame-retardancy of HIPS in the presence of tetrabromobisphenol-A. Iranian Polymer, 2004, 13:219[11] Grund, Jonson B. Compositional effect on fining and oxygen activity in mixed alkali silicate glass. Journal of Glass Science and Technology Part A, 2009, 50(1):62[12] Haberland H, Insepov Z, Moseler M. Molecular-dynamics simulation of thin-film growth by energetic cluster impact. Phys Rev, 1995, B51:11061[13] Fuchs G, Melinon P, Santos Aires F, et al. Cluster-beam deposition of thin metallic antimony films:cluster-size and deposition-rate effects. Phys Rev, 1991, B44:3926[14] Burguer P A, Cuendet P, Gratzel M. Ultrafine and specific catalysts affording efficient hydrogen evolution from water under visible light illumination. Am J Chem Soc, 1981, 103:2923 doi: 10.1021/ja00401a002[15] Meticos-Hucovic M, Loveric B. Semiconducting properties of anodically formed layer on antimony. Electrochim Acta, 1978, 23:1371 doi: 10.1016/0013-4686(78)80019-X[16] Nalin M, Poulain M, Riberio J L, et al. Antimony oxide based glasses. Non-Cryst Solids, 2001, 284:110 doi: 10.1016/S0022-3093(01)00388-X[17] Kim H J, Lee S H, Yon S J, et al. Effect of Sb2O3 on solarization of photosensitive glasses containing Ag and CeO. Korean J Ceram, 2001, 7:58[18] Satyanarayana T, Kityk I V, Ozga K, et al. Role of titanium valence states in optical and electronic features of PbO-Sb2O3-B=O3:TiO2 glass alloys. Alloys Compd, 2009, 482:283 doi: 10.1016/j.jallcom.2009.03.185[19] Halimi O, Boudine B, Sebais M, et al. Structural and optical characterization of ZnO nanocrystals embedded in bulk KBr single crystal. Mater Sci Eng C, 2003, 23:111[20] Boudine B, Sebais M, Halimi O, et al. Structural and optical properties of CdS nanocrystals embedded in NaCl single crystals. Mouras R Catalysis Today, 2004, 89:293 doi: 10.1016/j.cattod.2003.12.004[21] Bensouici A, Plaza J L, Diéguez E, et al. CdTe aggregates in KBr crystalline matrix. Luminescence, 2009, 129:948 doi: 10.1016/j.jlumin.2009.04.001[22] Samah M, Khalfane H, Bouhuerra, et al. Optic and structural properties of the aggregations of AgBr in an ionic matrix. Annales de Chimie Science des Matériaux, 2004, 29:49 doi: 10.3166/acsm.29.2.49-54[23] Othmani A, Plenet J C, Berstein E, et al. Nanocrystals of CdS dispersed in a sol-gel silica glass:optical properties. J Cryst Growth, 1994, 144:141 doi: 10.1016/0022-0248(94)90449-9[24] Ge S, Wang Q, Li J, et al. Controllable synthesis and formation mechanism of bow-tie-like Sb2O3 nanostructures via a surfactant-free solvothermal route. Alloys and Compounds, 2010, 494:169 doi: 10.1016/j.jallcom.2010.01.064[25] Zehani F, Sebais M. UV-visible emission of (O2-F+) centers in KBr. Cryst Res Technol, 2007, 42:1123 doi: 10.1002/(ISSN)1521-4079[26] Naidu B S, Pandey M, Sudarsan V, et al. Interaction of Sb+3 ions with Eu+3 ions during the room temperature synthesis of luminescent Sb2O3 nanorods:probed through Eu+3 luminescence. Luminescence, 2010, 113:177 -
Proportional views






 DownLoad:
DownLoad: