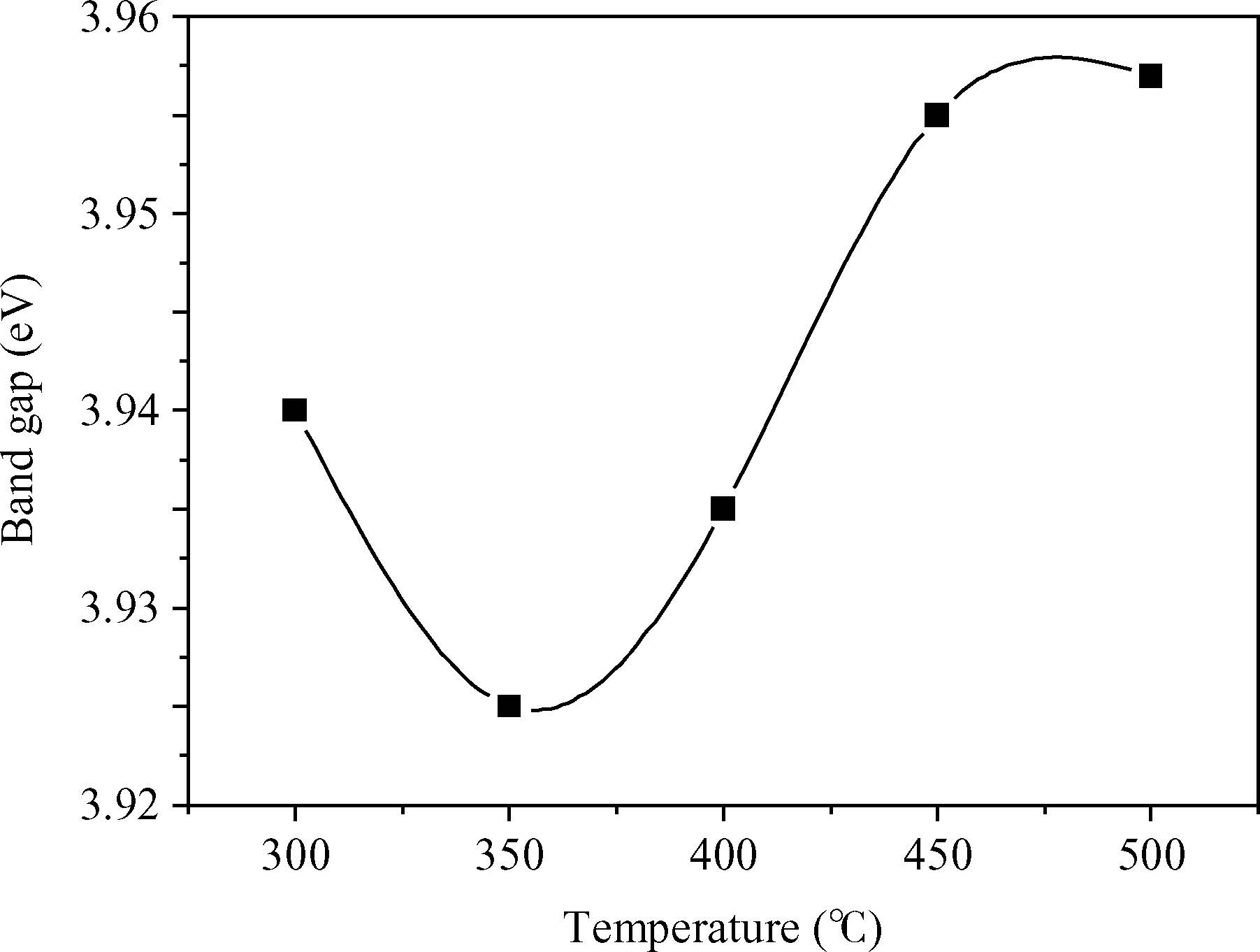
H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. Journal of Semiconductors, 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002 ****H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. J. Semicond., 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002.
| Citation:
|
H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO 2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. Journal of Semiconductors, 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002
****
H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. J. Semicond., 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002.
|
H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. Journal of Semiconductors, 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002 ****H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. J. Semicond., 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002.
| Citation:
|
H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO 2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. Journal of Semiconductors, 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002
****
H. Bendjedidi, A. Attaf, H. Saidi, M. S. Aida, S. Semmari, A. Bouhdjar, Y. Benkhetta. Properties of n-type SnO2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications[J]. J. Semicond., 2015, 36(12): 123002. doi: 10.1088/1674-4926/36/12/123002.
|





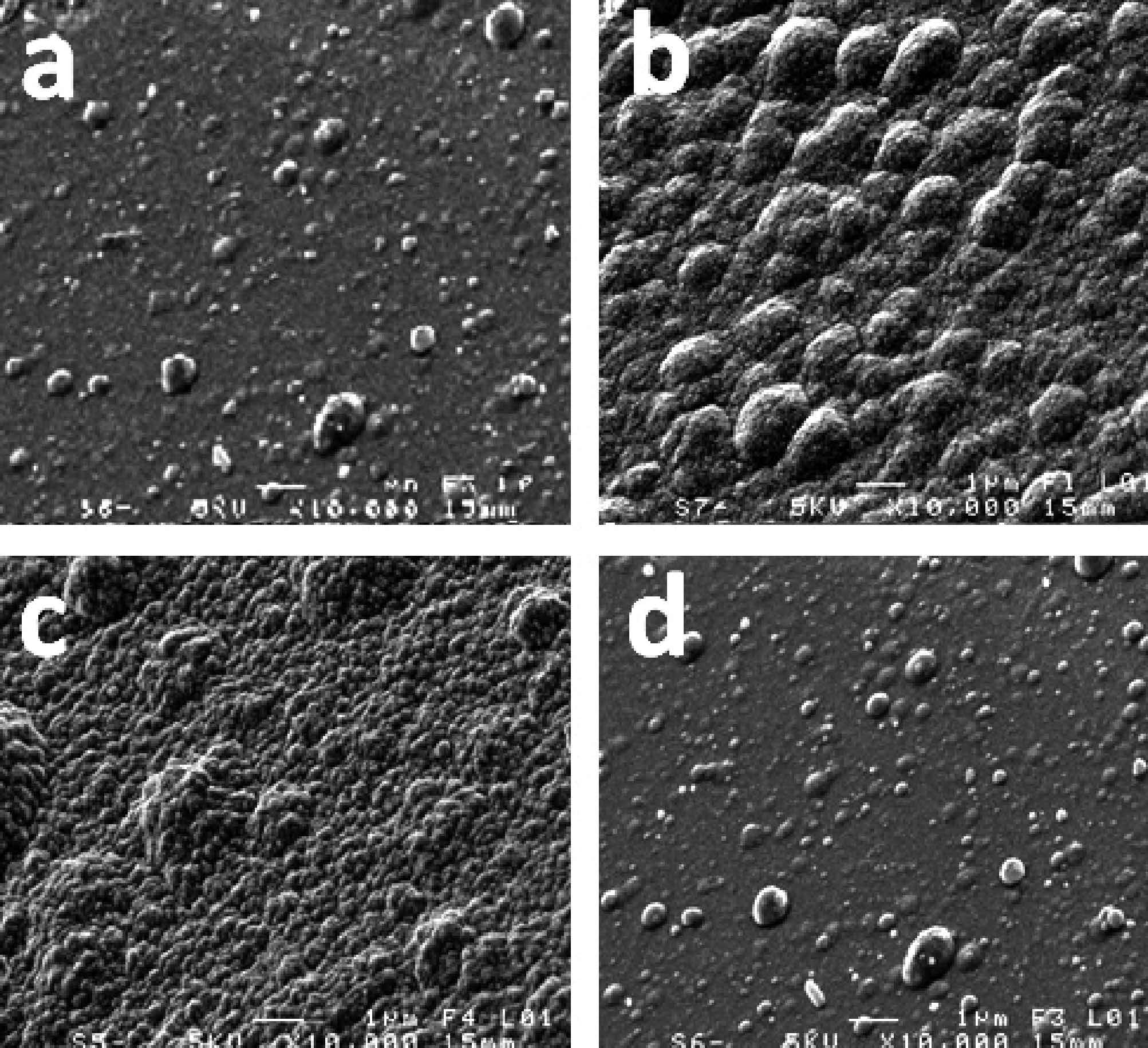
 DownLoad:
DownLoad: