| Citation: |
Shaohua Jia, Yuling Liu, Chenwei Wang, Chenqi Yan. Influence of oxidant passivation on controlling dishing in alkaline chemical mechanical planarization[J]. Journal of Semiconductors, 2015, 36(12): 126002. doi: 10.1088/1674-4926/36/12/126002
****
S H Jia, Y L Liu, C W Wang, C Q Yan. Influence of oxidant passivation on controlling dishing in alkaline chemical mechanical planarization[J]. J. Semicond., 2015, 36(12): 126002. doi: 10.1088/1674-4926/36/12/126002.
|
Influence of oxidant passivation on controlling dishing in alkaline chemical mechanical planarization
DOI: 10.1088/1674-4926/36/12/126002
More Information
-
Abstract
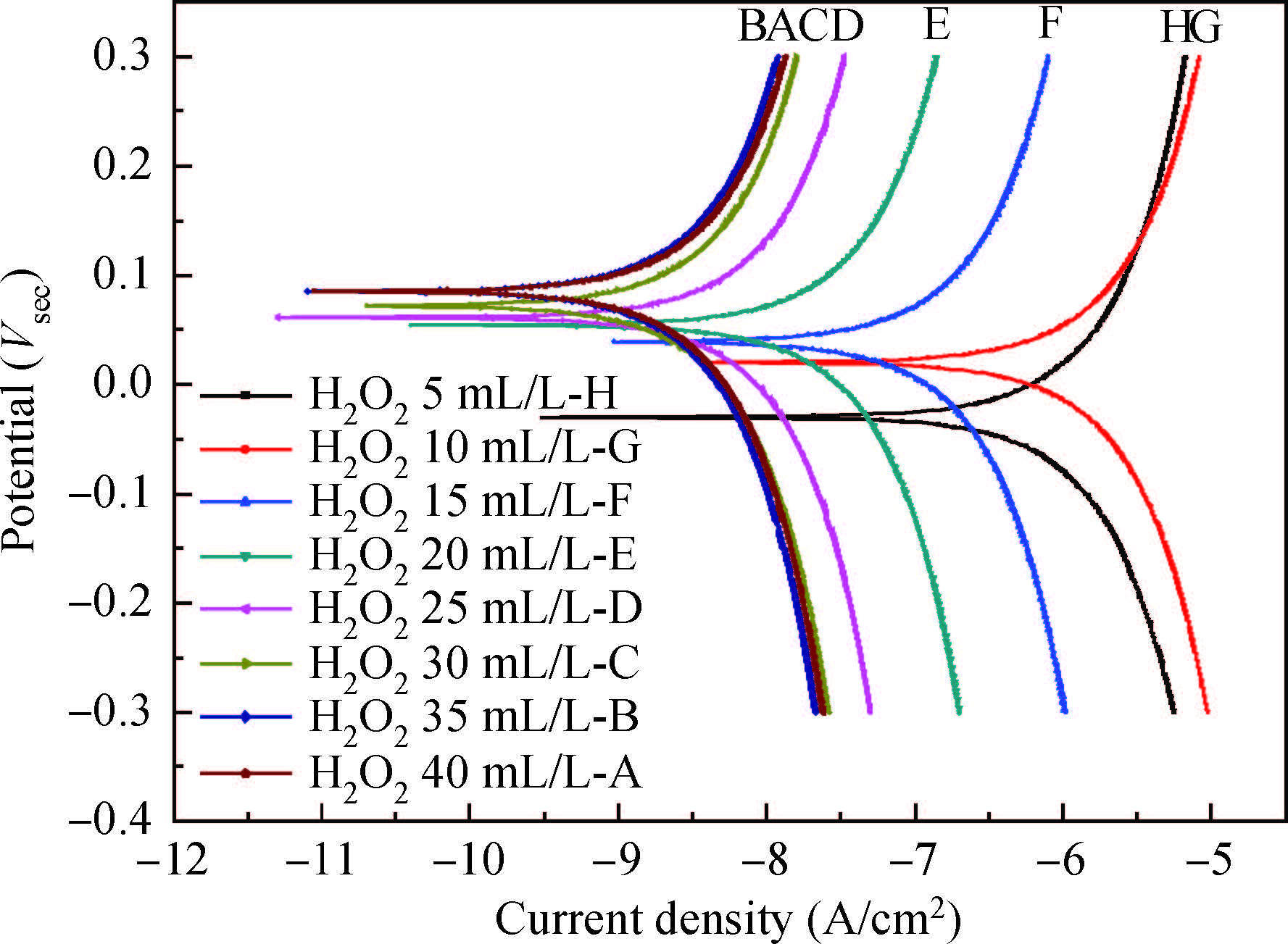
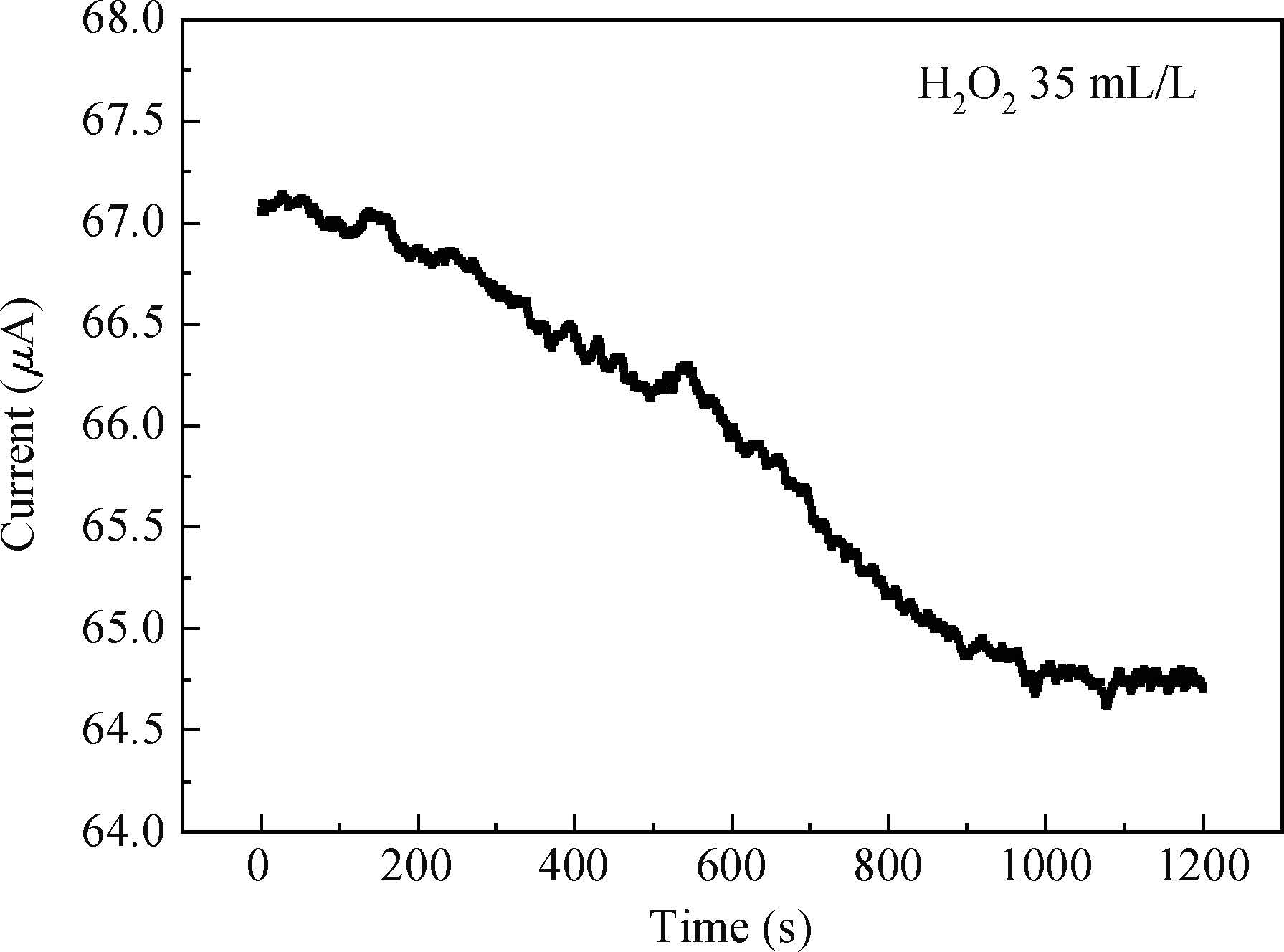
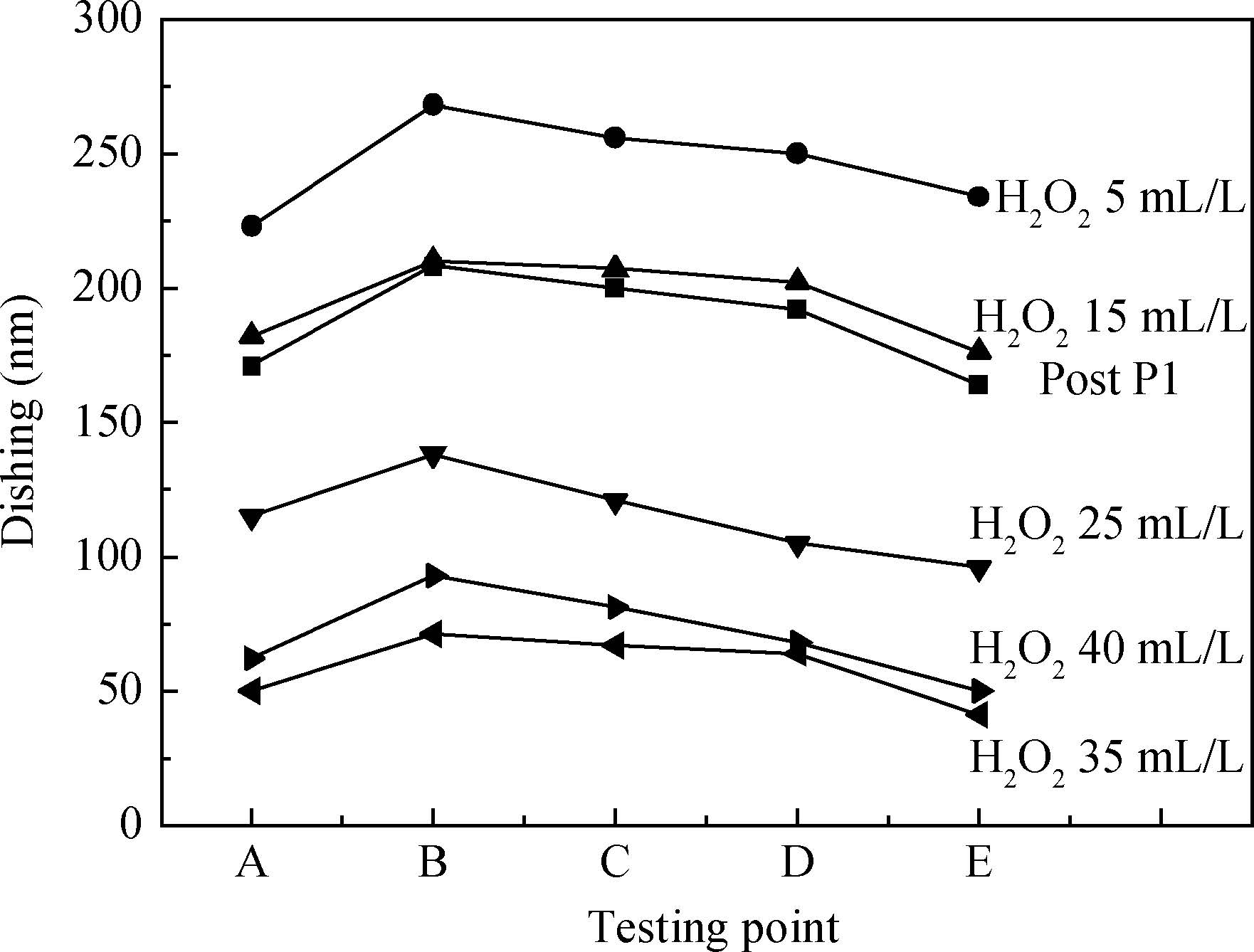
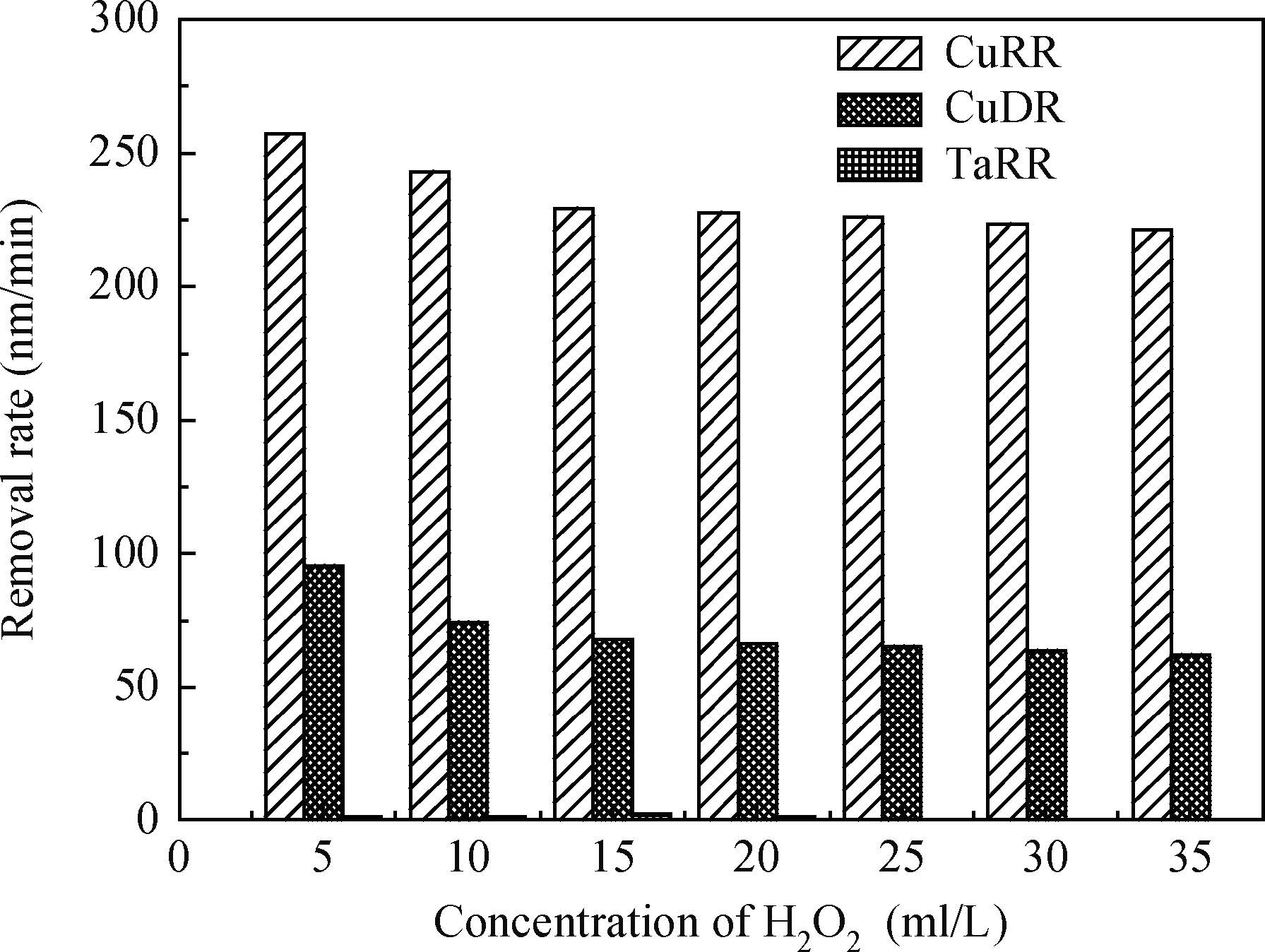
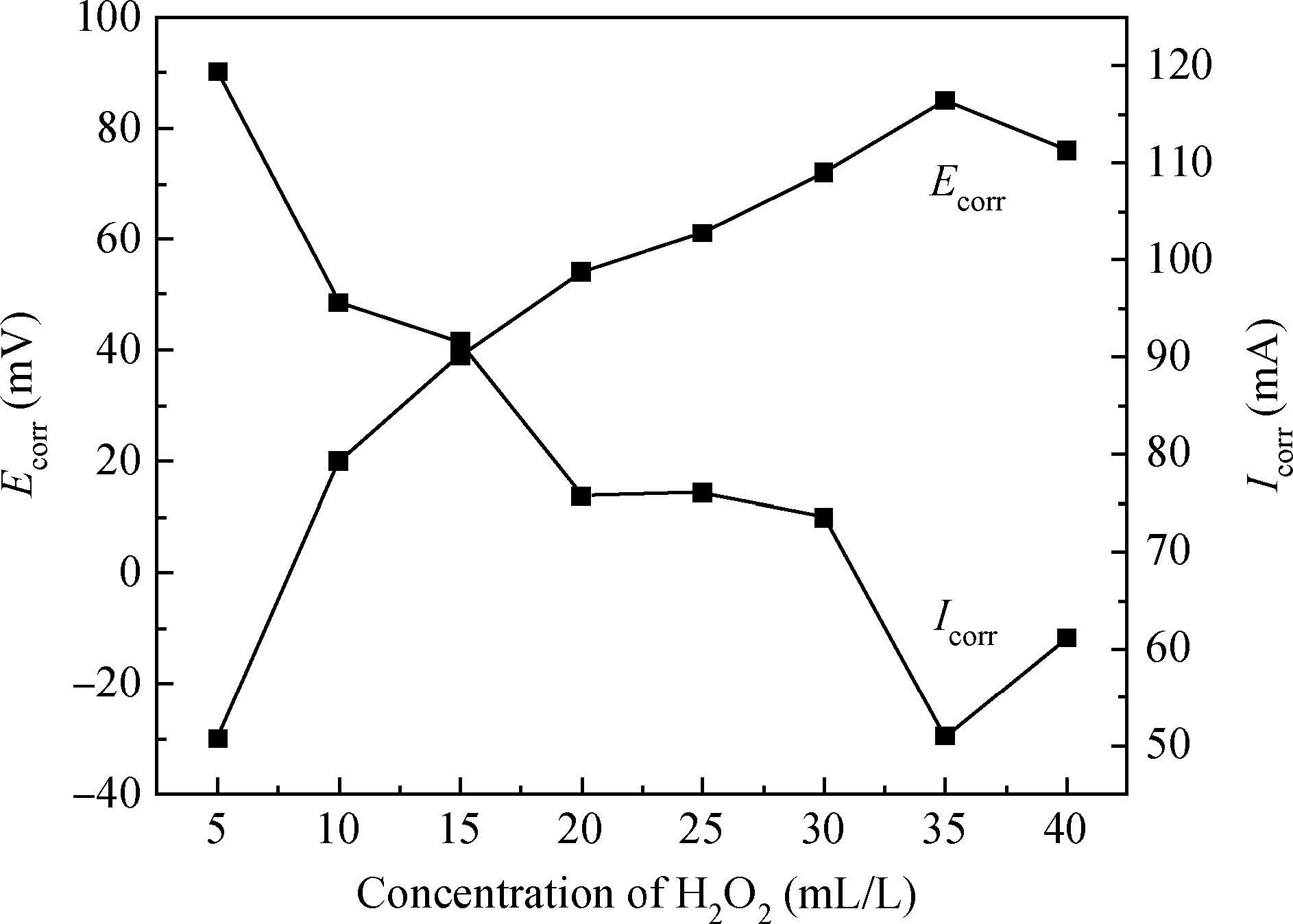
The article studied the electrochemical behavior of P2 alkaline polishing slurry. The main research is the changing discipline of Ecorr and Icorr in the Cu electrolyte at different concentrations of oxidant H2O2. It compares potentiodynamic polarization curves in different P2 slurries and analyzes the passivation function of H2O2 acting on controlling dishing. The result implies that the potential increases gradually and then levels off while the current density on the contrary decreases with the augment of H2O2 concentration. In addition, dishing declines with the increasing of H2O2 along with the optimization of planarization of the alkaline P2 slurry.-
Keywords:
- hydrogen peroxide,
- passivation,
- dishing,
- alkaline,
- Cu CMP
-
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] -
Proportional views





 DownLoad:
DownLoad: