| Citation: |
Weijuan Liu, Yuling Liu. Synergic effect of chelating agent and oxidant on chemical mechanical planarization[J]. Journal of Semiconductors, 2015, 36(2): 026001. doi: 10.1088/1674-4926/36/2/026001
****
W J Liu, Y L Liu. Synergic effect of chelating agent and oxidant on chemical mechanical planarization[J]. J. Semicond., 2015, 36(2): 026001. doi: 10.1088/1674-4926/36/2/026001.
|
Synergic effect of chelating agent and oxidant on chemical mechanical planarization
DOI: 10.1088/1674-4926/36/2/026001
More Information
-
Abstract
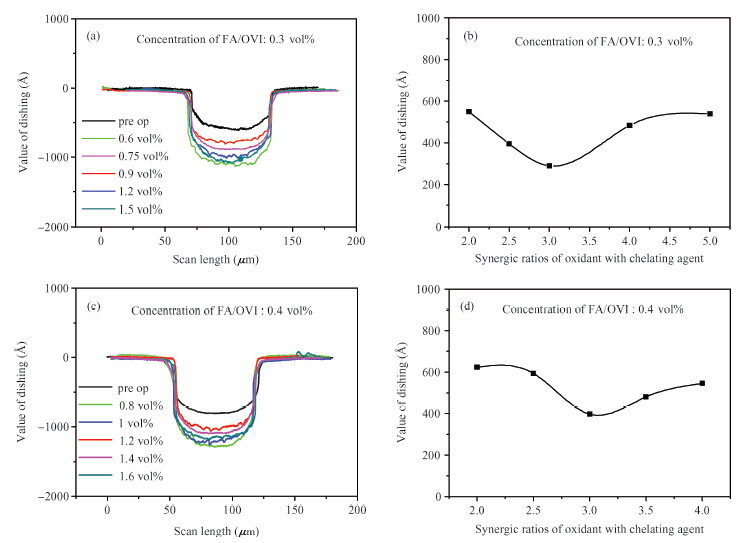
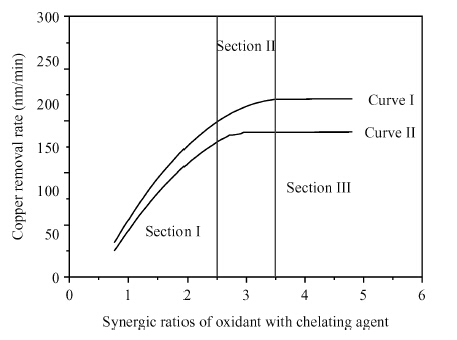
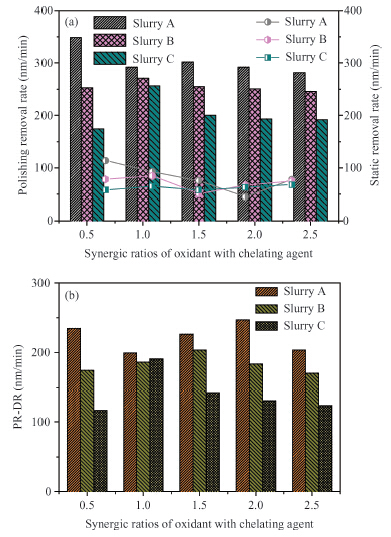
Chemically dominant alkaline slurry, which is free of BTA (benzotriazole) and other inhibitors, was investigated. The synergic effect of the chelating agent and oxidant on the chemical mechanical planarization (CMP) was taken into consideration. Copper CMP slurry is mainly composed of an oxidizer, nonionic surfactant, chelating agent and abrasive particles. The effect of different synergic ratios of oxidant with chelating agent on the polishing removal rate, static etch rate and planarization were detected. The planarization results reveal that with the increase of oxidant concentration, the dishing value firstly diminished and then increased again. When the synergic ratios is 3, the dishing increases the least. A theoretical model combined with chemical-mechanical kinetics process was proposed in the investigation, which can explain this phenomenon. Based on the theoretical model, the effect of synergic ratios of oxidant with chelating agent on velocity D-value (convex removal rate minus recessed removal rate) was analyzed. The results illustrate that when the synergic ratio is between 2.5—3.5, the velocity D-value is relatively higher, thereby good planarization can be achieved in this interval. This investigation provides a new guide to analyze and study copper line corrosion in the recessed region during copper clearing polishing.-
Keywords:
- CMP,
- theoretical model,
- synergic ratios,
- dishing,
- static etch rates
-
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] -
Proportional views





 DownLoad:
DownLoad: