| Citation: |
Shadia J. Ikhmayies, Naseem M. Abu El-Haija, Riyad N. Ahmad-Bitar. A comparison between different ohmic contacts for ZnO thin films[J]. Journal of Semiconductors, 2015, 36(3): 033005. doi: 10.1088/1674-4926/36/3/033005
****
S. J. Ikhmayies, N. M. A. El-Haija, R. N. Ahmad-Bitar. A comparison between different ohmic contacts for ZnO thin films[J]. J. Semicond., 2015, 36(3): 033005. doi: 10.1088/1674-4926/36/3/033005.
|
A comparison between different ohmic contacts for ZnO thin films
DOI: 10.1088/1674-4926/36/3/033005
More Information
-
Abstract
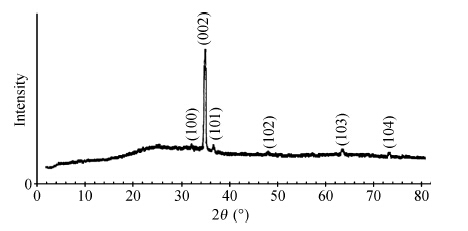
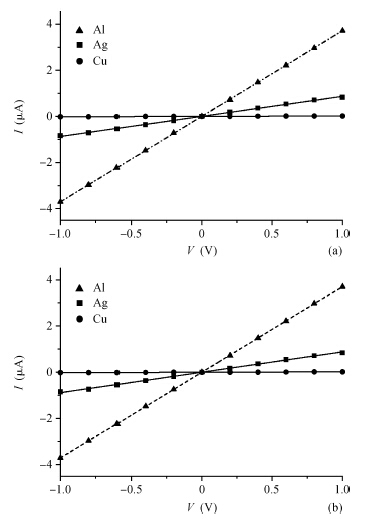
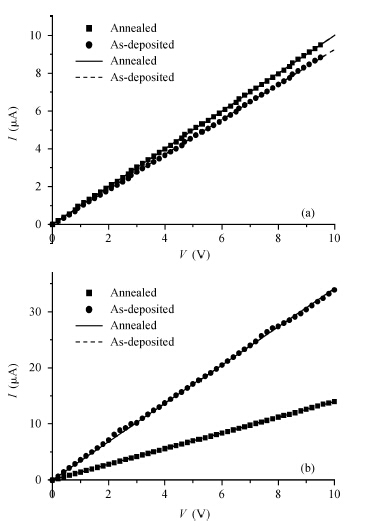
There are several metals that form ohmic contacts for ZnO thin films, such as copper, aluminum and silver. The aim of this work is to make a comparison between these ohmic contacts. To achieve this purpose, polycrystalline ZnO thin films were prepared by the spray pyrolysis technique, and characterized by the I—V measurements at room temperature. Two strips of each metal were thermally evaporated on the surface of the film and measurements were first recorded in the dark and room light, then in the dark before and after annealing for Al, which was found to be the best in the set. Films with aluminum contacts gave the smallest resistivity, best ohmicity and they are slightly affected by light as required. On the other hand, copper was found to be the worst, and films with copper contacts gave the largest resistivity, worst ohmicity and they are the most affected by light. Annealing improved the aluminum contacts due to alloying and doping.-
Keywords:
- transparent conducting oxide,
- ohmic contacts,
- annealing,
- solar cells
-
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] -
Proportional views





 DownLoad:
DownLoad: