| Citation: |
Bo Duan, Weijing An, Jianwei Zhou, Shuai Wang. Effect of FA/O complexing agents and H2O2 on chemical mechanical polishing of ruthenium in weakly alkaline slurry[J]. Journal of Semiconductors, 2015, 36(7): 076002. doi: 10.1088/1674-4926/36/7/076002
****
B Duan, W J An, J W Zhou, S Wang. Effect of FA/O complexing agents and H2O2 on chemical mechanical polishing of ruthenium in weakly alkaline slurry[J]. J. Semicond., 2015, 36(7): 076002. doi: 10.1088/1674-4926/36/7/076002.
|
Effect of FA/O complexing agents and H2O2 on chemical mechanical polishing of ruthenium in weakly alkaline slurry
DOI: 10.1088/1674-4926/36/7/076002
More Information
-
Abstract
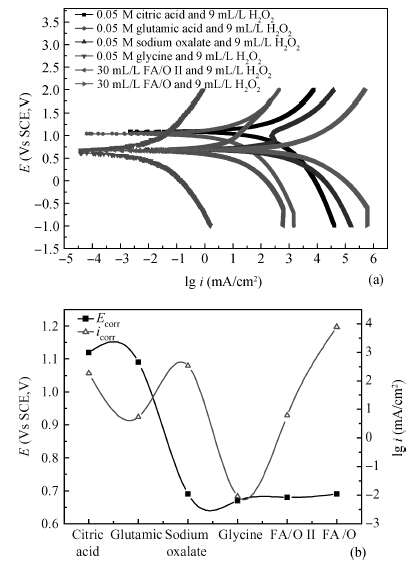
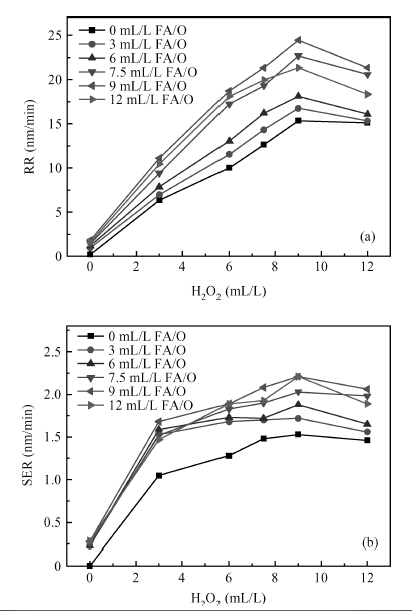
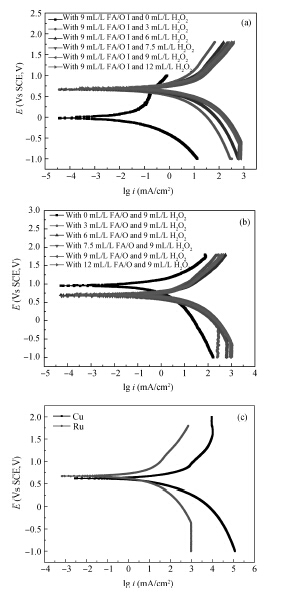
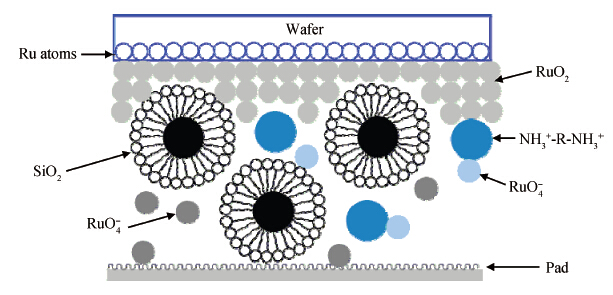
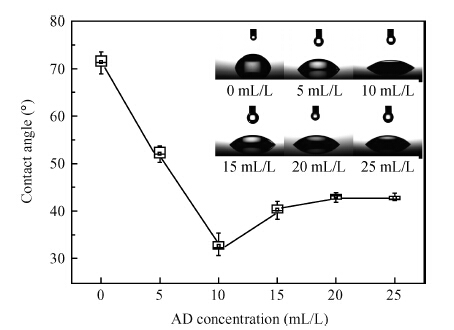
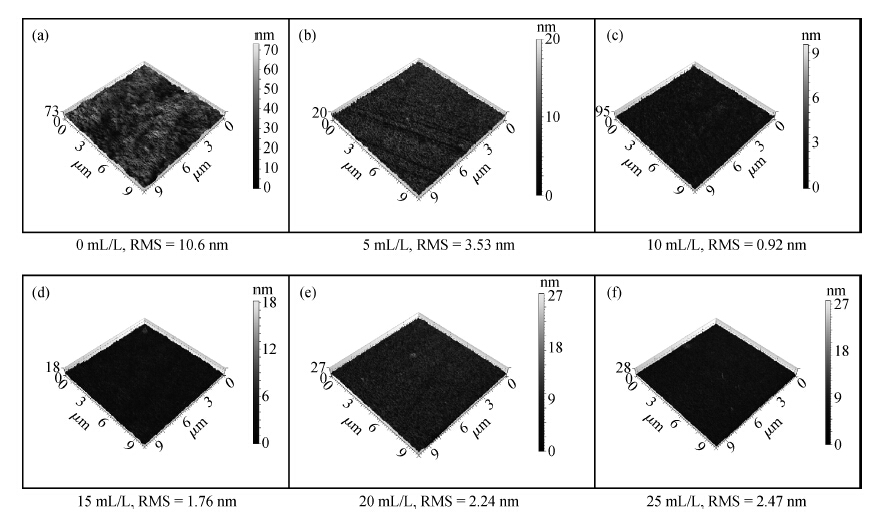
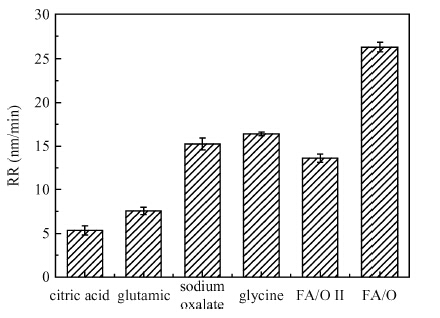
This paper investigated the effect of FA/O and hydrogen peroxide (H2O2) on ruthenium (Ru) removal rate (RR) and static etching rate (SER). It was revealed that Ru RR and SER first linearly increased then slowly decreaseed with the increasing H2O2 probably due to the formation of uniform Ru oxides on the surface during polishing. Their corrosion behaviors and states of surface oxidation were analyzed. In addition, FA/O could chelate Ru oxides (such as (RuO4)2- and RuO4- changed into soluble amine salts [R(NH3)4] (RuO4)2) and enhance Ru RR. The non-ionic surfactant AD was used to improve the Ru CMP performance. In particular, the addition of AD can lead to significant improvement of the surface roughness. -
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] -
Proportional views





 DownLoad:
DownLoad: