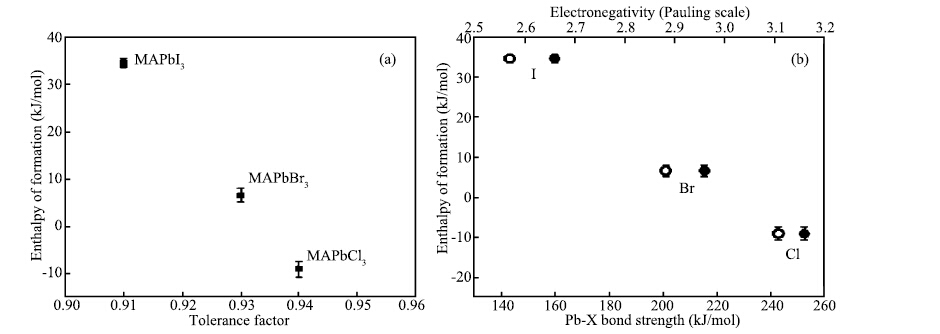
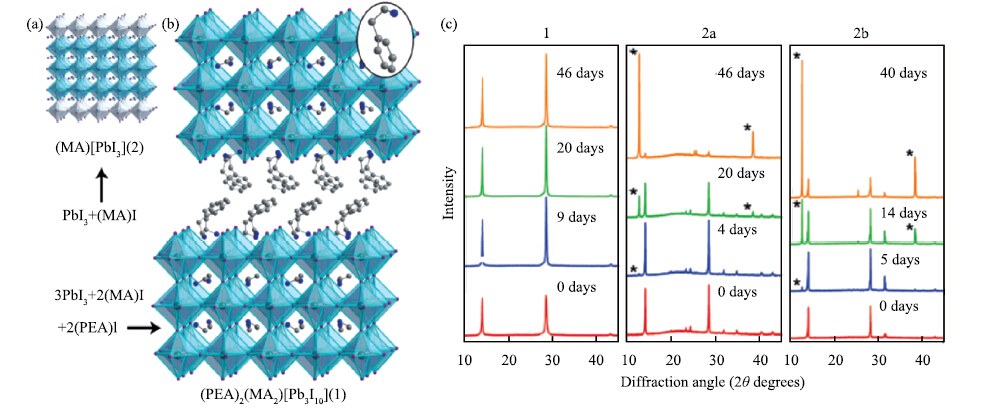
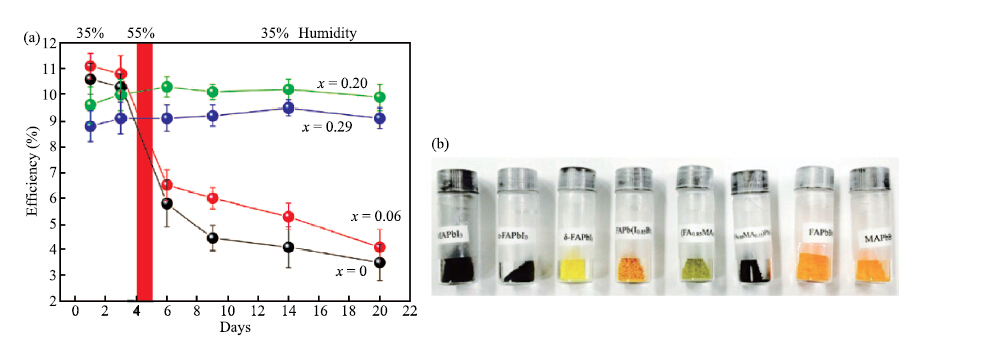
| Citation: |
Xiaojun Qin, Zhiguo Zhao, Yidan Wang, Junbo Wu, Qi Jiang, Jingbi You. Recent progress in stability of perovskite solar cells[J]. Journal of Semiconductors, 2017, 38(1): 011002. doi: 10.1088/1674-4926/38/1/011002
****
X J Qin, Z G Zhao, Y D Wang, J B Wu, Q Jiang, J B You. Recent progress in stability of perovskite solar cells[J]. J. Semicond., 2017, 38(1): 011002. doi: 10.1088/1674-4926/38/1/011002.
|
Recent progress in stability of perovskite solar cells
DOI: 10.1088/1674-4926/38/1/011002
More Information
-
Abstract
Perovskite solar cells have attracted significant attention in just the past few years in solar cell research fields, where the power conversion efficiency was beyond 22. 1%. Now, the most important challenge for perovskite solar cells in practical applications is the stability issue. In this mini-review, we will summarize the degradation mechanism of perovskite solar cells, including the perovskite material itself and also the interfaces. While we also provide our opinion on improving the stability of perovskite solar cells.-
Keywords:
- perovskite,
- solar cells,
- stability
-
References
[1] Kojima A, Teshima K, Shirai Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc, 2009, 131: 6050 doi: 10.1021/ja809598r[2] Kim H S, Lee C R, Im J H, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci Rep, 2012, 2: 591 https://www.researchgate.net/profile/Jacques-E_Moser/publication/230716542_Lead_iodide_perovskite_sensitized_all-solid-state_submicron_thin_film_mesoscopic_solar_cell_with_efficiency_exceeding_9/links/09e41506f09cb81b07000000.pdf[3] Lee M M, Teuscher J, Miyasaka T, et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science, 2012, 338: 643 doi: 10.1126/science.1228604[4] Burschka J, Pellet N, Moon S J, et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature, 2013, 499: 316 doi: 10.1038/nature12340[5] Jeon N J, Noh J H, Kim Y C, et al. Solvent engineering for highperformance inorganic-organic hybrid perovskite solar cells. Nat Mater, 2014, 13: 897 doi: 10.1038/nmat4014[6] Jeon N J, Noh J H, Yang W S, et al. Compositional engineering of perovskite materials for high-performance solar cells. Nature, 2015, 517: 476 doi: 10.1038/nature14133[7] Yang W S, Noh J H, Jeon N J, et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science, 2015, 348: 1234 doi: 10.1126/science.aaa9272[8] National renewable energy laboratory best research-cell efficiencies. www.nrel.gov/ncpv/images/efficiency_chart.jpg, 2016[9] Christians J A, Herrera P, Kamat P V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J Am Chem Soc, 2015, 137: 1530 doi: 10.1021/ja511132a[10] Habisreutinger S N, Leijtens T, Eperon E, et al. Carbon nanotube/polymer composites as a highly stable hole collection layer in perovskite solar cells. Nano Lett, 2014, 14: 5561 doi: 10.1021/nl501982b[11] Supasai T, Rujisamphan N, Ullrich K, et al. Formation of a passivating CH3NH3PbI3/PbI2 interface during moderate heating of CH3NH3PbI3 layers. Appl Phys Lett, 2013, 103: 183906 doi: 10.1063/1.4826116[12] Conings B, Drijkoningen J, Gauquelin N, et al. Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv Energy Mater, 2015, 5: 1500477 doi: 10.1002/aenm.201500477[13] Chen Q, Zhou H P, Song T B, et al. Controllable self-induced passivation of hybrid lead iodide perovskites toward high perfor mance solar cells. Nano Lett, 2014, 14: 4158 doi: 10.1021/nl501838y[14] Azpiroz J M, Mosconi E, Bisquert J, et al. Defect migration in methylammonium lead iodide and its role in perovskite solar cell operation. Energy Environ Sci, 2015, 8: 2118 doi: 10.1039/C5EE01265A[15] Berhe T A, Su W N, Chen C H, et al. Organometal halide perovskite solar cells: degradation and stability. Energy Environ Sci, 2016, 9: 323 doi: 10.1039/C5EE02733K[16] You J B, Meng L, Song T B, et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat Nanotech, 2016, 11: 75 http://cn.bing.com/academic/profile?id=cfa525fe83a9f619904826ac20a799fb&encoded=0&v=paper_preview&mkt=zh-cn[17] Kim J H, Liang P W, Williams S T, et al. High-performance and environmentally stable planar heterojunction perovskite solar cells based on a solution-processed copper-doped nickel oxide hole-transporting layer. Adv Mater, 2015, 27: 695 doi: 10.1002/adma.201404189[18] Ono L K, Raga S R, Remeika M, et al. Pinhole-free hole transport layers significantly improve the stability of MAPbI3-based perovskite solar cells under operating conditions. J Mater Chem A, 2015, 3: 15451 doi: 10.1039/C5TA03443D[19] Leijtens T, Eperon G E, Pathak S, et al. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat Commun, 2013, 4: 2885 https://www.researchgate.net/profile/Antonio_Abate/publication/259154549_Overcoming_ultraviolet_light_instability_of_sensitized_TiO2_with_meso-superstructured_organometal_tri-halide_perovskite_solar_cells/links/0a85e52e256d97db57000000.pdf?origin=publication_list[20] Nagabhushana G P, Shivaramaiah R, Navrotsky A. Direct calorimetric verification of thermodynamic instability of lead halide hybrid perovskites. PNAS, 2016, 113: 7717 doi: 10.1073/pnas.1607850113[21] Eperon G E, Stranks S D, Menelaou C, et al. Formamidinium lead trihalide: a broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ Sci, 2014, 7: 982 doi: 10.1039/c3ee43822h[22] Li Z, Yang M J, Park J S, et al. Stabilizing perovskite structures by tuning tolerance factor: formation of formamidinium and cesium lead iodide solid-state alloys. Chem Mater, 2016, 28: 284 doi: 10.1021/acs.chemmater.5b04107[23] Lee J W, Kim D H, Kim H S, et al. Formamidinium and cesium hybridization for photo- and moisture-stable perovskite solar cell. Adv Energy Mater, 2015, 5: 1501310 doi: 10.1002/aenm.201501310[24] Saliba M, Matsui T, Seo J Y, et al. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ Sci, 2016, 9: 1989 doi: 10.1039/C5EE03874J[25] Saliba M, Matsui T, Domanski K, et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science, 2016, 354: 206 doi: 10.1126/science.aah5557[26] Choi H, Jeong J, Kim H B, et al. Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells. Nano Energy, 2014, 7: 80 doi: 10.1016/j.nanoen.2014.04.017[27] Mei A, Li X, Liu L, et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science, 2014, 345: 295 doi: 10.1126/science.1254763[28] Smith I C, Hoke E T, Solis-Ibarra D. A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew Chem, 2014, 126: 11414 doi: 10.1002/ange.201406466[29] Quan L N, Yuan M J, Comin R, et al. Ligand-stabilized reduceddimensionality perovskites. J Am Chem Soc, 2016, 138: 2649 doi: 10.1021/jacs.5b11740[30] Tsai H, Nie W Y, Jean-Christophe B, et al. High-efficiency twodimensional Ruddlesden-Popper perovskite solar cells. Nature, 2016, 536: 312 doi: 10.1038/nature18306[31] Colella S, Mosconi E, Fedeli P, et al. MAPbI3-xClx mixed halide perovskite for hybrid solar cells: the role of chloride as dopant on the transport and structural properties. Chem Mater, 2013, 25: 4613 doi: 10.1021/cm402919x[32] You J, Hong Z, Yang Y, et al. Low-temperature solutionprocessed perovskite solar cells with high efficiency and flexibility. ACS Nano, 2014, 8: 1674 doi: 10.1021/nn406020d[33] Noh J H, Im S H, Heo J H, et al. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett, 2013, 13: 1764 doi: 10.1021/nl400349b[34] Jeon N J, Noh J H, Yang W S, et al. Compositional engineering of perovskite materials for high-performance solar cells. Nature, 2015, 517: 476 doi: 10.1038/nature14133[35] Liu Y S, Hong Z R, Chen Q, et al. Perovskite solar cells employing dopant-free organic hole transport materials with tunable energy levels. Adv Mater, 2016, 28: 440 doi: 10.1002/adma.v28.3[36] Liu Y S, Chen Q, Duan H S, et al. A dopant-free organic hole transport material for efficient planar heterojunction perovskite solar cells. J Mater Chem A, 2015, 3: 11940 doi: 10.1039/C5TA02502H[37] Qin P, Tanaka S, Ito S, et al. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat Commun, 2014, 5: 3834 http://repository.kaust.edu.sa/kaust/handle/10754/597000[38] Christians J A, Fung R C M, Kamat P V. An inorganic hole conductor for organo-lead halide perovskite solar cells improved hole conductivity with copper iodide. J Am Chem Soc, 2014, 136: 758 doi: 10.1021/ja411014k[39] Jeng J Y, Chen K C, Chiang T Y, et al. Nickel oxide electrode interlayer in CH3NH3PbI3 perovskite/PCBM planarheterojunction hybrid solar cells. Adv Mater, 2014, 26: 4107 doi: 10.1002/adma.v26.24[40] Kim J H, Liang P W, Williams S T, et al. High-performance and environmentally stable planar heterojunction perovskite solar cells based on a solution-processed copper-doped nickel oxide hole-transporting layer. Adv Mater, 2015, 27: 695 doi: 10.1002/adma.201404189[41] Park J H, Seo J, Park S, et al. Efficient CH3NH3PbI3 perovskite solar cells employing nanostructured p-type NiO electrode formed by a pulsed laser deposition. Adv Mater, 2015, 27: 4013 doi: 10.1002/adma.201500523[42] Ye S, Sun W, Li Y, et al. CuSCN-based inverted planar perovskite solar cell with an average PCE of 15.6%. Nano Lett, 2015, 15: 3723 doi: 10.1021/acs.nanolett.5b00116[43] Liu D Y, Kelly T L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat Photonics, 2014, 8: 133 http://cn.bing.com/academic/profile?id=e544adea4a5a7d33adfd0597fb77b8d6&encoded=0&v=paper_preview&mkt=zh-cn[44] Dong Q, Shi Y T, Wang K, et al. Insight into perovskite solar cells based on SnO2 compact electron-selective layer. J Phys Chem C, 2015, 119: 10212 doi: 10.1021/acs.jpcc.5b00541?selectedTab=citation&volume=119[45] Song J, Zheng E, Bian J, et al. Low-temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells. J Mater Chem A, 2015, 3: 10837 doi: 10.1039/C5TA01207D[46] Li Y, Zhu J, Huang Y, et al. Mesoporous SnO2 nanoparticle films as electron transporting material in perovskite solar cells. RSC Adv, 2015, 5: 28424 doi: 10.1039/C5RA01540E[47] Ke W, Fang G J, Liu Q, et al. Low-temperature solutionprocessed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J Am Chem Soc, 2015;137: 6730 doi: 10.1021/jacs.5b01994[48] Baena J P C, Steier L, Tress W, et al. A highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ Sci, 2015, 8: 2928 doi: 10.1039/C5EE02608C[49] Jiang Q, Zhang L Q, Wang H L, et al. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat Energy, 2016, 1: 16117 doi: 10.1038/nenergy.2016.117[50] Chen W, Wu Y Z, Yue Y F, et al., Efficient and stable largearea perovskite solar cells with inorganic charge extraction layers. Science, 2015, 350: 944 doi: 10.1126/science.aad1015[51] Zhu Z, Bai Y, Liu X, et al., Enhanced efficiency and stability of inverted perovskite solar cells using highly crystalline SnO2 nanocrystals as the robust electron-transporting layer. Adv Mater, 2016, 28: 6478 doi: 10.1002/adma.201600619 -
Proportional views






 DownLoad:
DownLoad: