| Citation: |
Zohreh Shadrokh, Ahmad Yazdani, Hosein Eshghi. Preparation and characterization of sphere-like Cu2SnS3 nanoparticles and their dropcasted thin films[J]. Journal of Semiconductors, 2017, 38(1): 013001. doi: 10.1088/1674-4926/38/1/013001
****
Z Shadrokh, A Yazdani, H Eshghi. Preparation and characterization of sphere-like Cu2SnS3 nanoparticles and their dropcasted thin films[J]. J. Semicond., 2017, 38(1): 013001. doi: 10.1088/1674-4926/38/1/013001.
|
Preparation and characterization of sphere-like Cu2SnS3 nanoparticles and their dropcasted thin films
DOI: 10.1088/1674-4926/38/1/013001
More Information
-
Abstract
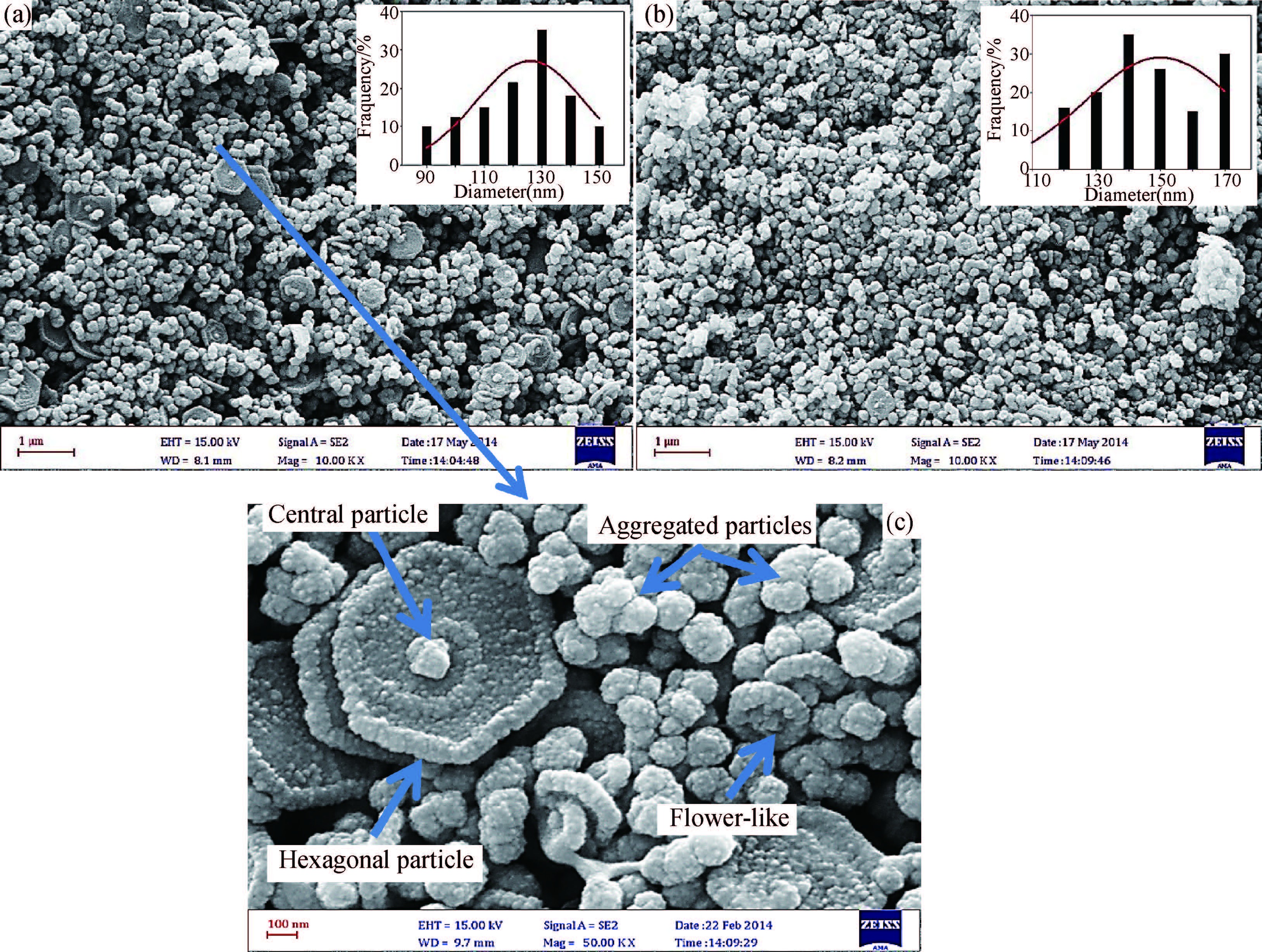
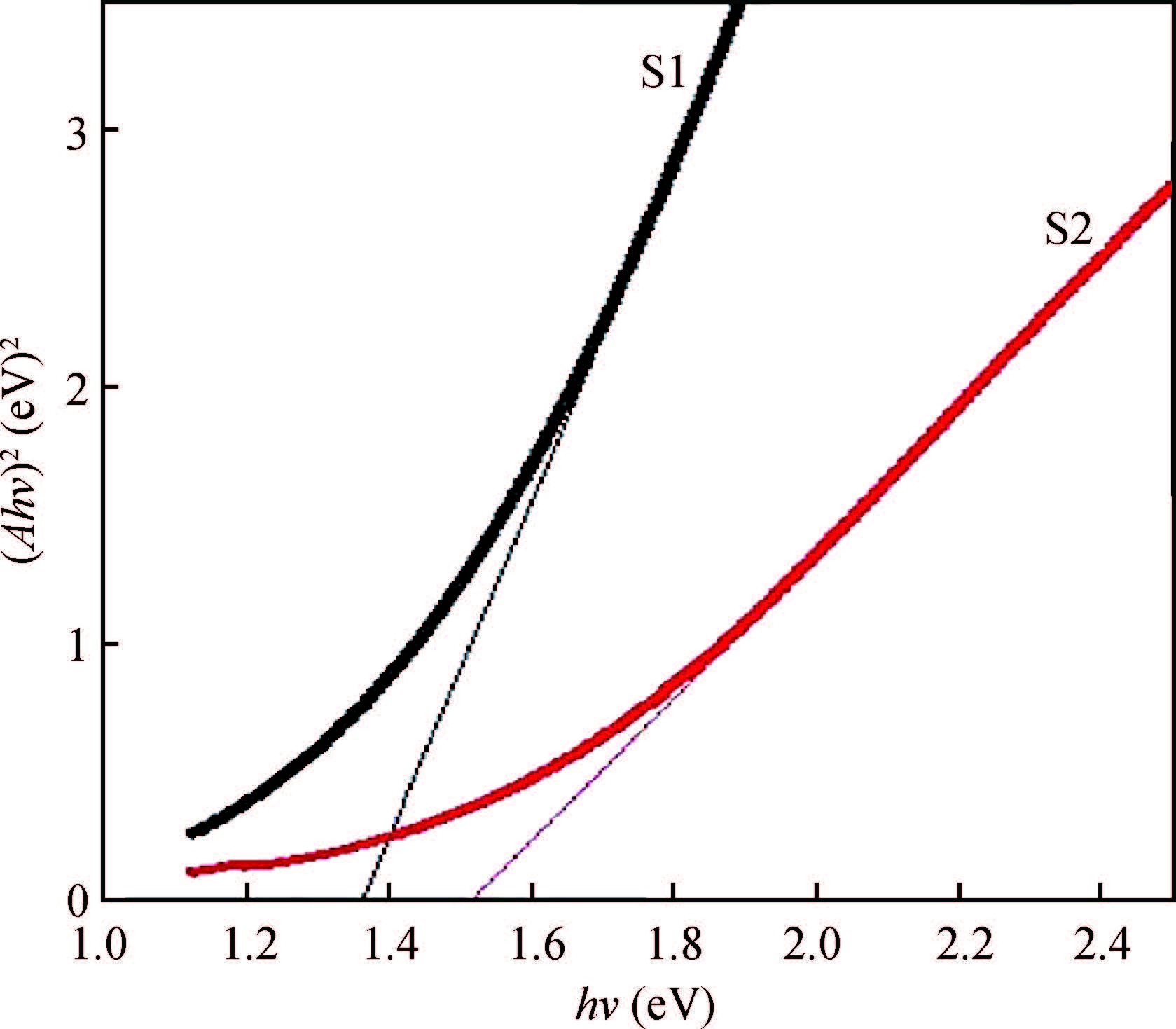
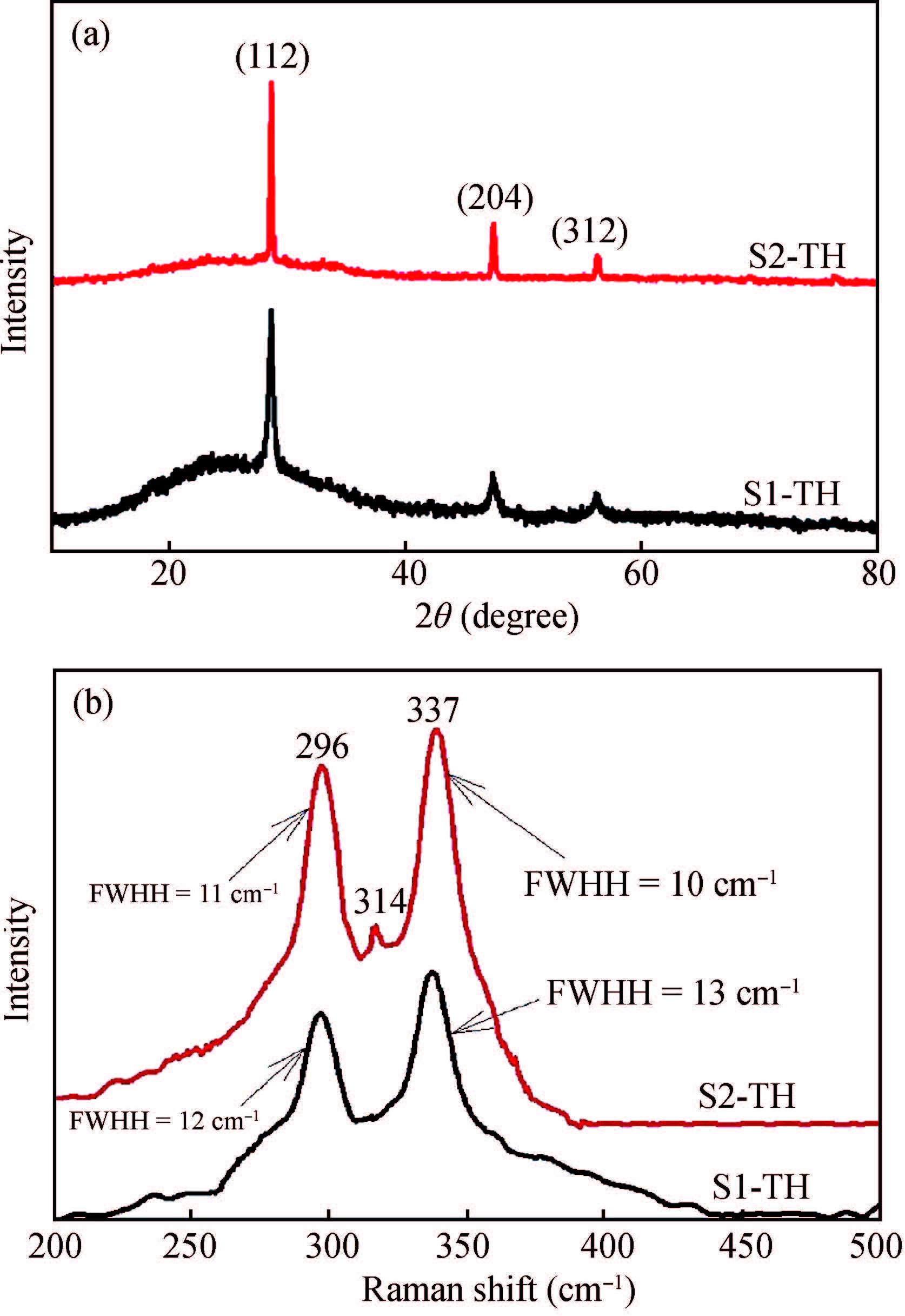
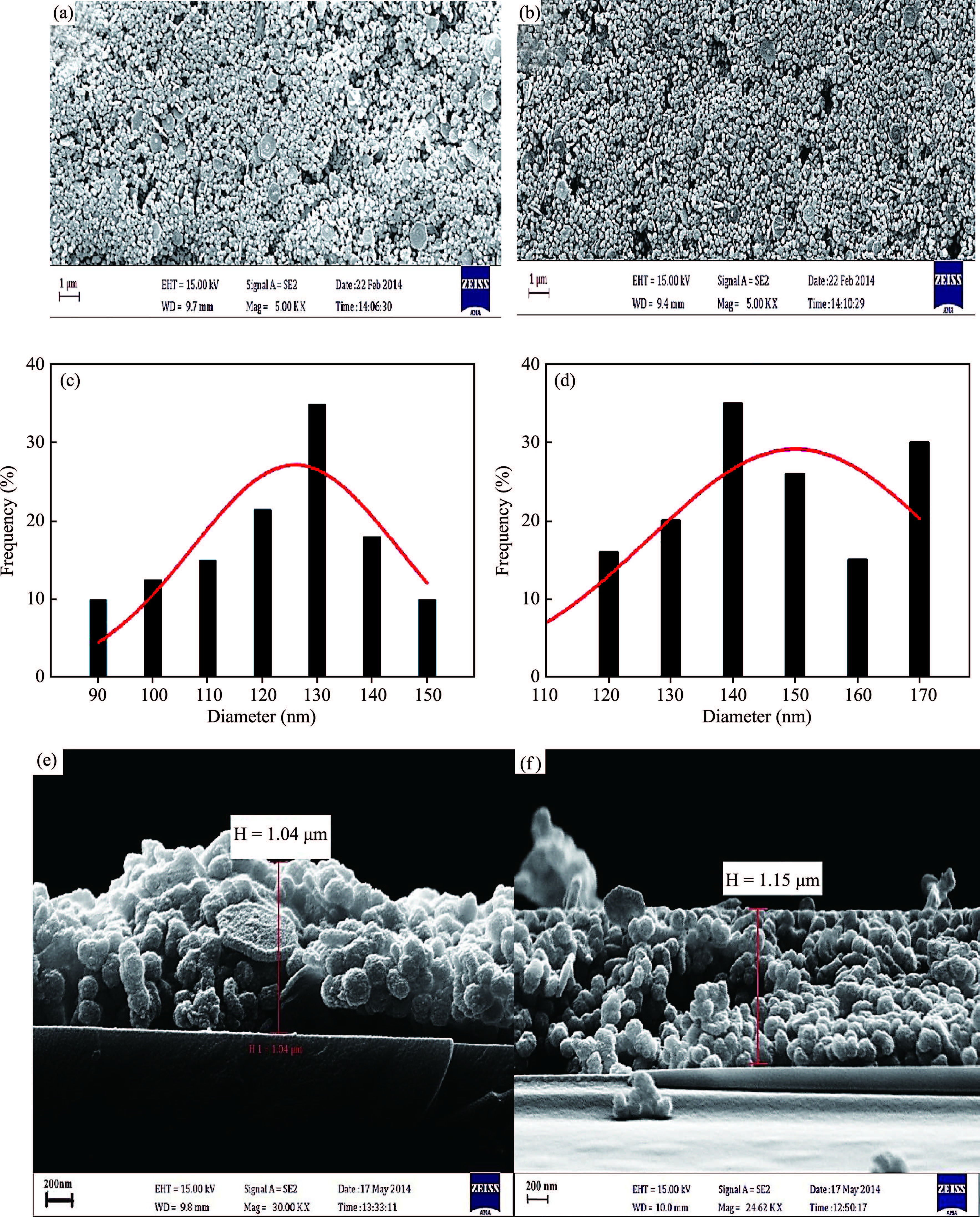
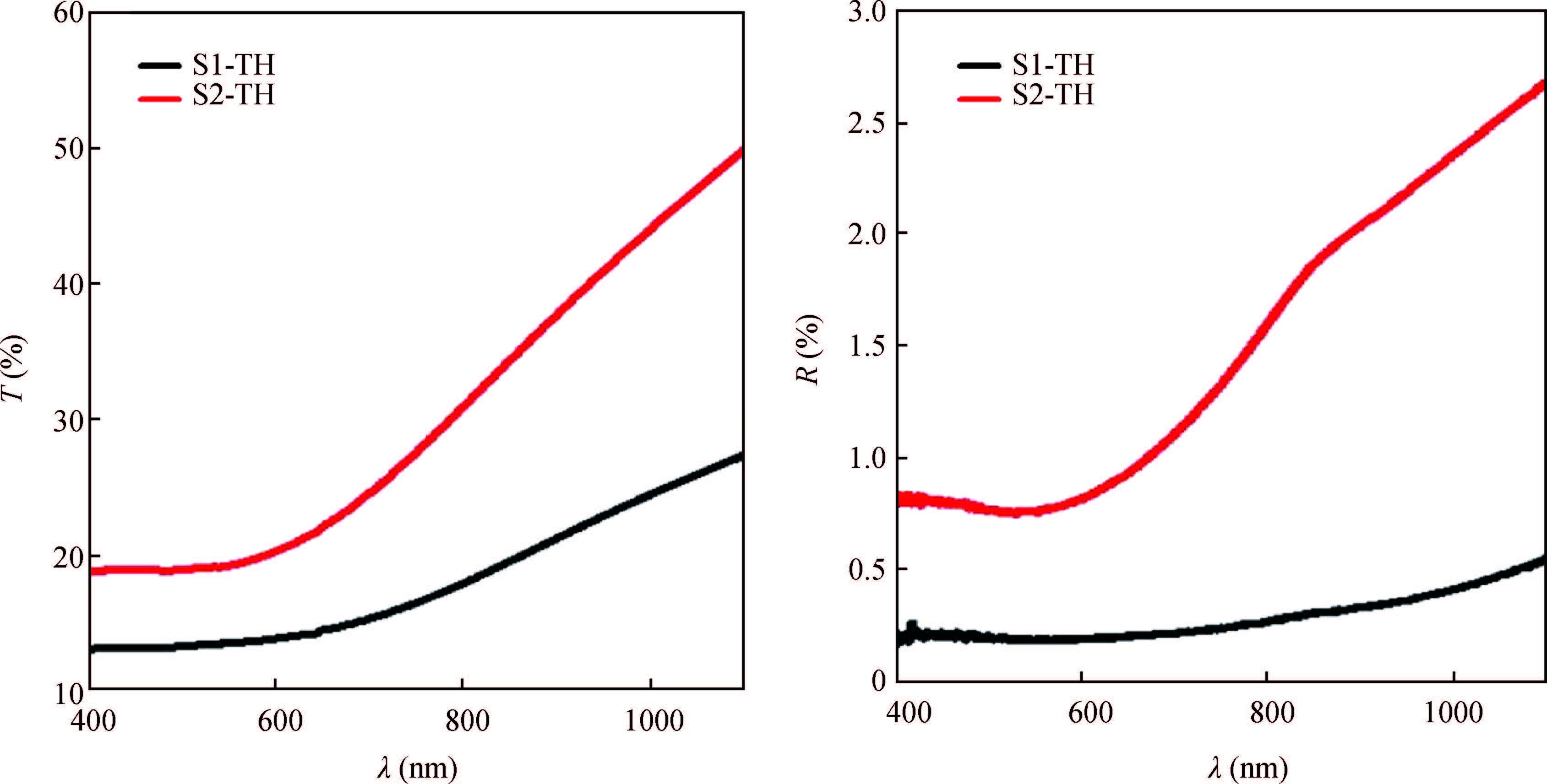
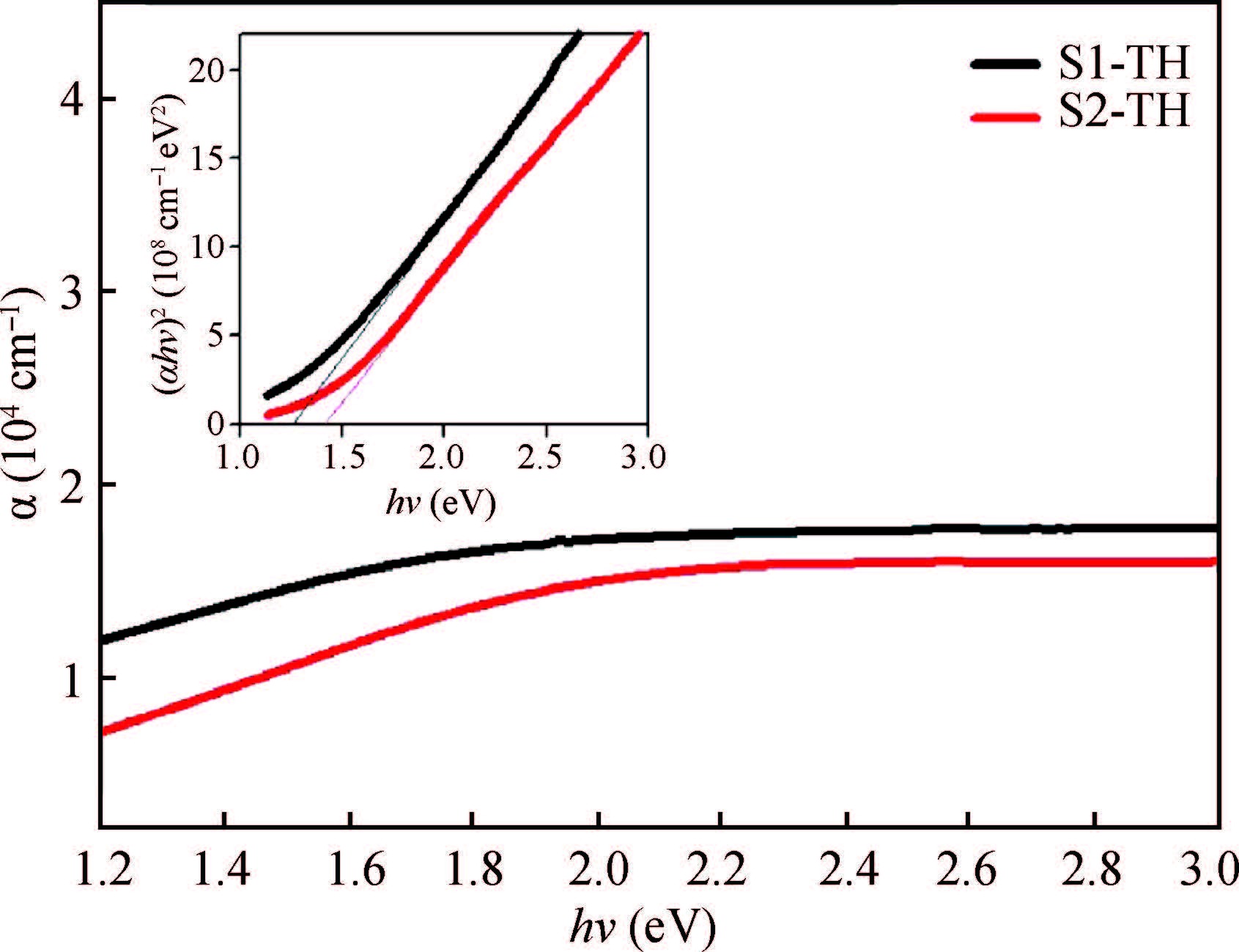
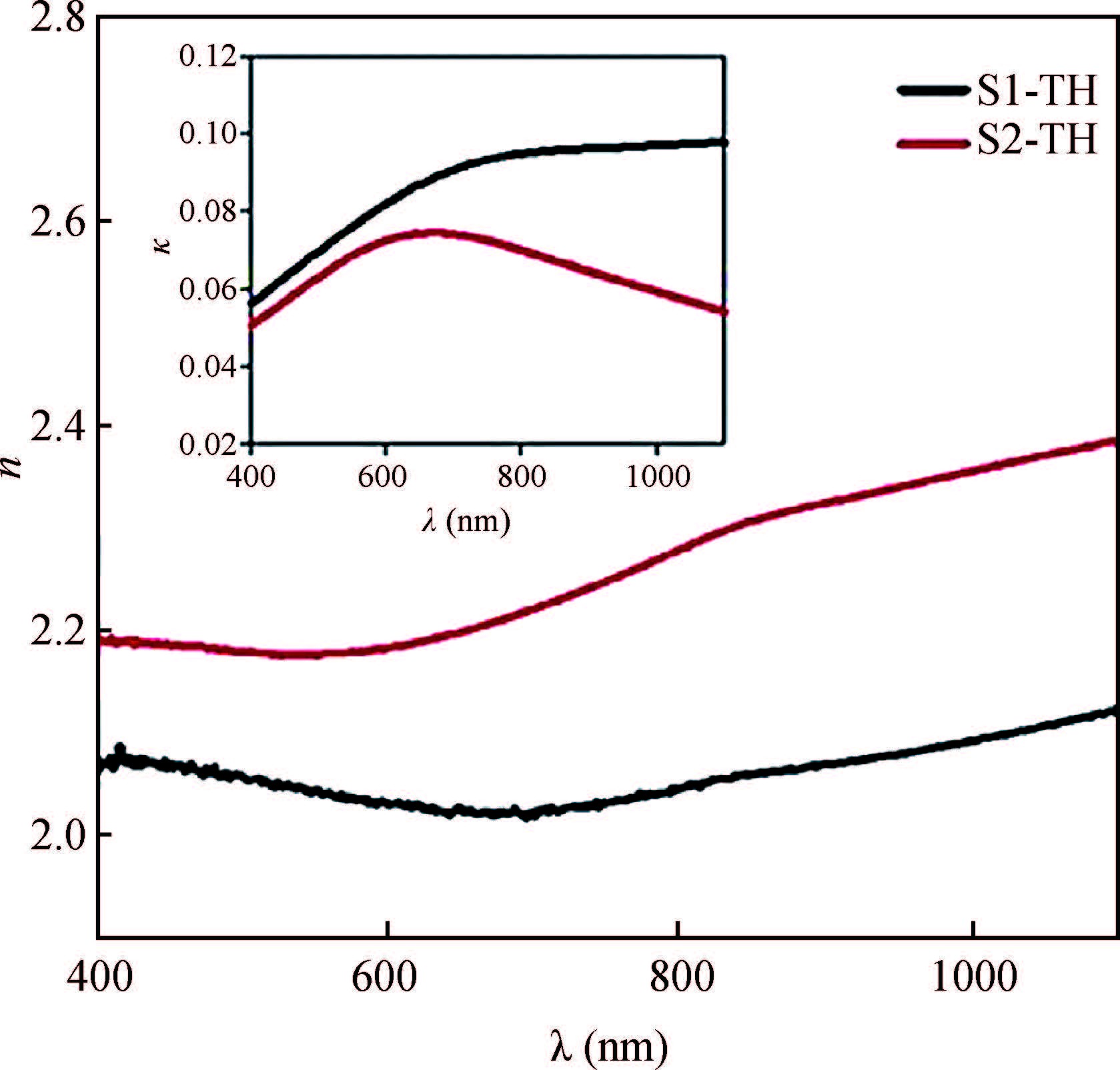
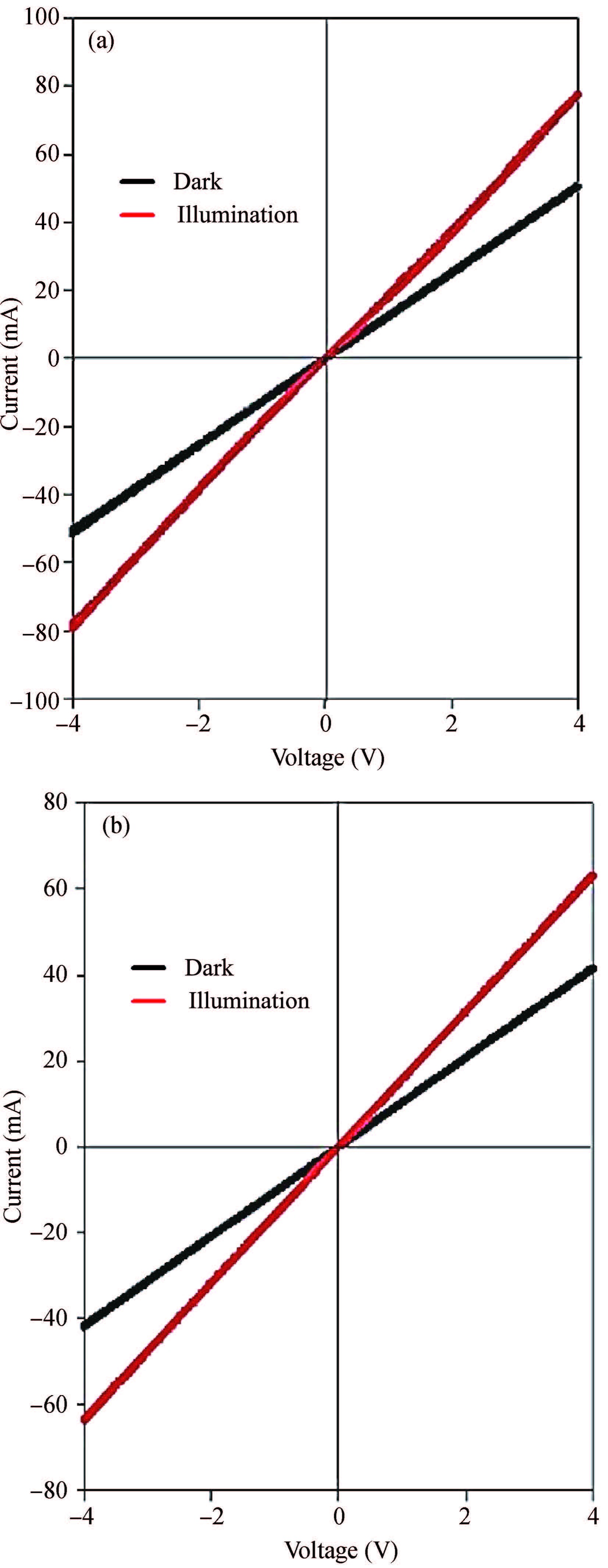
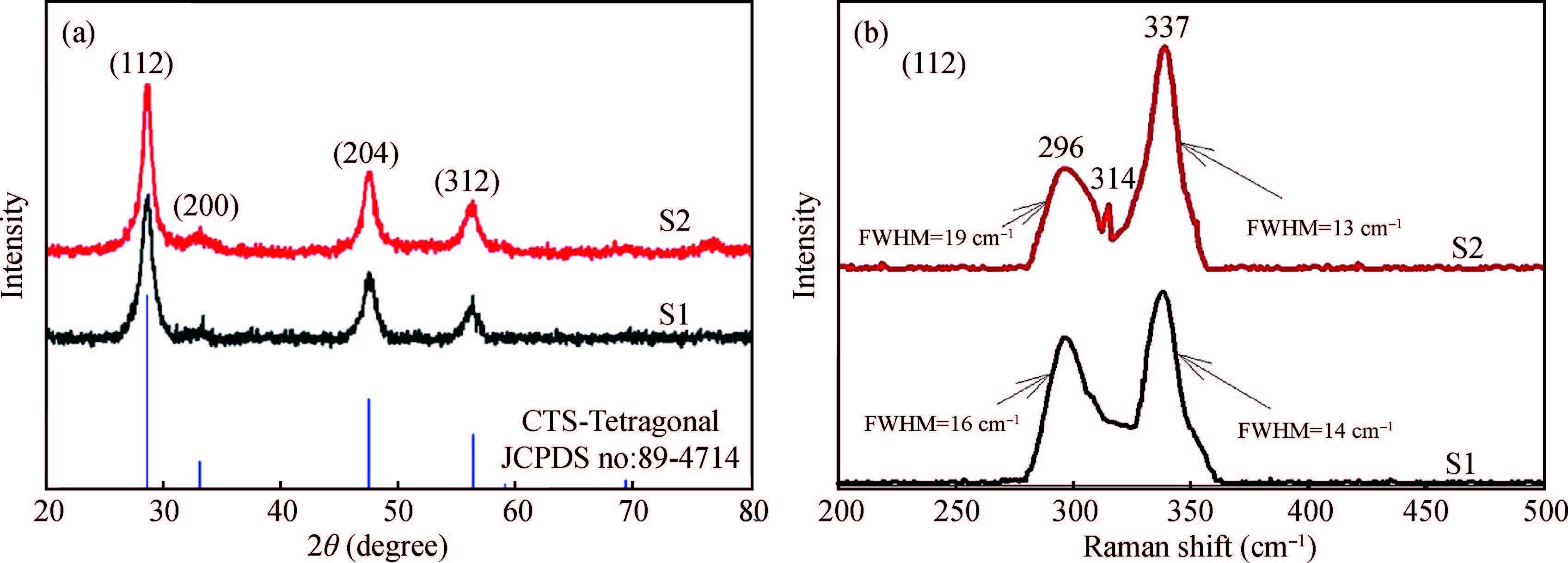
Ternary sphere-like Cu2SnS3(CTS) semiconductor and 2D hexagonal sheets were synthesized via a simple solvothermal method using PVP as the surface ligand at two temperatures of 180 and 220℃. The structural, morphological, and chemical compositions as well as optical properties of as-synthesized CTS particles were characterized using X-ray diffraction (XRD), Raman spectroscopy, energy dispersive X-ray spectrometry (EDS), field emission scanning electron microscopy (FESEM), and UV-Vis spectroscopy. The size of sphere-like particles and the side length of hexagonal sheets were within the range of 120-140 nm and 500 nm-2 μm, respectively. FESEM, XRD, and EDS were analyzed to investigate the mechanism of the morphological evolution of CTS particles. CTS particles showed proliferation of Sn atomic ratio, which is strongly sensitive to reaction temperature and, highly affects the increase of band gap energy from 1.36 to 1.53 eV due to generation metal defects and formation SnS2. The optical analysis via the transmittance and reflectance reveals that the band-gap energy of dropcasted CTS thin films decreases after annealing due to grain growth and change of chemical compositions. Photo-responses of CTS nanocrystal thin films indicated a considerable increase in the conductivity of the films under light illumination. All these results showed the potential of these films for solar cell applications. -
References
[1] Mitzi D B, Gunawan O, Todorov T K, et al. The path towards a high performance solution-processed kesterite solar cell. Sol Energy Mater Sol Cells, 2011, 95: 1421 doi: 10.1016/j.solmat.2010.11.028[2] Araki H, MikadukiA, Kubo Y, et al. Preparation of Cu2ZnSnS4 thin films by sulfurization of stacked metallic layers. Thin Solid Films, 2008, 517: 1457 doi: 10.1016/j.tsf.2008.09.058[3] Tiwari D, Chaudhuri T K, Shripathei T, et al. Non-toxic, earth-abundant 2% efficient Cu2SnS3 solar cell based on tetragonal films direct-coated from single metal-organic precursor solution. Sol Energy Mater Sol Cells, 2013, 113: 165 doi: 10.1016/j.solmat.2013.02.017[4] Guan H, Shen H, Gao C, et al. Structural and optical properties of Cu2SnS3 and Cu3SnS4 thin films by successive ionic layer adsorption and reaction. J Mater Sci: Mater Electron, 2013, 24: 1490 doi: 10.1007/s10854-012-0960-x[5] Kahraman S, çetinkaya S, Yasar S, et al. Polyethylene glycol-assisted growth of Cu2SnS3 promising absorbers for thin film solar cell applications. Philos Mag, 2014, 94: 3149 doi: 10.1080/14786435.2014.952257[6] Aihara N, Kanai A, et al. Sulfurization temperature dependences of photovoltaic properties in Cu2SnS3-based thin-film solar cells. Jpn J Appl Phys, 2014, 53: 05FW13[7] Chino K, Koike J, Kimura K, et al. Preparation of Cu2SnS3 thin films by sulfurization of Cu/Sn stacked precursors. Jpn J Appl Phys, 2012, 51: 10NC35[8] Qu B, Zhang M, Lei D, et al. Facile solvothermal synthesis of mesoporous Cu2SnS3 spheres and their application in lithium-ion batteries. Nanoscale, 2011, 3: 3646 doi: 10.1039/c1nr10401b[9] Qu B, Li H, Zhang M, et al. Ternary Cu2SnS3 cabbage-like nanostructures: large-scale synthesis and their application in Li-ion batteries with superior reversible capacity. Nanoscale, 2011, 3: 4389 doi: 10.1039/c1nr10784d[10] Fernandes P A, Salomé P M P, da Cunha A F, et al. Study of ternary Cu2SnS3 and Cu3SnS4 thin films prepared by sulfurizing stacked metal precursors. J Phys D, 2010, 43: 215403 doi: 10.1088/0022-3727/43/21/215403[11] Bouaziz M, Amlouk M, Belgacem S, et al. Structural and optical properties of Cu2SnS3 sprayed thin films. Thin Solid Films, 2009, 517: 2527 doi: 10.1016/j.tsf.2008.11.039[12] Berg D M, Djemour R, Gütaye L, et al. Thin film solar cells based on the ternary compound Cu2SnS3. Thin Solid Films, 2012, 520: 6291 doi: 10.1016/j.tsf.2012.05.085[13] Adelifard M, Mohagheghi M M B, Eshghi H. Preparation and characterization of Cu2SnS3 ternary semiconductor nanostructures via the spray pyrolysis technique for photovoltaic applications. Phys Scr, 2012, 85: 035603 doi: 10.1088/0031-8949/85/03/035603[14] Chen Q, Ma D. Preparation of nanostructured Cu2SnS3 photocatalysts by solvothermal method. Int J Photoenergy, 2013, 2013: 593420[15] Chen X, Wang X, An C, et al. Preparation and characterization of ternary Cu-Sn-E (E=S, Se) semiconductor nanocrystallites via a solvothermal element reaction route. J Cryst Growth, 2003, 256: 368 doi: 10.1016/S0022-0248(03)01338-1[16] Liang X, Cai Q, Xiang W, et al. Preparation and characterization of flower-like Cu2SnS3 nanostructures by solvothermal route. J Mater Sci Technol, 2013 29: 231 doi: 10.1016/j.jmst.2012.12.011[17] Chang J, Waclawik E R. Controlled synthesis of CuInS2, Cu2SnS3 and Cu2ZnSnS4 nano-structures: insight into the universal phase selectivity mechanism. Cryst Eng Comm, 2013, 15: 5577 doi: 10.1039/c3ce90095a[18] Wu C, Hu Z, Wang C, et al. Hexagonal Cu2SnS3 with metallic character: another category of conducting sulfides. Appl Phys Lett, 2007, 91: 143104 doi: 10.1063/1.2790491[19] Li Q, Ding Y, Liu X, et al. Preparation of ternary I-IV-VI nanocrystallines via a mild solution route. Mater Res Bull, 2001 36: 2649 doi: 10.1016/S0025-5408(01)00759-0[20] Xu J, Yang X, Wong T L, et al. Large-scale synthesis of Cu2SnS3 and Cu1.8S hierarchical microspheres as efficient counter electrode materials for quantum dot sensitized solar cells. Nanoscale, 2012, 4: 6537 doi: 10.1039/c2nr31724a[21] Singh J, Verma N K. Structural, optical and magnetic properties of cobalt-doped CdSe Nanoparticles. Bull Mater Sci, 2014, 37: 541 doi: 10.1007/s12034-014-0671-4[22] Deshpande M P, Chaki S H, Patel N H, et al. Study on nanoparticles of ZnSe synthesized by chemical method and their charactrization. J Nano-Electron Phys, 2011, 3: 193 http://jnep.sumdu.edu.ua/download/numbers/2011/1,%20Part%201/articles/jnep_2011_V3_N1(Part1)_193-202.pdf[23] Gonçalves N S, Carvalho J A, Lima Z M, et al. Size-strain study of NiO nanoparticles by X-ray powder diffraction line broadening. Mater Lett, 2012, 72: 36 doi: 10.1016/j.matlet.2011.12.046[24] Choudhury B, Choudhury A. Local structure modification and phase transformation of TiO2 nanoparticles initiated by oxygen defects, grain size, and annealing temperature. Int Nano Lett, 2013, 3: 55 doi: 10.1186/2228-5326-3-55[25] Gouadec G, Colomban P. Raman spectroscopy of nanomaterials: how spectra relate to disorder, particle size and mechanical properties. Prog Cryst Growth Charact Mater, 2007, 53: 1 doi: 10.1016/j.pcrysgrow.2007.01.001[26] Fernandesa P A, Salomé P M P, da Cunhae A F, et al. Study of polycrystalline Cu2ZnSnS4 films by Raman scattering. J Alloys Compd, 2011, 509: 7600 doi: 10.1016/j.jallcom.2011.04.097[27] Pearson R G. Hard and soft acids and bases. J Am Chem Soc,1963, 85: 3533 doi: 10.1021/ja00905a001[28] Shadrokh Z, Eshghi H, Yazdani A. Investigating the effects of temperature and metal ion ratio on physical and optical properties of Cu2ZnSnS4 nanoparticles and thin films. Mater Sci Semicond Process, 2015, 40: 752 doi: 10.1016/j.mssp.2015.06.082[29] Jia Z, Chen Q, Chen J, et al. The photovoltaic properties of novel narrow band gap Cu2SnS3 film prepared by spray pyrolysis method. RSC Adv, 2015, 5: 28885 doi: 10.1039/C5RA01610J[30] Singh J. Optical properties of condensed matter and applications. John Wiley & Sons Ltd, 2006 http://cn.bing.com/academic/profile?id=2264287b9dde57b8554814acb6a1cea5&encoded=0&v=paper_preview&mkt=zh-cn[31] Belgacem S, Bennaceur R. Propriétés optiques des couches minces de SnO2 et CuInS2 airless spray. Revue de Physique Appliquée, 1990, 25: 1245 doi: 10.1051/rphysap:0199000250120124500[32] Aissa Z, Amlouk M, Ben Nasrallahe T, et al. Effect of S/In concentration ratio on the physical properties of AgInS2-sprayed thin films. Sol Energy Mater Sol Cells, 2007, 91: 489 doi: 10.1016/j.solmat.2006.10.022[33] Lu X, Zhuang Z, Peng Q, et al. Wurtzite Cu2ZnSnS4 nanocrystals: a novel quaternary semiconductor. Chem Commun, 2011, 47: 3141 doi: 10.1039/c0cc05064d[34] Cao M, Li C, Zhang B, et al. PVP assisted solvothermal synthesis of uniform Cu2FeSnS4 nanospheres. J Alloys Compd, 2015, 622: 695 doi: 10.1016/j.jallcom.2014.10.164 -
Proportional views





 DownLoad:
DownLoad: