| Citation: |
Khizar-ul Haq, M. Irfan, Muhammad Masood, Murtaza Saleem, Tahir Iqbal, Ishaq Ahmad, M. A. Khan, M. Zaffar, Muhammad Irfan. Enhanced room temperature ferromagnetism in Cr-doped ZnO nanoparticles prepared by auto-combustion method[J]. Journal of Semiconductors, 2018, 39(4): 043001. doi: 10.1088/1674-4926/39/4/043001
****
K Haq, M Irfan, M Masood, M Saleem, T Iqbal, I Ahmad, M A Khan, M Zaffar, M Irfan. Enhanced room temperature ferromagnetism in Cr-doped ZnO nanoparticles prepared by auto-combustion method[J]. J. Semicond., 2018, 39(4): 043001. doi: 10.1088/1674-4926/39/4/043001.
|
Enhanced room temperature ferromagnetism in Cr-doped ZnO nanoparticles prepared by auto-combustion method
DOI: 10.1088/1674-4926/39/4/043001
More Information
-
Abstract
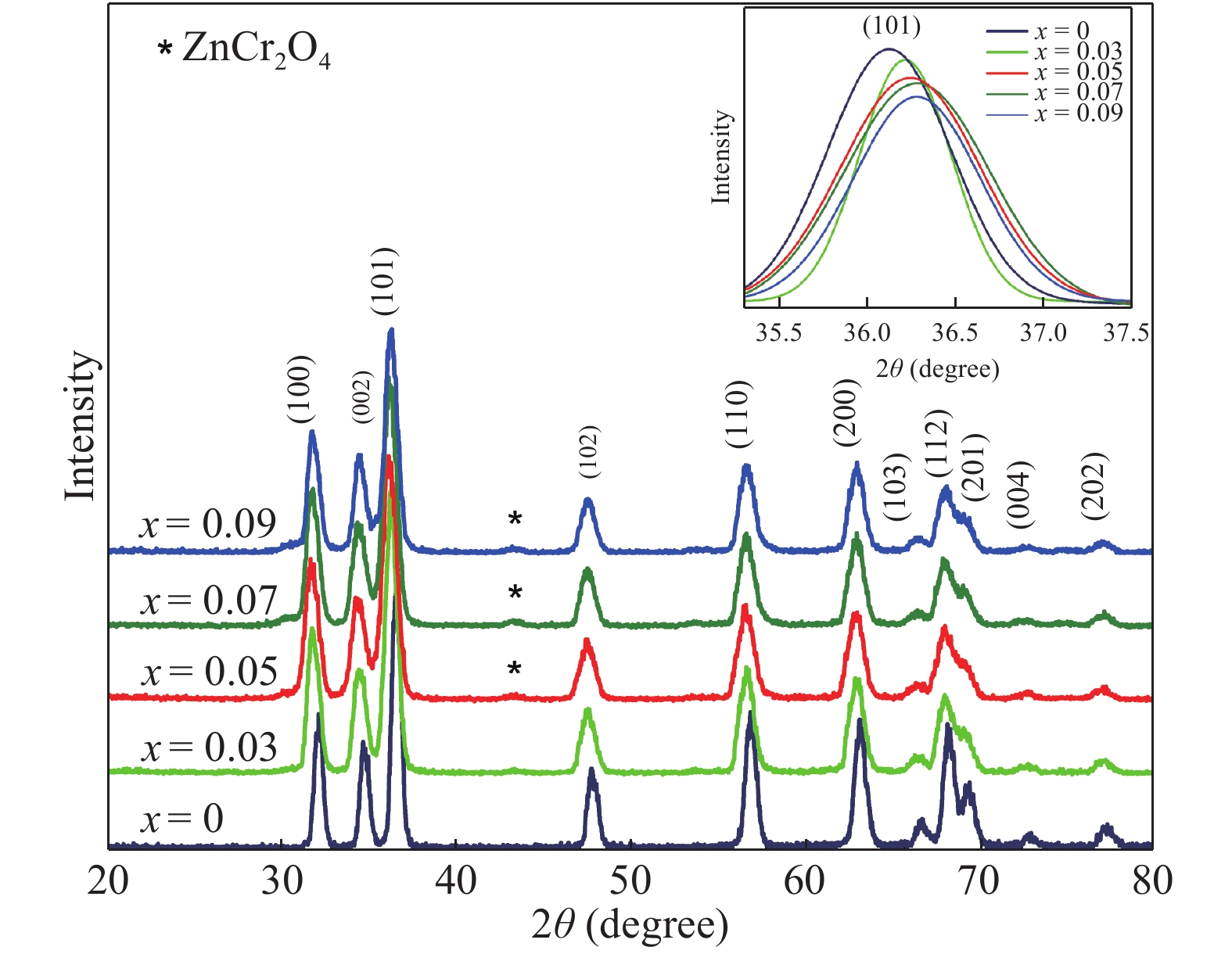
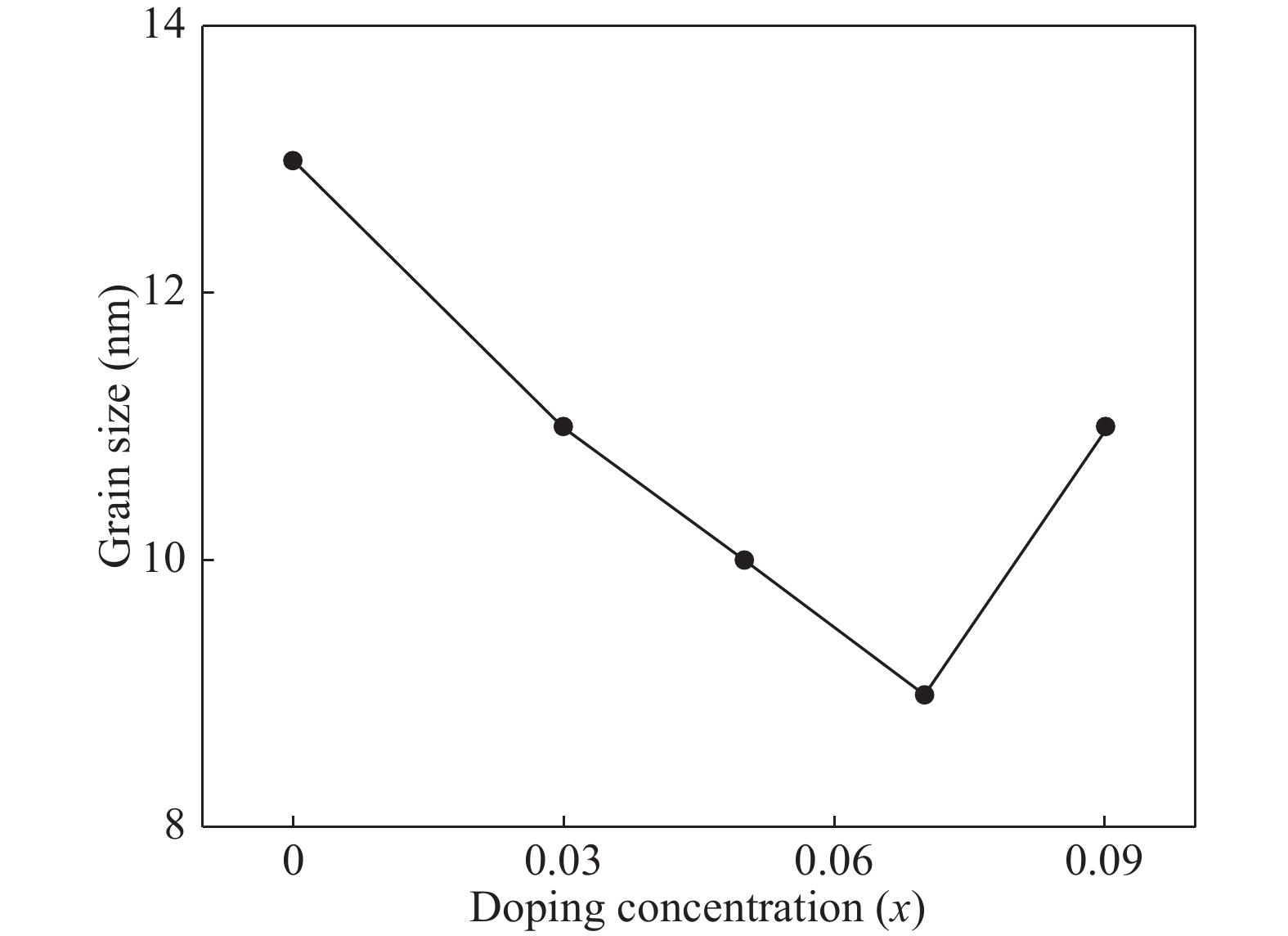
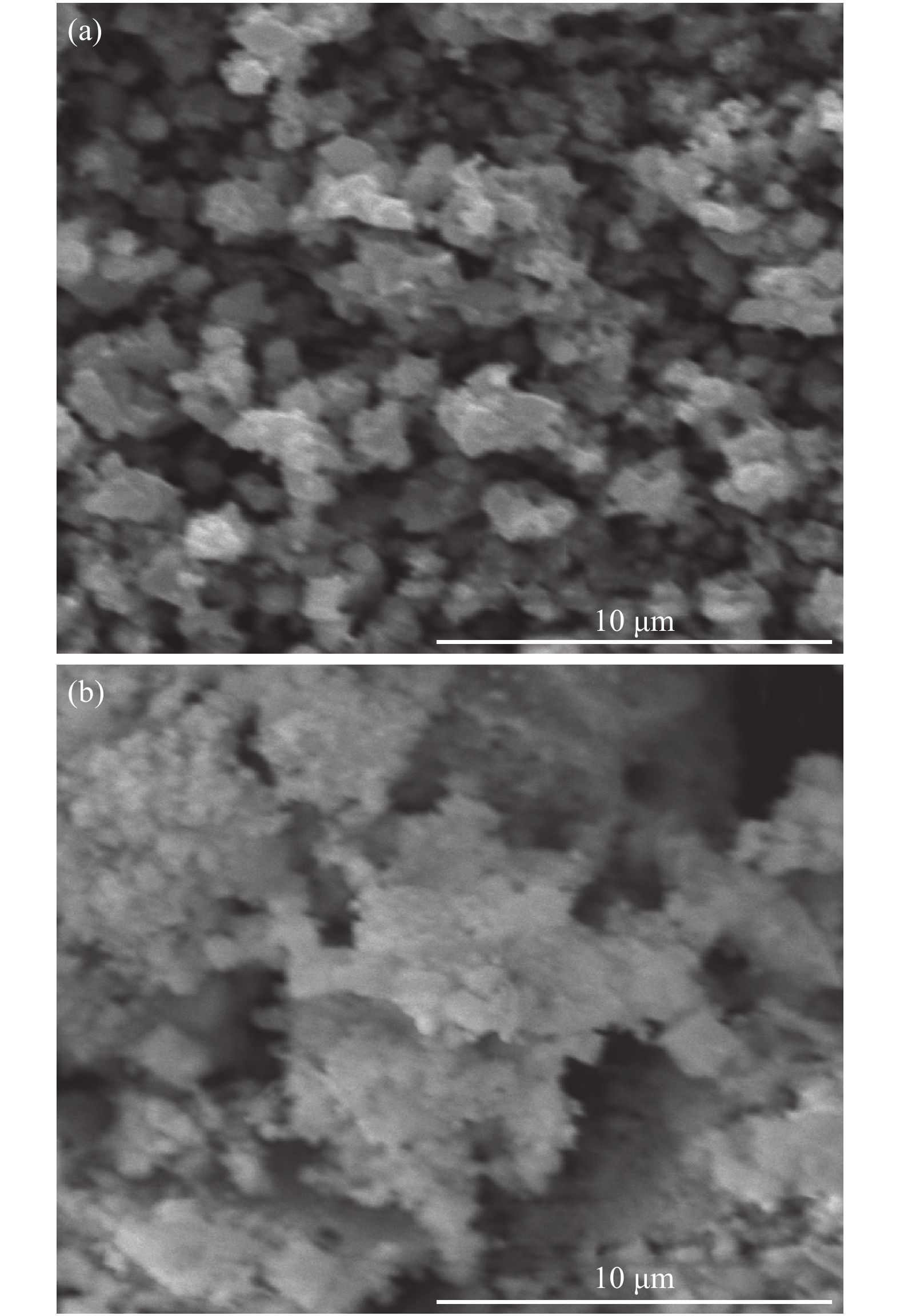
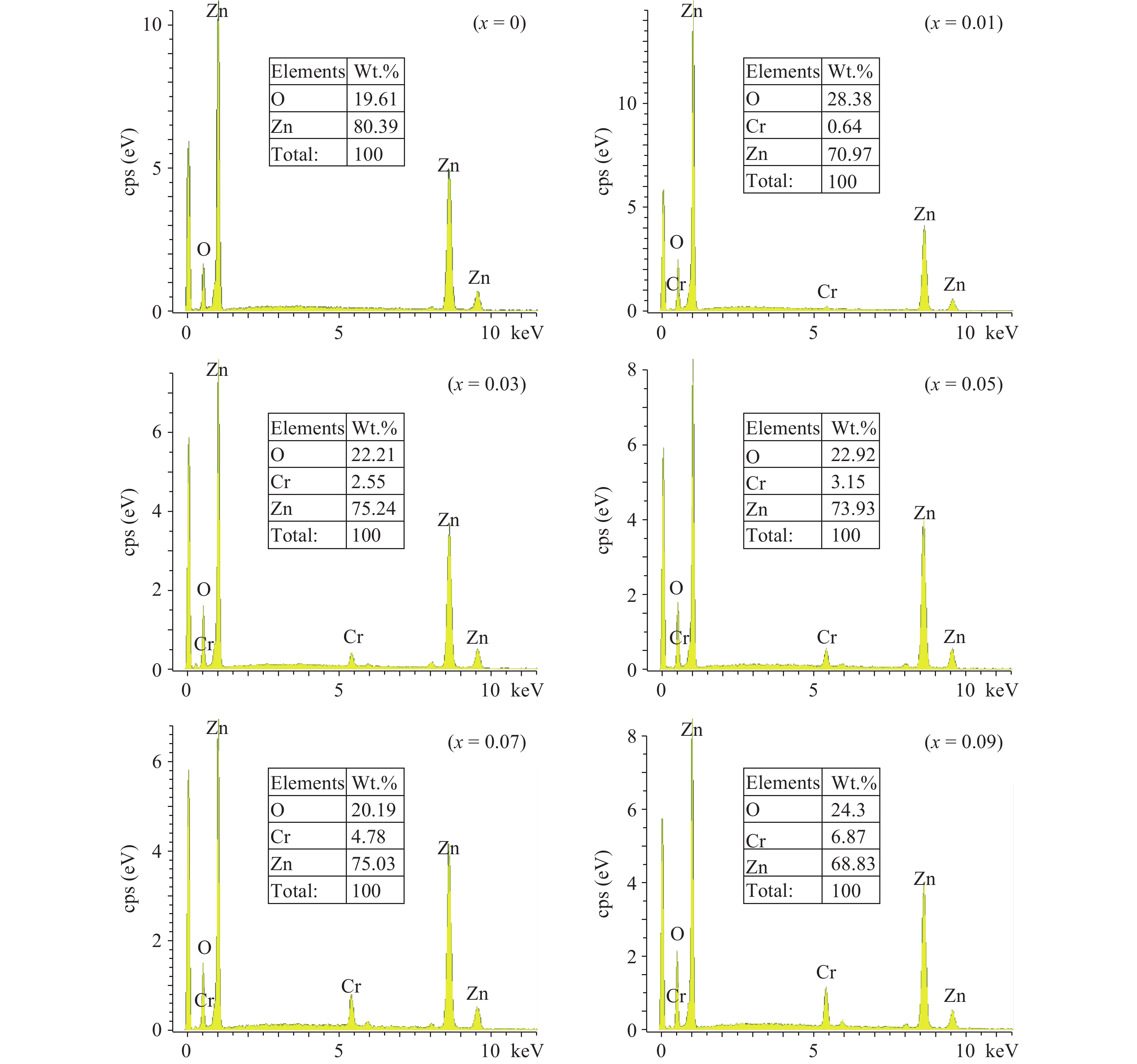
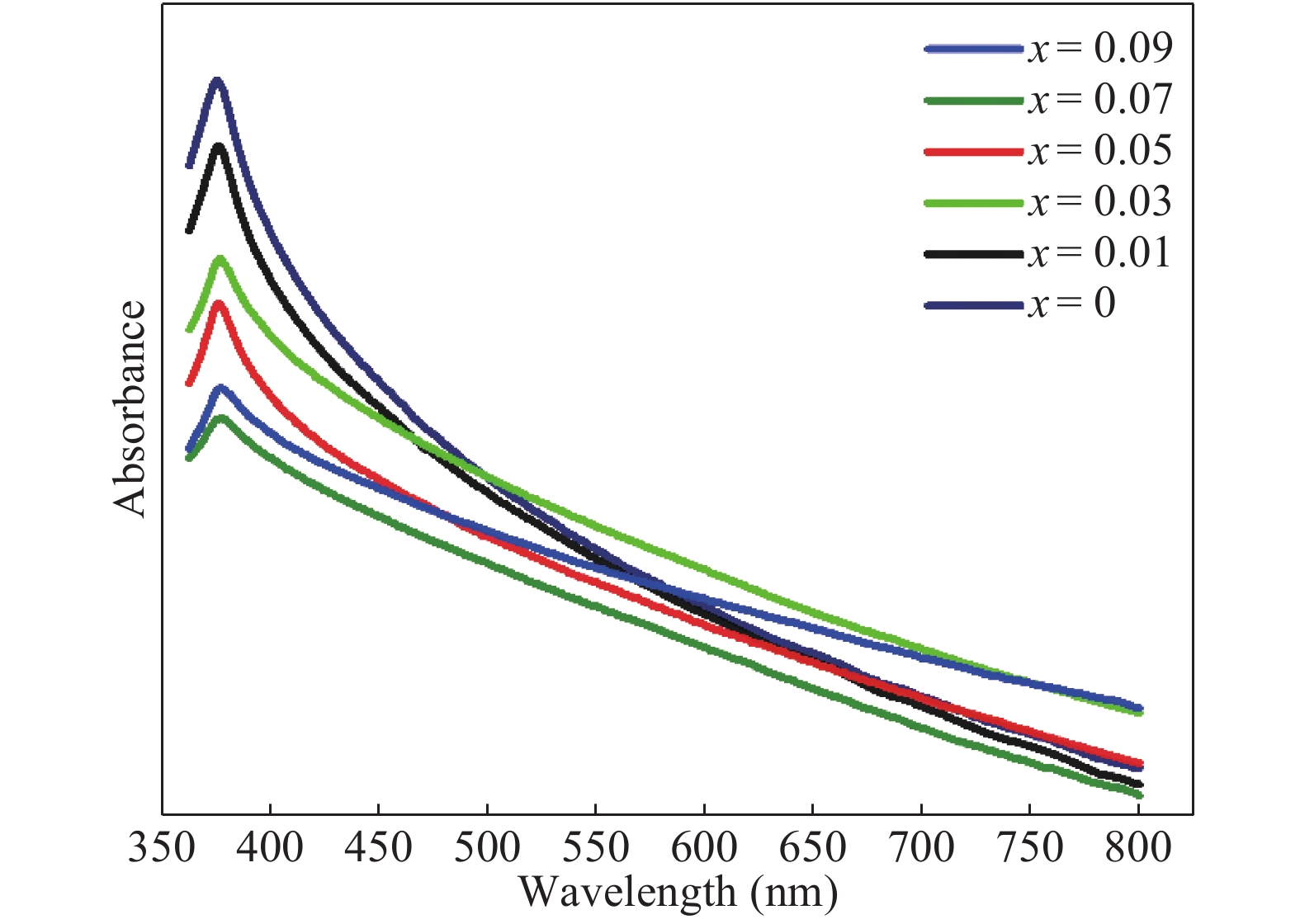
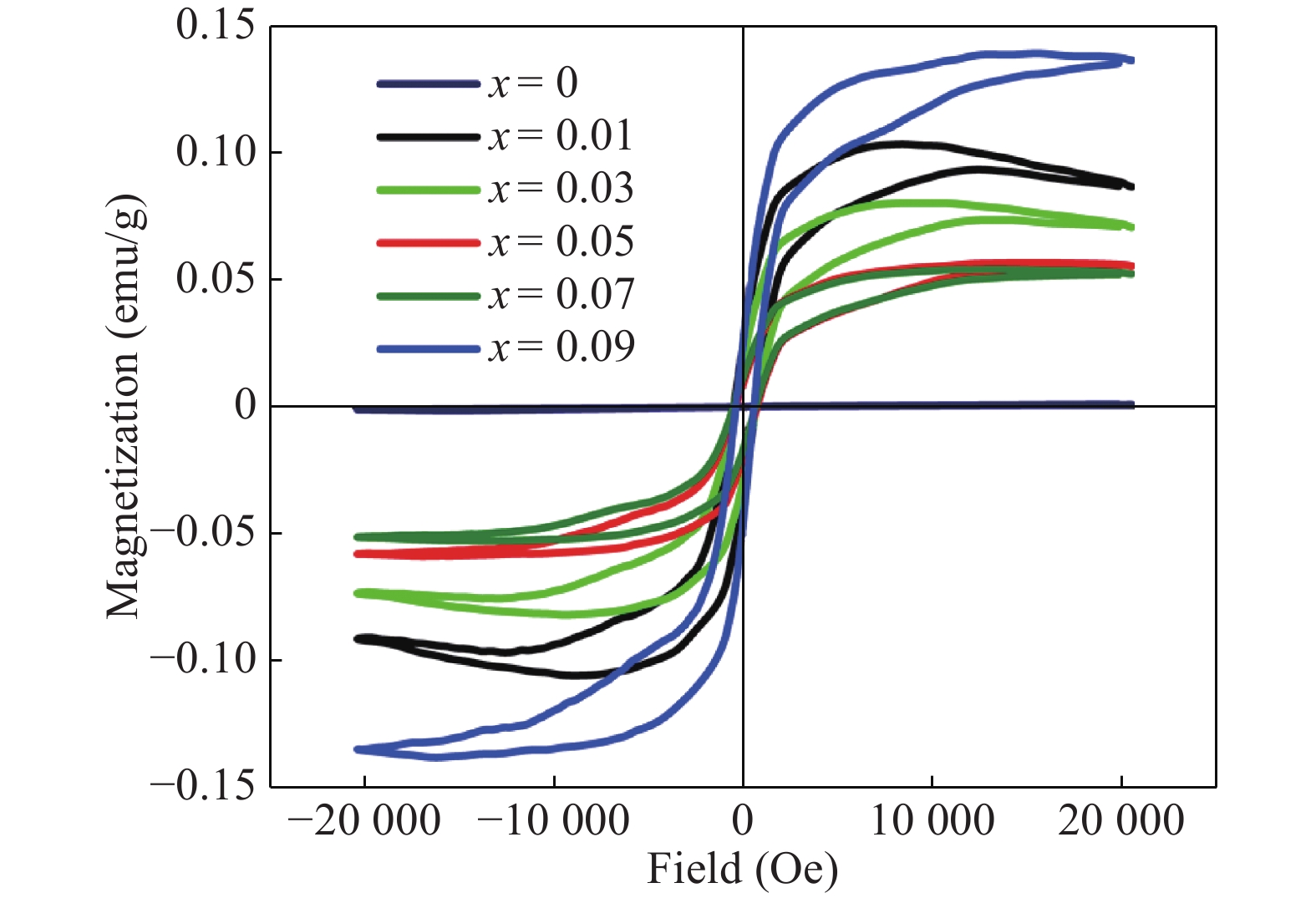
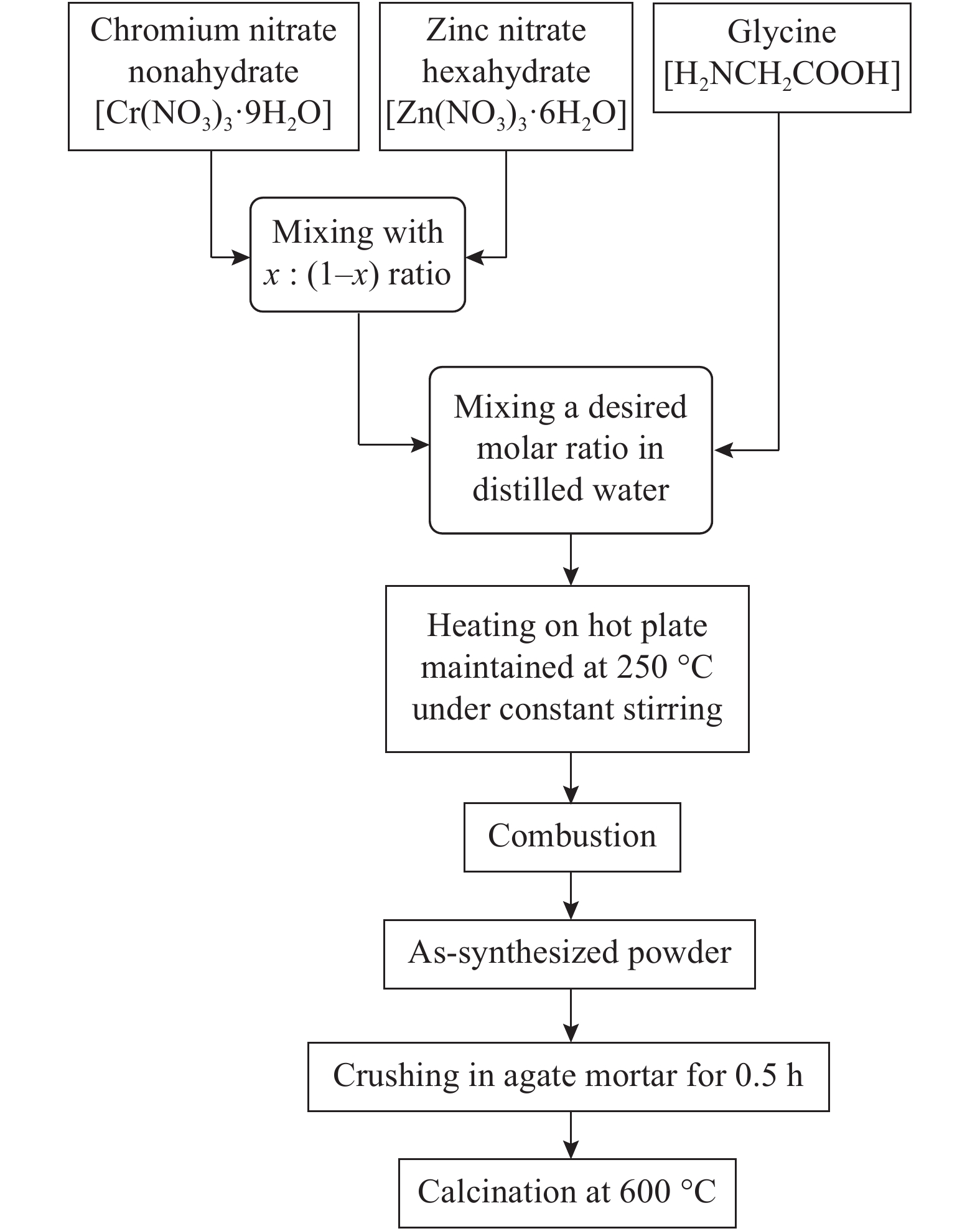
Zn1−xCrxO (x = 0.00, 0.01, 0.03, 0.05, 0.07, and 0.09) nanoparticles were synthesized, by an auto-combustion method. Structural, optical, and magnetic characteristics of Cr-doped ZnO samples calcined at 600 °C have been analyzed by using X-ray diffraction (XRD), field emission scanning electron microscope (FESEM), UV–Vis spectroscopy and vibrating sample magnetometer (VSM). The XRD data confirmed the hexagonal wurtzite structure of pure and Cr-doped ZnO nanoparticles. The calculated values of grain size using Scherrer's formula are in the range of 30.7–9.2 nm. The morphology of nanopowders has been observed by FESEM, and EDS results confirmed a systematic increase of Cr content in the samples and clearly indicate with no impurity element. The band gaps, computed by UV–Vis spectroscopy, are in the range of 2.83–2.35 eV for different doping concentrations. By analyzing VSM data, significantly enhanced room temperature ferromagnetism is identified in Cr-doped ZnO samples. The value of magnetization is a 12 times increased of the value reported by Daunet al. (2010). Room temperature ferromagnetism of the nanoparticles is of vital prominence for spintronics applications.-
Keywords:
- Cr-doped ZnO,
- auto-combustion,
- DMS,
- ferromagnetism
-
References
[1] Mang A, Reimann K, Rübenacke S. Band gaps, crystal-field splitting, spin-orbit coupling, and exciton binding energies in ZnO under hydrostatic pressure. Solid State Commun, 1995, 94(4): 251 doi: 10.1016/0038-1098(95)00054-2[2] Reynolds D, Look D, Jogai B. Optically pumped ultraviolet lasing from ZnO. Solid State Commun, 1996, 99(12): 873 doi: 10.1016/0038-1098(96)00340-7[3] Dietl T. Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science, 2000, 287(5455): 1019 doi: 10.1126/science.287.5455.1019[4] Liu C, Yun F, Morkoç H. Ferromagnetism of ZnO and GaN: a review. J Mater Sci: Mater Electron, 2005, 16(9): 555 doi: 10.1007/s10854-005-3232-1[5] Pearton S, Norton D, Ivill M, et al. Ferromagnetism in transition-metal doped ZnO. J Electron Mater, 2006, 36(4): 462[6] Sima M, Enculescu I, Sima M, et al. ZnO:Mn:Cu nanowires prepared by template method. Phys Status Solidi B, 2007, 244(5): 1522 doi: 10.1002/(ISSN)1521-3951[7] Bououdina M, Omri K, El-Hilo M, et al. Structural and magnetic properties of Mn-doped ZnO nanocrystals. Physica E, 2014, 56: 107 doi: 10.1016/j.physe.2013.08.024[8] Ahmadipour M, Hatami M, Sadabadi H, et al. Magnetic property of Zn0.8CO0.2O diluted magnetic semiconductors by auto combustion method. Int J Curr Eng Technol, 2013, 3(1): 85[9] Raja K, Ramesh P, Geetha D. Synthesis, structural and optical properties of ZnO and Ni-doped ZnO hexagonal nanorods by Co-precipitation method. Spectrochima Acta A, 2014, 120: 19 doi: 10.1016/j.saa.2013.09.103[10] More D, Phadnis C, Basu S, et al. Correlation of structural and magnetic properties of Ni-doped ZnO nanocrystals. J Phys D, 2013, 47(4): 045308[11] Jin Z, Fukumura T, Kawasaki M, et al. High throughput fabrication of transition-metal-doped epitaxial ZnO thin films: A series of oxide-diluted magnetic semiconductors and their properties. Appl Phys Lett, 2001, 78(24): 3824 doi: 10.1063/1.1377856[12] Ueda K, Tabata H, Kawai T. Magnetic and electric properties of transition-metal-doped ZnO films. Appl Phys Lett, 2001, 79(7): 988 doi: 10.1063/1.1384478[13] Wang Q, Sun Q, Jena P, et al. Magnetic coupling between Cr atoms doped at bulk and surface sites of ZnO. Appl Phys Lett, 2005, 87(16): 162509 doi: 10.1063/1.2106023[14] Duan L, Zhao X, Liu J, et al. Room-temperature ferromagnetism in lightly Cr-doped ZnO nanoparticles. Appl Phys A, 2010, 99(3): 679 doi: 10.1007/s00339-010-5590-7[15] Pang X, Zhang J, Gao K, et al. Room temperature ferromagnetism in sputtered Zn1−xCrxO thin films. Mater Lett, 2011, 65(17/18): 2728 doi: 10.1016/j.matlet.2011.05.074[16] Li L, Wang W, Liu H, et al. First principles calculations of electronic band structure and optical properties of Cr-doped ZnO. J Phys Chem C, 2009, 113(19): 8460 doi: 10.1021/jp811507r[17] Naqvi S, Soleimani H, Yahya N, et al. Structural and optical properties of chromium doped zinc oxide nanoparticles synthesized by sol–gel method. AIP Conference Proceedings, 2014, 1621: 530 doi: 10.1063/1.4898517[18] Mehedi Hassan M, Khan W, Azam A, et al. Influence of Cr incorporation on structural, dielectric and optical properties of ZnO nanoparticles. J Ind Eng Chem, 2015, 21: 283 doi: 10.1016/j.jiec.2014.01.047[19] Safa S, Nejad A. Influence of Cr dopant on the microstructure and optical properties of ZnO nanorods. J Adv Mater Process, 2014, 2(1): 19[20] Sartiman S, Djaja N, Saleh R. Chromium-doped ZnO nanoparticles synthesized by Co-precipitation: chromium effects. Mater Sci Appl, 2013, 4: 528[21] Mou P, Pal U, Pérez-Rodríguez P, et al. Effects of crystallization and dopant concentration on the emission behavior of TiO2:Eu nanophosphors. Nanoscale Res Lett, 2012, 7: 1 doi: 10.1186/1556-276X-7-1[22] Kelsall R W, Hamley I W, Geoghegan M. Nanoscale science and technology. John Wiley & Sons, 2006[23] Wang X, Zheng R, Liu Z, et al. Structural, optical and magnetic properties of Co-doped ZnO nanorods with hidden secondary phases. J Nanotechnol, 2008, 19: 455702 doi: 10.1088/0957-4484/19/45/455702[24] Rasouli S, Moeen S. Combustion synthesis of Co-doped zinc oxide nanoparticles using mixture of citric acid–glycine fuels. J Alloys Compnds, 2011, 509(5): 1915 doi: 10.1016/j.jallcom.2010.10.087[25] Mote V, Huse V, Dole B. Synthesis and characterization of Cr doped ZnO nanocrystals. World J Conden Matter Phys, 2012, 02(04): 208 doi: 10.4236/wjcmp.2012.24035[26] Carrero A, Sagredo V, Pernechele C, et al. Synthesis and characterization of Co-doped ZnO manocompound. IEEE Trans Magns, 2013, 49(8): 4614 doi: 10.1109/TMAG.2013.2260857[27] Ren T, Baker H, Poduska K. Optical absorption edge shifts in electrodeposited ZnO thin films. Thin Solid Films, 2007, 515(20/21): 7976[28] Zhuge L, Wu X, Wu Z, et al. Effect of defects on room-temperature ferromagnetism of Cr-doped ZnO films. Scripta Materialia, 2009, 60(4): 214 doi: 10.1016/j.scriptamat.2008.10.002[29] Yılmaz S, Parlak M, Özcan Ş, et al. Structural, optical and magnetic properties of Cr doped ZnO microrods prepared by spray pyrolysis method. Appl Surf Sci, 2011, 257(22): 9293 doi: 10.1016/j.apsusc.2011.05.017[30] Suryanarayana C, Norton M. X-ray diffraction. 1st ed. New York: Plenum Press, 1998[31] Chen Y, Ding K, Yang L, et al. Nanoscale ferromagnetic chromium oxide film from gas-phase nanocluster deposition. Appl Phys Lett, 2008, 92(17): 173112 doi: 10.1063/1.2919077[32] Karmakar D, Mandal S, Kadam R, et al. Ferromagnetism in Fe-doped ZnO nanocrystals: experiment and theory. Phys Rev B, 2007, 75(14): 144404 doi: 10.1103/PhysRevB.75.144404[33] Jin C, Yang Y, Wu Z, et al. Tunable ferromagnetic behavior in Cr doped ZnO nanorod arrays through defect engineering. J Mater Chem C, 2014, 2(16): 2992 doi: 10.1039/C4TC00074A[34] Sundaresan A, Bhargavi R, Rangarajan N, et al. Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys Rev B, 2006, 74(16): 161306 doi: 10.1103/PhysRevB.74.161306[35] Ghosh S, Khan G, Mandal K. Defect-driven magnetism in luminescent n/p-type pristine and gd-substituted SnO2 nanocrystalline thin films. ACS Appl Mater Interfaces, 2012, 4(4): 2048 doi: 10.1021/am300023a[36] Cong C J, Hong J H, Zhang K L. Effect of atmosphere on the magnetic properties of the Co-doped ZnO magnetic semiconductors. Mater Chem Phys, 2009, 113: 435 doi: 10.1016/j.matchemphys.2008.06.062[37] Xiong Z, Liu X C, Zhuo S Y, et al. Oxygen enhanced ferromagnetism in Cr-doped ZnO films. Appl Phys Lett, 2011, 99: 052513 doi: 10.1063/1.3624589[38] Ramachandran S, Narayan J, Prater J T. Effect of oxygen annealing on Mn doped ZnO diluted magnetic semiconductors. Appl Phys Lett, 2006, 88: 023112 doi: 10.1063/1.2164924[39] Wibowo J A, Djaja N F, Saleh R. Cu- and Ni-doping effect on structure and magnetic properties of Fe-doped ZnO nanoparticles. Adv Mater Phys Chem, 2013, 3: 48 doi: 10.4236/ampc.2013.31008[40] An Y K, Wang S Q, Feng D Q, et al. Correlation between oxygen vacancies and magnetism in Fe-doped In2O3 films. Appl Surf Sci, 2013, 276: 535 doi: 10.1016/j.apsusc.2013.03.129[41] An Y K, Wang S Q, Duan L S, et al. Local Mn structure and room temperature ferromagnetism in Mn-doped In2O3 films. Appl Phys Lett, 2013, 102: 212411 doi: 10.1063/1.4808116[42] Spanhel L. Colloidal ZnO nanostructures and functional coatings: a survey. J Sol-Gel Sci Technol, 2006, 39: 4[43] Chand P, Gaur A, Kumar A. Structural and optical properties of ZnO nanoparticles synthesized at different pH values. J Alloys ad Compnds, 2012, 539: 174 doi: 10.1016/j.jallcom.2012.05.104[44] Pushpanathan K, Sathya S, Jaychithra M, et al. Influence of reaction temperature on crystal structure and band gap of ZnO nanoparticles. Mater Manuf Process, 2012, 27: 1334 doi: 10.1080/10426914.2012.700163 -
Proportional views





 DownLoad:
DownLoad: