| Citation: |
Xiuwen Zhang, Jianbai Xia. Approaches to design inorganic semiconductors while maintaining structural motifs[J]. Journal of Semiconductors, 2018, 39(7): 071002. doi: 10.1088/1674-4926/39/7/071002
****
X W Zhang, J B Xia, Approaches to design inorganic semiconductors while maintaining structural motifs[J]. J. Semicond., 2018, 39(7): 071002. doi: 10.1088/1674-4926/39/7/071002.
|
Approaches to design inorganic semiconductors while maintaining structural motifs
DOI: 10.1088/1674-4926/39/7/071002
More Information
-
Abstract
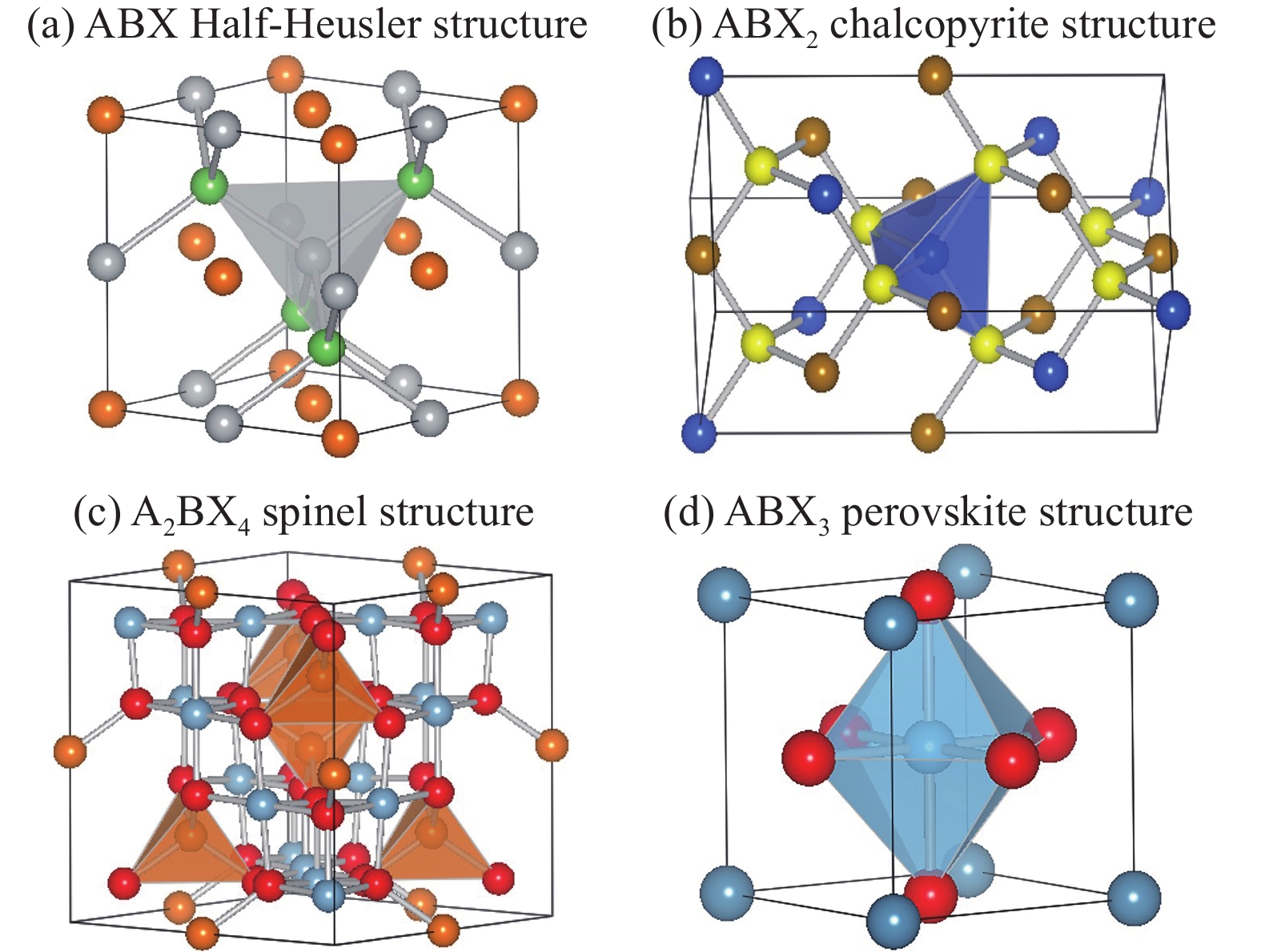
Inorganic semiconductors are the essential constituents of information society, having enabled most of the devices for microelectronics, optoelectronics, new energy technology, healthcare devices, artificial intelligence, etc. From the view of condensed matter physics and materials science, inorganic semiconductors are not intricate. However, this special class of materials are keeping inspiring new inventions and consequently new technologies, which in turn promote the design of new semiconductors. The kinds of semiconductors in nature are finite. On one hand, the new techniques need semiconductors with better properties than existing ones, on the other hand, the new computers’ software and hardware develop so rapid that it is possible to design new semiconductors according to people’s desire before manufacturing them. In this paper, we review the rational design of inorganic semiconductors by transformation of their elemental constituents, while maintaining their structure motifs. -
References
[1] Xia J B. Morden semiconductor physics. Beijing: Peking University Press, 2000 (in Chinese)[2] Yu P Y, Cardona M. Fundamentals of semiconductors: physics and materials properties. 4th ed. Heidelberg: Springer, 2010.[3] Parthé E. Crystal chemistry of tetrahedral structures. New York: Gordon and Breach, 1964[4] Berger L I. Semiconductor materials. Boca Raton: CRC Press, 1997[5] Xia J B, Ge W K, Chang K. Semiconductor spintronics, Beijing: Science Press, 2008 (in Chinese)[6] Baliga B J. Fundamentals of power semiconductor devices. New York: Springer, 2008[7] Li S S, Long G L, Bai F S, et al. Quantum computing. Proc Natl Acad Sci, 2001, 98: 11847 doi: 10.1073/pnas.191373698[8] Campbell S A. Fabrication engineering at the micro and nanoscale. New York: Oxford University Press, 2007[9] Sze S M. Physics of semiconductor devices. New York: Wiley-Interscience, 1969[10] Goodman C H L . The prediction of semiconducting properties in inorganic compounds. J Phys Chem Solids, 1958, 6: 305 doi: 10.1016/0022-3697(58)90050-7[11] Pamplin B R. Super-cell structure of semiconductors. Nature, 1960, 188: 136 doi: 10.1038/188136a0[12] Goldschmidt V M . Geochemische verteilungsgesetze der elemente. Skrifter der Norske Videnskaps-Akad, 1926, 8: 529[13] Johnson W C, Parsons J B, Crew M C. Nitrogen compounds of gallium III. Gallic nitride. J Phys Chem, 1932, 36: 2651[14] Inorganic Crystal Structure Database, Fachinformationszentrum Karlsruhe, Germany, 2006[15] ICDD PDF: International Centre For Diffraction Data, Powder Diffraction File, Newtown Square, PA, USA, 1997[16] Gruhn T. Comparative ab initio study of half-Heusler compounds for optoelectronic applications. Phys Rev B, 2010, 82: 125210 doi: 10.1103/PhysRevB.82.125210[17] Al-Sawai W, Lin H, Markiewicz R S, et al. Topological electronic structure in half-Heusler topological insulators. Phys Rev B, 2010, 82: 125208 doi: 10.1103/PhysRevB.82.125208[18] Chadov S, Qi X, Kübler J, et al. Tunable multifunctional topological insulators in ternary Heusler compounds. Nat Mater, 2010, 9: 541 doi: 10.1038/nmat2770[19] Lin H, Wray L A, Xia Y, et al. Half-Heusler ternary compounds as new multifunctional experimental platforms for topological quantum phenomena. Nat Mater, 2010, 9: 546 doi: 10.1038/nmat2771[20] Xiao D, Yao Y, Feng W, et al. Half-Heusler compounds as a new class of three-dimensional topological insulators. Phys Rev Lett, 2010, 105: 096404 doi: 10.1103/PhysRevLett.105.096404[21] Wang S, Wang Z, Setyawan W, et al. Assessing the thermoelectric properties of sintered compounds via high-throughput ab-initio calculations. Phys Rev X, 2011, 1: 021012 doi: 10.1103/PhysRevX.1.021012[22] Canepa P, Bo S H, Gautam G S, et al. High magnesium mobility in ternary spinel chalcogenides. Nat Commun, 2017, 8: 1759 doi: 10.1038/s41467-017-01772-1[23] Zhang X, Yu L, Zakutayev A, et al. Sorting stable versus unstable hypothetical compounds: the case of multi-functional ABX half-Heusler filled tetrahedral structures. Adv Funct Mater, 2012, 22: 1425 doi: 10.1002/adfm.v22.7[24] Zhang X, Stevanovi Vc, d’Avezac M, et al. Prediction of A2BX4 metal-chalcogenide compounds via first-principles thermodynamics. Phys Rev B, 2012, 86: 014109 doi: 10.1103/PhysRevB.86.014109[25] Gautier R, Zhang X, Hu L, et al. Prediction and accelerated laboratory discovery of previously unknown 18-electron ABX compounds. Nat Chem, 2015, 7: 308 doi: 10.1038/nchem.2207[26] Schon J C, Jansen M. First step towards planning of syntheses in solid-state chemistry: determination of promising structure candidates by global optimization. Angew Chem Int Ed Engl, 1996, 35: 1286 doi: 10.1002/(ISSN)1521-3773[27] Oganov A R, Glass C W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J Chem Phys, 2006, 124: 244704 doi: 10.1063/1.2210932[28] Trimarchi T, Zunger A. Global space-group optimization problem: Finding the stablest crystal structure without constraints. Phys Rev B, 2007, 75: 104113 doi: 10.1103/PhysRevB.75.104113[29] Wu S Q, Ji M, Wang C Z, et al. An adaptive genetic algorithm for crystal structure prediction. J Phys: Condens Matter, 2014, 26: 035402 doi: 10.1088/0953-8984/26/3/035402[30] Pickard C J, Needs R J. High-pressure phases of silane. Phys Rev Lett, 2006, 97: 045504 doi: 10.1103/PhysRevLett.97.045504[31] Wang Y, Lv J, Zhu L. High-pressure phases of silane crystal structure prediction via particle-swarm optimization. Phys Rev B, 2010, 82: 094116 doi: 10.1103/PhysRevB.82.094116[32] Hautier G, Fischer C C, Jain A, et al. Finding natureos missing ternary oxide compounds using machine learning and density functional theory. Chem Mater, 2010, 22: 3762 doi: 10.1021/cm100795d[33] Meredig B, Agrawal A, Kirklin S, et al. Combinatorial screening for new materials in unconstrained composition space with machine learning. Phys Rev B, 2014, 89: 094104 doi: 10.1103/PhysRevB.89.094104[34] Zhang X, Zunger A. Diagrammatic separation of different crystal structures of A2BX4 compounds without energy minimization: a pseudopotential orbital radii approach. Adv Funct Mater, 2010, 20: 1944 doi: 10.1002/adfm.v20:12[35] Zunger A. Systematization of the stable crystal structure of all AB-type binary compounds: A pseudopotential orbital-radii approach. Phys Rev B, 1980, 22: 5839 doi: 10.1103/PhysRevB.22.5839[36] Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 77: 3865 doi: 10.1103/PhysRevLett.77.3865[37] Kohn W, Sham L J. Self-consistent equations including exchange and correlation effects. Phys Rev A, 1965, 140: 1133 doi: 10.1103/PhysRev.140.A1133[38] Stevanovic V, Lany S, Zhang X, et al. Correcting density functional theory for accurate predictions of compound enthalpies of formation: Fitted elemental-phase reference energies. Phys Rev B, 2012, 85: 115104 doi: 10.1103/PhysRevB.85.115104[39] Yan F , Zhang X, Yu Y, et al. Design and discovery of a novel half-Heusler transparent hole conductor made of all-metallic heavy elements. Nat Commun, 2015, 6: 7308 doi: 10.1038/ncomms8308[40] Zakutayev A, Zhang X, Nagaraja A, et al. Theoretical prediction and experimental realization of new stable inorganic materials using the inverse design approach. J Am Chem Soc, 2013, 135: 10048 doi: 10.1021/ja311599g[41] Vermeer M J D, Zhang X, Trimarchi G, et al. Prediction and synthesis of strain tolerant RbCuTe crystals based on rotation of one-dimensional nano ribbons within a three-dimensional inorganic network. J Am Chem Soc, 2015, 137: 11383 doi: 10.1021/jacs.5b06182[42] Trimarchi G, Zhang X, Vermeer M J D, et al. Emergence of a few distinct structures from a single formal structure type during high-throughput screening for stable compounds: The case of RbCuS and RbCuSe. Phys Rev B, 2015, 92: 165103 doi: 10.1103/PhysRevB.92.165103[43] Yu Y G, Zhang X, Zunger A. Natural off-stoichiometry causes carrier doping in half-Heusler filled tetrahedral structures. Phys Rev B, 2017, 95: 085201 doi: 10.1103/PhysRevB.95.085201[44] Mahlab E, Volterra V, Low W, et al. Orthorhombic electron spin resonance spectrum of U3 in CaF2. Phys Rev, 1963, 131: 920 doi: 10.1103/PhysRev.131.920[45] Gai Y, Li J, Li S S, et al. Design of narrow-gap TiO2: a passivated codoping approach for enhanced photoelectrochemical activity. Phys Rev Lett, 2009, 102: 036402 doi: 10.1103/PhysRevLett.102.036402[46] Wei S H. Overcoming the doping bottleneck in semiconductors. Comput Mater Sci, 2004, 30: 337 doi: 10.1016/j.commatsci.2004.02.024[47] Palmer G B, Poeppelmeier K R, Mason T O. Conductivity and transparency of ZnO/SnO2-cosubstituted In2O3. Chem Mater, 1997, 9: 3121 doi: 10.1021/cm9704037[48] Zhao S, Kang L, Shen Y, et al. Designing a beryllium-free deep-ultraviolet nonlinear optical material without a structural instability problem. J Am Chem Soc, 2016, 138: 2961 doi: 10.1021/jacs.6b00436[49] McClure E T, Ball M R, Windl W, et al. Cs2AgBiX6 (X = Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors. Chem Mater, 2016, 28: 1348 doi: 10.1021/acs.chemmater.5b04231[50] Xia Z, Ma C, Molokeev M S, et al. Chemical unit cosubstitution and tuning of photoluminescence in the Ca2(Al1–xMgx) (Al1–xSi1+x)O7:Eu2+ phosphor. J Am Chem Soc, 2015, 137: 12494 doi: 10.1021/jacs.5b08315[51] Xia Z, Poeppelmeier K R. Chemistry-inspired adaptable framework structures. Acc Chem Res, 2017, 50: 1222 doi: 10.1021/acs.accounts.7b00033[52] Wang C, Chen S, Yang J H, et al. Design of I2–II–IV–VI4 semiconductors through element substitution: the thermodynamic stability limit and chemical trend. Chem Mater, 2014, 26: 3411 doi: 10.1021/cm500598x[53] Cai Z H, Narang P, Atwater H A, et al. Cation-mutation design of quaternary nitride semiconductors lattice-matched to GaN. Chem Mater, 2015, 27: 7757 doi: 10.1021/acs.chemmater.5b03536[54] Zhao X G, Yang J H, Fu Y, et al. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J Am Chem Soc, 2017, 139: 2630 doi: 10.1021/jacs.6b09645[55] Yang J, Zhang P, Wei S H. Band structure engineering of Cs2AgBiBr6 perovskite through order–disordered transition: a first-principle study. J Phys Chem Lett, 2018, 9: 31 doi: 10.1021/acs.jpclett.7b02992[56] Zhang S B, Wei S H, Zunger A . Stabilization of ternary compounds via ordered arrays of defect pairs. Phys Rev Lett, 1997, 78: 4059 doi: 10.1103/PhysRevLett.78.4059[57] Zhang S B, Wei S H, Zunger A, et al. Defect physics of the CuInSe2 chalcopyrite semiconductor. Phys Rev B, 1998, 57: 9642 doi: 10.1103/PhysRevB.57.9642[58] Nowotny H, Bachmayer K. Die Verbindungen LiMgP, LiZnP und LiZnAs. Monatsh Chem, 1950, 81: 488 doi: 10.1007/BF00906437[59] Juza R, Hund F. Die ternären Nitride LiMgN und LiZnN. 16. Mitteilung über Metallamide und Metallnitride. Z Anorg Allg Chem, 1948, 257: 1 doi: 10.1002/zaac.v257:1/3[60] Wood D M, Zunger A, de Groot R. Electronic structure of filled tetrahedral semiconductors. Phys Rev B, 1985, 31: 2570 doi: 10.1103/PhysRevB.31.2570[61] Wei S H, Zunger A. Electronic structure and phase stability of LiZnAs: A half ionic and half covalent tetrahedral semiconductor. Phys Rev Lett, 1986, 56: 528 doi: 10.1103/PhysRevLett.56.528 -
Proportional views





 DownLoad:
DownLoad:





















