| Citation: |
Yong Chen, Yang Zhao, Qiufeng Ye, Zema Chu, Zhigang Yin, Xingwang Zhang, Jingbi You. Improved efficiency and photo-stability of methylamine-free perovskite solar cells via cadmium doping[J]. Journal of Semiconductors, 2019, 40(12): 122201. doi: 10.1088/1674-4926/40/12/122201
****
Y Chen, Y Zhao, Q F Ye, Z M Chu, Z G Yin, X W Zhang, J B You, Improved efficiency and photo-stability of methylamine-free perovskite solar cells via cadmium doping[J]. J. Semicond., 2019, 40(12): 122201. doi: 10.1088/1674-4926/40/12/122201.
|
Improved efficiency and photo-stability of methylamine-free perovskite solar cells via cadmium doping
DOI: 10.1088/1674-4926/40/12/122201
More Information
-
Abstract
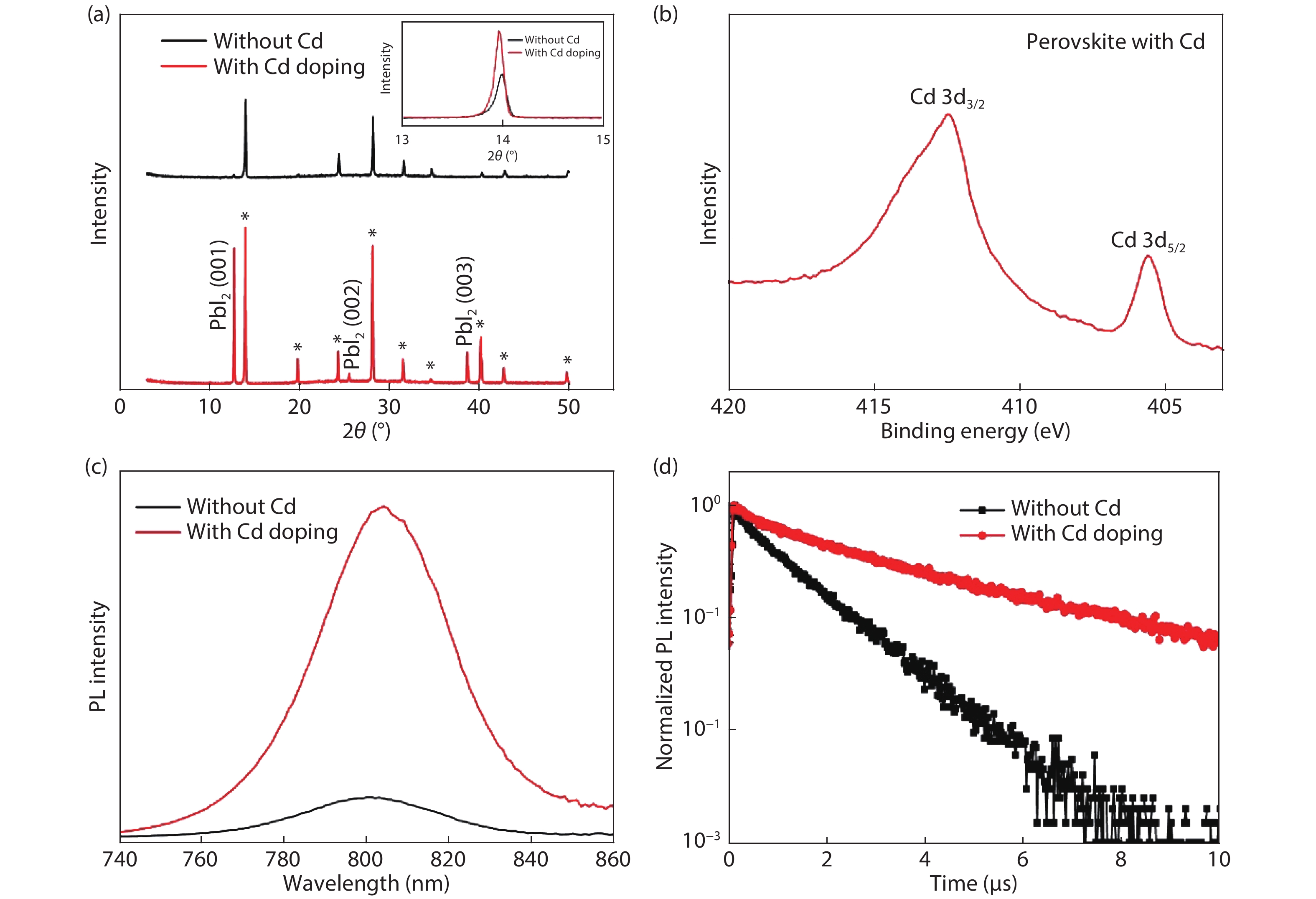
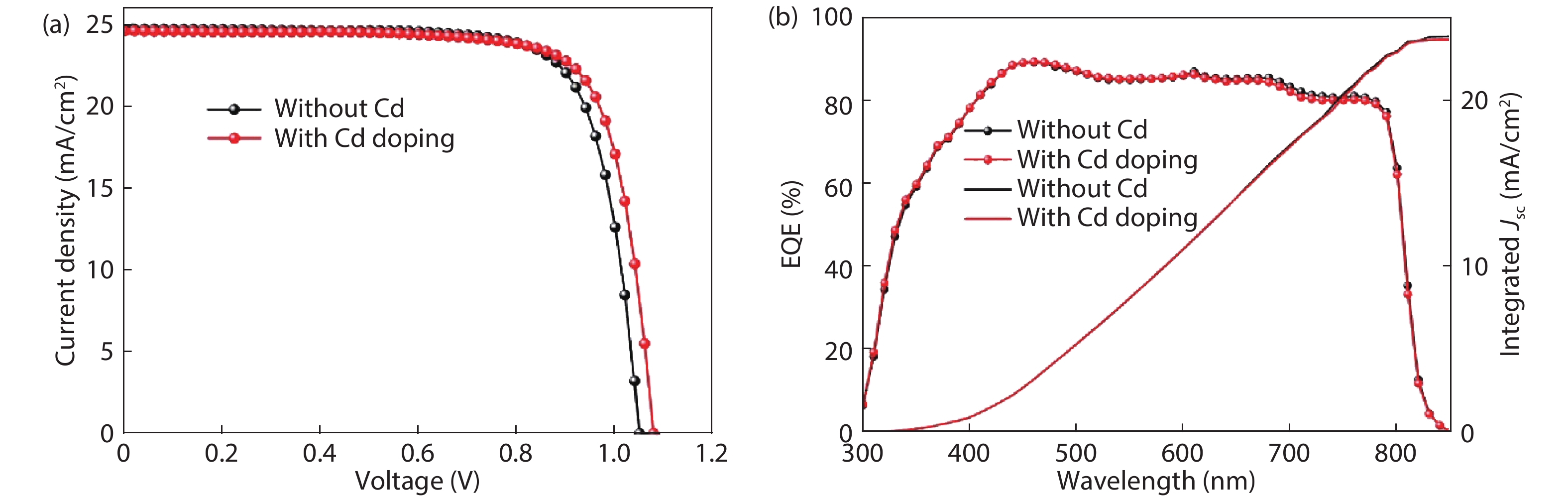
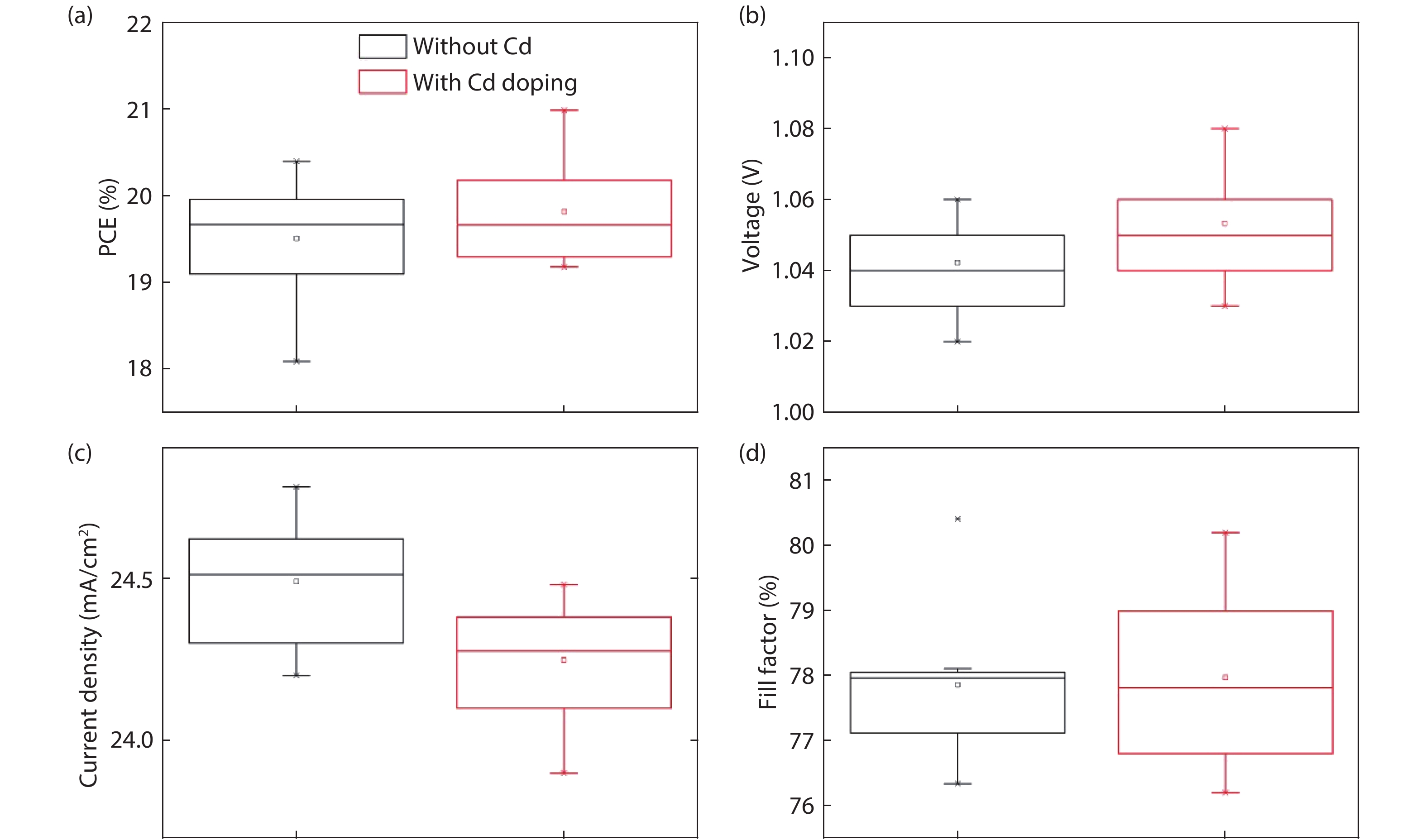
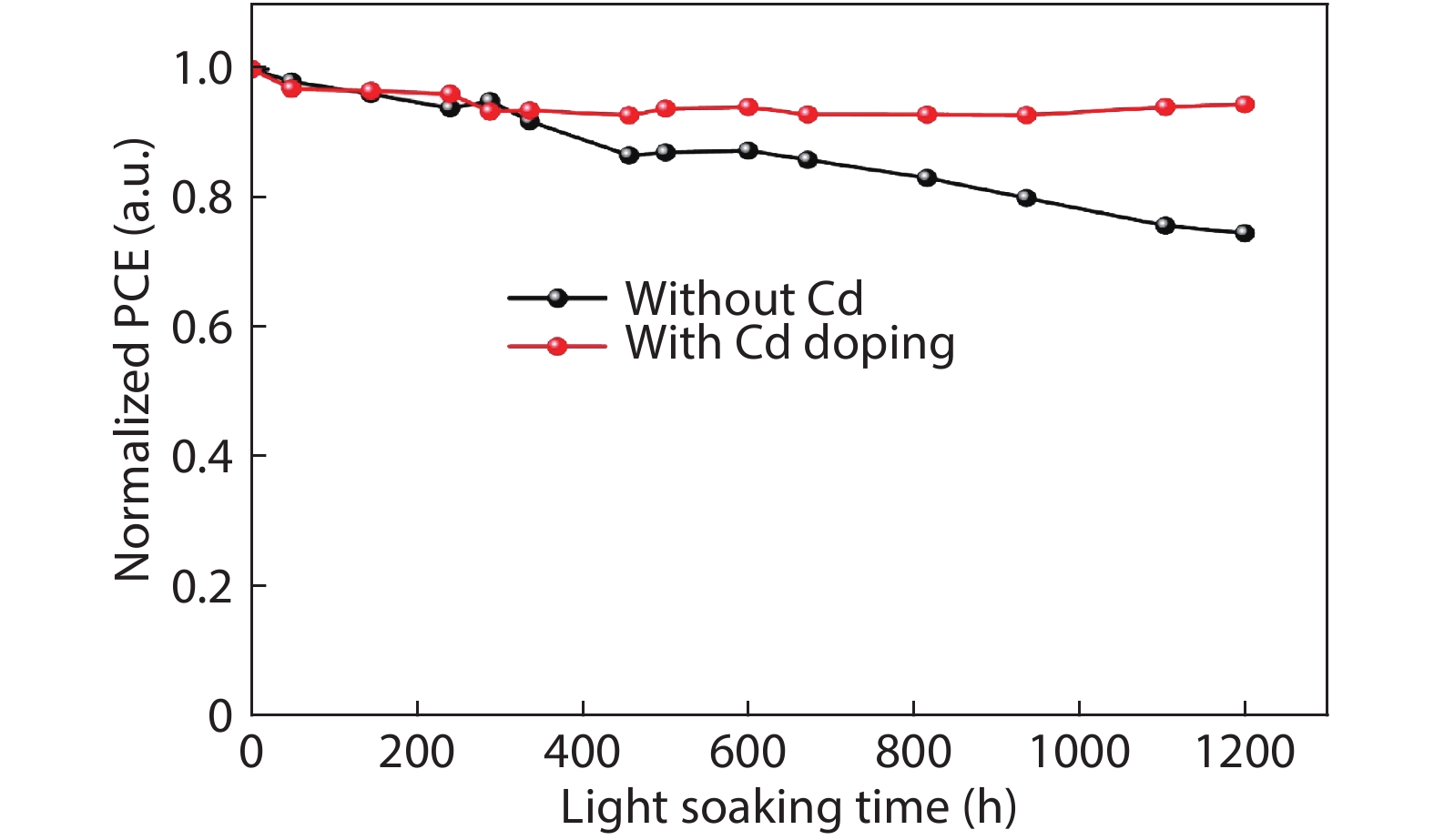
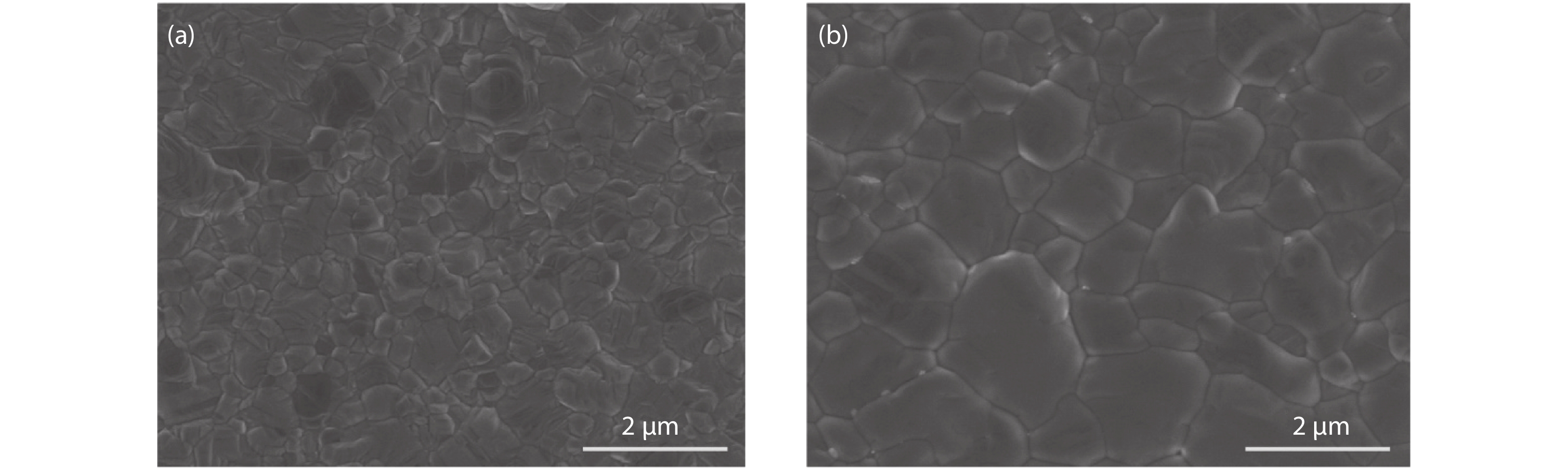
Although perovskite solar cells containing methylamine cation can show high power conversion efficiency, stability is a concern. Here, methylamine-free perovskite material CsxFA1–xPbI3 was synthesized by a one-step method. In addition, we incorporated smaller cadmium ions into mixed perovskite lattice to partially replace Pb ions to address the excessive internal strain in perovskite structure. We have found that the introduction of Cd can improve the crystallinity and the charge carrier lifetime of perovskite films. Consequently, a power conversion efficiency as high as 20.59% was achieved. More importantly, the devices retained 94% of their initial efficiency under 1200 h of continuous illumination.-
Keywords:
- methylamine-free,
- photo-stability,
- lattice strain,
- cadmium doping
-
References
[1] Kojima A, Teshima K, Shirai Y, et al. Organometal halide perovskitesas visible-light sensitizers for photovoltaic cells. J Am Chem Soc, 2009, 131, 6050 doi: 10.1021/ja809598r[2] Xing G, Mathews N, Lim S S, et al. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat Mater, 2014, 13, 476 doi: 10.1038/nmat3911[3] Miyata A, Mitioglu A, Plochocka P, et al. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic-inorganic tri-halide perovskites. Nat Phys, 2015, 11, 582 doi: 10.1038/nphys3357[4] Green M A, Ho-Baillie A, Snaith H J. The emergence of perovskite solar cells. Nat Photonics, 2014, 8, 506 doi: 10.1038/nphoton.2014.134[5] Zhao J, Kong G, Chen S, et al. Single crystalline CH3NH3PbI3 self-grown on FTO/TiO2 substrate for high efficiency perovskite solar cells. Sci Bull, 2017, 62, 1173 doi: 10.1016/j.scib.2017.08.022[6] Im J H, Lee C R, Lee J W, et al. 6.5% effcient perovskite quantum-dot-sensitized solar cell. Nanoscale, 2011, 3, 4088 doi: 10.1039/c1nr10867k[7] Kim H S, Lee C R, Im J H, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with effciency exceeding 9%. Sci Rep, 2012, 2, 591 doi: 10.1038/srep00591[8] Burschka J, Pellet N, Moon S J, et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature, 2013, 499, 316 doi: 10.1038/nature12340[9] Hao F, Stoumpos C C, Liu Z, et al. Controllable perovskite crystallization at a gas–solid interface for hole conductor-free solar cells with steady power conversion efciency over 10%. J Am Chem Soc, 2014, 136, 16411 doi: 10.1021/ja509245x[10] Im J H, Jang I H, Pellet N, et al. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat Nanotech, 2014, 9, 927 doi: 10.1038/nnano.2014.181[11] Tan W, Bowring A R, Meng A C, et al. Thermal stability of mixed cation metal halide perovskites in air. ACS Appl Mater Interfaces, 2018, 10, 5485 doi: 10.1021/acsami.7b15263[12] Wang S H, Jiang Y, Juarez-Perez E J, et al. Accelerated degradation of methylammonium lead iodide perovskites induced by exposure to iodine vapour. Nat Energy, 2017, 2, 16195 doi: 10.1038/nenergy.2016.195[13] Zhang T, Meng X, Bai Y, et al. Profiling the organic cation-dependent degradation of organolead halide perovskite solar cells. J Mater Chem A, 2017, 5, 1103 doi: 10.1039/C6TA09687E[14] Zhao Y C, Zhou W, Tan H, et al. Mobile-ion-induced degradation of organic hole-selective layers in perovskite solar cells. J Phys Chem C, 2017, 121, 14517 doi: 10.1021/acs.jpcc.7b04684[15] Lee J W, Kim D H, Kim H S, et al. Formamidinium and cesium hybridization for photo- and moisture-stable perovskite solar cell. Adv Energy Mater, 2015, 5, 1501310 doi: 10.1002/aenm.201501310[16] McMeekin D P, Sadoughi G, Rehman W, et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science, 2016, 351, 151 doi: 10.1126/science.aad5845[17] Saliba M, Matsui T, Domanski K, et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science, 2016, 354, 206 doi: 10.1126/science.aah5557[18] Duong T, Mulmudi H K, Shen H, et al. Structural engineering using rubidium iodide as a dopant under excess lead iodide conditions for high efficiency and stable perovskites. Nano Energy, 2016, 30, 330 doi: 10.1016/j.nanoen.2016.10.027[19] Turren-Cruz S H, Hagfeldt A, Saliba M. Methylammonium-free, high-performance and perovskite solar cells on a planar architecture. Science, 2018, 362, 6413 doi: 10.1126/science.aat3583[20] Li W, Li J, Niu G, et al. Effect of cesium chloride modification on the film morphology and UV-induced stability of planar perovskite solar cells. J Mater Chem A, 2016, 4, 11688 doi: 10.1039/C5TA09165A[21] Zhao Y, Zhu K. CH3NH3Cl-assisted one-step solution growth of CH3NH3PbI3: Structure, charge-carrier dynamics, and photovoltaic properties of perovskite.solar cells. J Phys Chem C, 2014, 118, 9412 doi: 10.1021/jp502696w[22] Dar M I, Arora N, Gao P, et al. Investigation regarding the role of chloride in organic–inorganic halide perovskites obtained from chloride containing precursors. Nano Lett, 2014, 14, 6991 doi: 10.1021/nl503279x[23] Colella S, Mosconi E, Pellegrino G, et al. Elusive presence of chloride in mixed halide perovskite solar cells. J Phys Chem Lett, 2014, 5, 3532 doi: 10.1021/jz501869f[24] Chen Q, Zhou H P, Fang Y H, et al. Te optoelectronic role of chlorine in CH3NH3PbI3(Cl)-based perovskite solar cells. Nat Commun, 2015, 6, 7269 doi: 10.1038/ncomms8269[25] Saidaminov M I, Kim J, Jain A, et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat Energy, 2018, 3, 648 doi: 10.1038/s41560-018-0192-2[26] Zheng X J, Wu C C, Jha S K, et al. Improved phase stability of formamidinium lead triiodide perovskite by strain relaxation. ACS Energy Lett, 2016, 1, 1014 doi: 10.1021/acsenergylett.6b00457[27] Kieslich G, Sun S, Cheetham A K. Solid-state principles applied to organic-inorganic perovskites: New tricks for an old dog. Chem Sci, 2014, 5, 4712 doi: 10.1039/C4SC02211D[28] Navas J, Sánchez-Coronilla A, Gallardo J J, et al. New insights on the organic-inorganic hybrid perovskite CH3NH3PbI3 nanoparticles. Experimental and theoretical study of doping in Pb2+ sites with Sn2+, Sr2+, Cd2+ and Ca2+. Nanoscale, 2015, 7, 6216 doi: 10.1039/C5NR00041F[29] Son D Y, Lee J W, Choi Y J, et al. Self-formed grain boundary healing layer for highly efficient CH3NH3PbI3 perovskite solar cells. Nat Energy, 2016, 1, 16081 doi: 10.1038/nenergy.2016.81[30] Zheng G H J, Li L, Wang L G, et al. The investigation of anamidine-based additive in the perovskite films and solar cells. J Semicond, 2017, 38(1), 014001 doi: 10.1088/1674-4926/38/1/014001[31] Wang F, Bai S, Tress W, et al. Defects engineering for high performance perovskite solar cells. npj Flex Electron, 2018, 2, 22 doi: 10.1038/s41528-018-0035-z -
Proportional views





 DownLoad:
DownLoad: