| Citation: |
Jiamin Cao, Lifei Yi, Liming Ding. The origin and evolution of Y6 structure[J]. Journal of Semiconductors, 2022, 43(3): 030202. doi: 10.1088/1674-4926/43/3/030202
****
J M Cao, L F Yi, L M Ding, The origin and evolution of Y6 structure[J]. J. Semicond., 2022, 43(3): 030202. doi: 10.1088/1674-4926/43/3/030202.
|
-
References
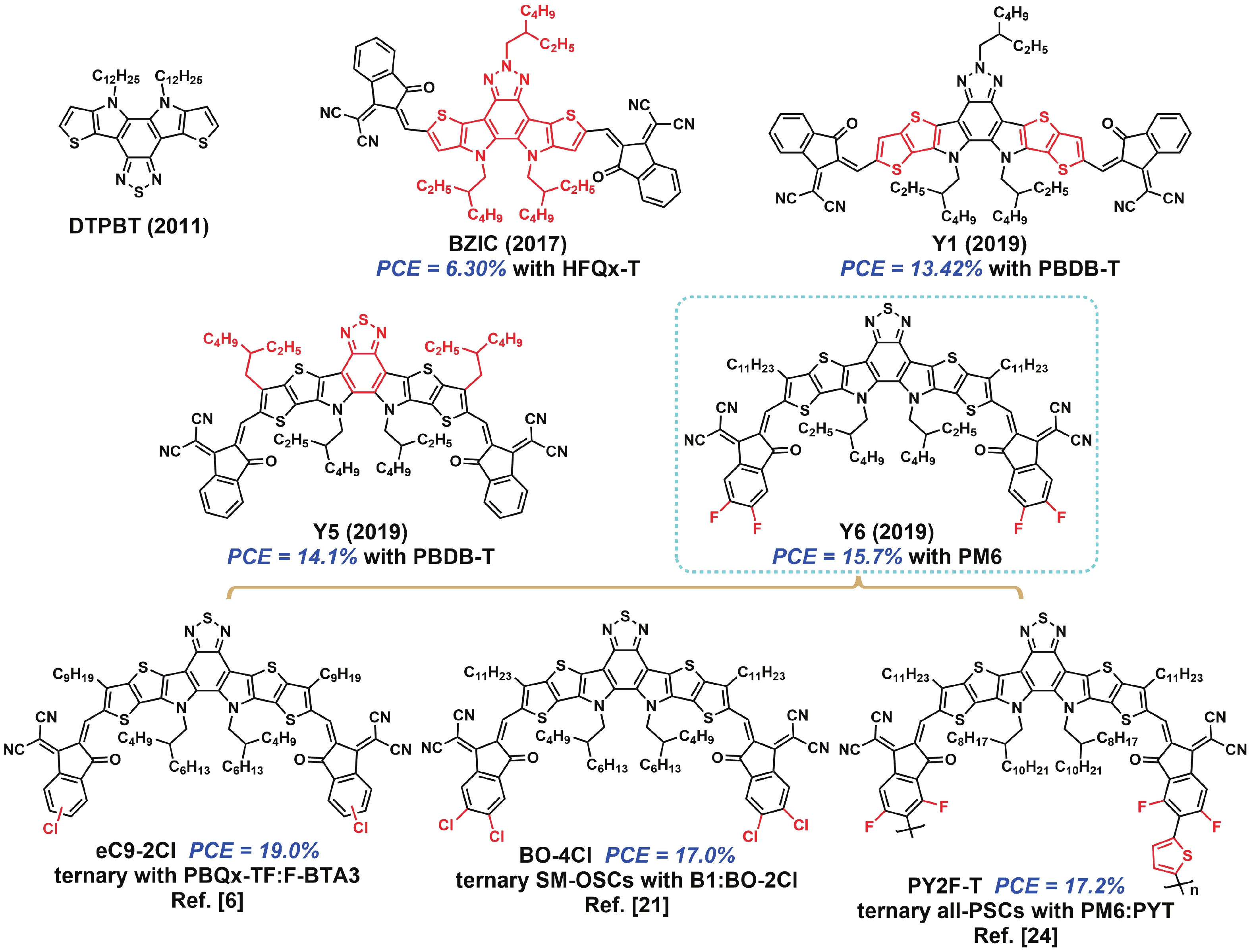
[1] Lin Y, Wang J, Zhang Z G, et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv Mater, 2015, 27, 1170 doi: 10.1002/adma.201404317[2] Xiao Z, Jia X, Li D, et al. 26 mA cm–2 Jsc from organic solar cells with a low-bandgap nonfullerene acceptor. Sci Bull, 2017, 62, 1494 doi: 10.1016/j.scib.2017.10.017[3] Xiao Z, Jia X, Ding L, et al. Ternary organic solar cells offer 14% power conversion efficiency. Sci Bull, 2017, 62, 1562 doi: 10.1016/j.scib.2017.11.003[4] Yuan J, Zhang Y, Zhou L, et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule, 2019, 3, 1140 doi: 10.1016/j.joule.2019.01.004[5] Li S, Li C Z, Shi M, et al. New phase for organic solar cell research: emergence of Y-series electron acceptors and their perspectives. ACS Energy Lett, 2020, 5, 1554 doi: 10.1021/acsenergylett.0c00537[6] Cui Y, Xu Y, Yao H, et al. Single-junction organic photovoltaic cell with 19% efficiency. Adv Mater, 2021, 33, 2102420 doi: 10.1002/adma.202102420[7] Liu Q, Jiang Y, Jin K, et al. 18% Efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[8] Qin J, Zhang L, Zuo C, et al. A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency. J Semicond, 2021, 42, 010501 doi: 10.1088/1674-4926/42/1/010501[9] Jin K, Xiao Z, Ding L. 18.69% PCE from organic solar cells. J Semicond, 2021, 42, 060502 doi: 10.1088/1674-4926/42/6/060502[10] Meng X, Jin K, Xiao Z, et al. Side chain engineering on D18 polymers yields 18.74% power conversion efficiency. J Semicond, 2021, 42, 100501 doi: 10.1088/1674-4926/42/10/100501[11] Cheng Y J, Chen C H, Ho Y J, et al. Thieno[3, 2-b]pyrrolo donor fused with benzothiadiazolo, benzoselenadiazolo and quinoxalino acceptors: synthesis, characterization, and molecular properties. Org Lett, 2011, 13, 5484 doi: 10.1021/ol202199v[12] Cheng Y J, Ho Y J, Chen C H, et al. Synthesis, photophysical and photovoltaic properties of conjugated polymers containing fused donor-acceptor dithienopyrrolobenzothiadiazole and dithienopyrroloquinoxaline arenes. Macromolecules, 2012, 45, 2690 doi: 10.1021/ma202764v[13] Feng L, Yuan J, Zhang Z, et al. Thieno[3, 2-b]pyrrolo-fused pentacyclic benzotriazole-based acceptor for efficient organic photovoltaics. ACS Appl Mater Interfaces, 2017, 9, 31985 doi: 10.1021/acsami.7b10995[14] Yuan J, Huang T, Cheng P, et al. Enabling low voltage losses and high photocurrent in fullerene-free organic photovoltaics. Nat Commun, 2019, 10, 570 doi: 10.1038/s41467-019-08386-9[15] Yuan J, Zhang Y, Zhou L, et al. Fused benzothiadiazole: a building block for n-type organic acceptor to achieve high-performance organic solar cells. Adv Mater, 2019, 31, 1807577 doi: 10.1002/adma.201807577[16] Wei Q, Liu W, Leclerc M, et al. A-DA′D-A non-fullerene acceptors for high-performance organic solar cells. Sci China Chem, 2020, 63, 1352 doi: 10.1007/s11426-020-9799-4[17] Xie L, Zhang Y, Zhuang W, et al. Low-bandgap nonfullerene acceptor based on thieno[3, 2-b]indole core for highly efficient binary and ternary organic solar cells. Chem Eng J, 2022, 427, 131674 doi: 10.1016/j.cej.2021.131674[18] Duan C, Ding L. The new era for organic solar cells: non-fullerene small molecular acceptors. Sci Bull, 2020, 65, 1231 doi: 10.1016/j.scib.2020.04.030[19] Yuan J, Zhang C, Chen H, et al. Understanding energetic disorder in electron-deficient-core-based non-fullerene solar cells. Sci China Chem, 2020, 63, 1159 doi: 10.1007/s11426-020-9747-9[20] Liu S, Yuan J, Deng W, et al. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat Photonics, 2020, 14, 300 doi: 10.1038/s41566-019-0573-5[21] Qin J, Chen Z, Bi P, et al. 17% efficiency all-small-molecule organic solar cells enabled by nanoscale phase separation with a hierarchical branched structure. Energy Environ Sci, 2021, 14, 5903 doi: 10.1039/d1ee02124a[22] Jin K, Xiao Z, Ding L. D18, an eximious solar polymer. J Semicond, 2021, 42, 010502 doi: 10.1088/1674-4926/42/3/010502[23] Ji X, Xiao Z, Sun H, et al. Polymer acceptors for all-polymer solar cells. J Semicond, 2021, 42, 080202 doi: 10.1088/1674-4926/42/8/080202[24] Sun R, Wang W, Yu H, et al. Achieving over 17% efficiency of ternary all-polymer solar cells with two well-compatible polymer acceptors. Joule, 2021, 5, 1548 doi: 10.1016/j.joule.2021.04.007[25] Fan Q, Xiao Z, Wang E, et al. Polymer acceptors based on Y6 derivatives for all-polymer solar cells. Sci Bull, 2021, 66, 1950 doi: 10.1016/j.scib.2021.07.002[26] Yuan J, Zhang H, Zhang R, et al. Reducing voltage losses in the A-DA′D-A acceptor-based organic solar cells. Chem, 2020, 6, 2147 doi: 10.1016/j.chempr.2020.08.003 -
Proportional views





 DownLoad:
DownLoad:














