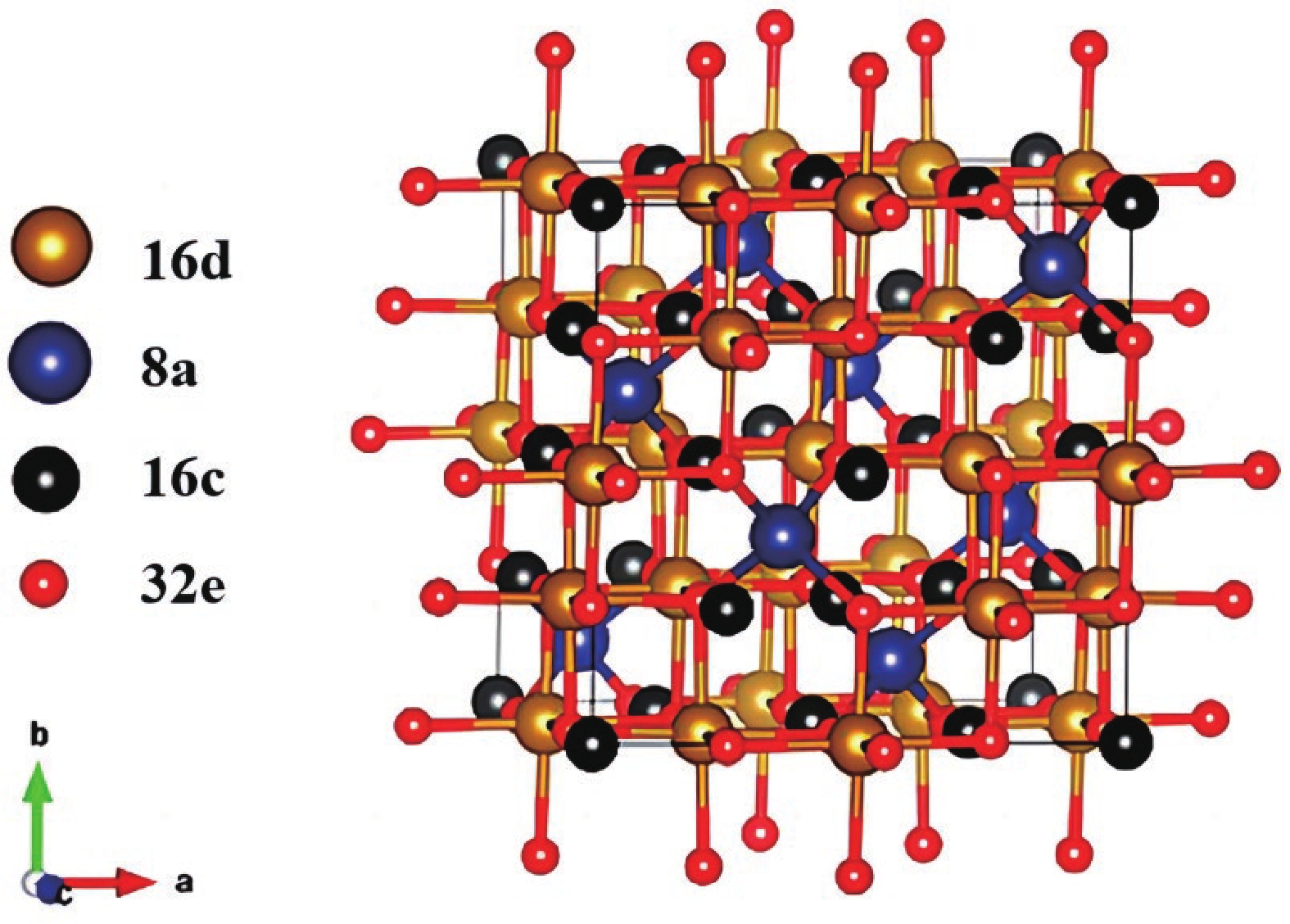
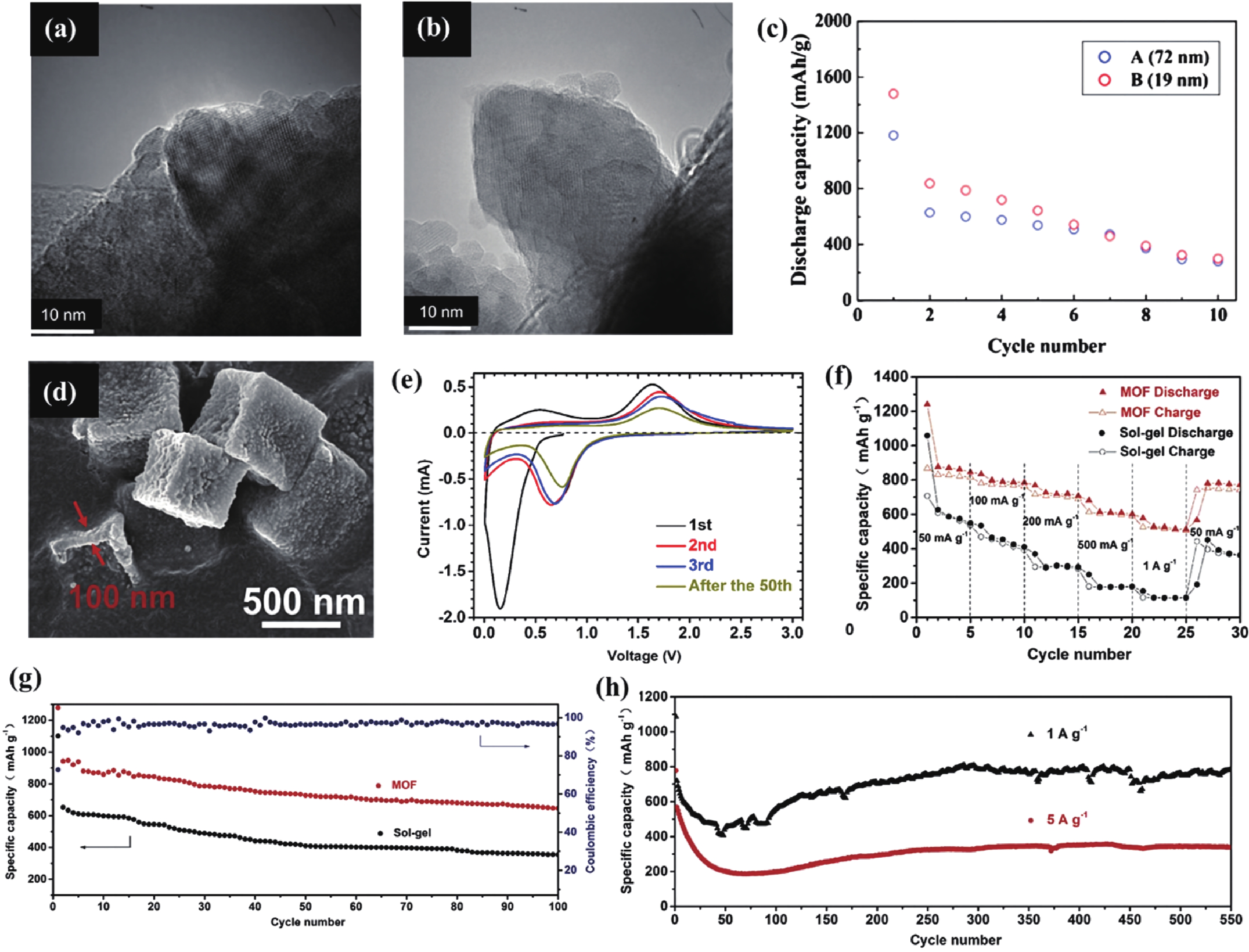
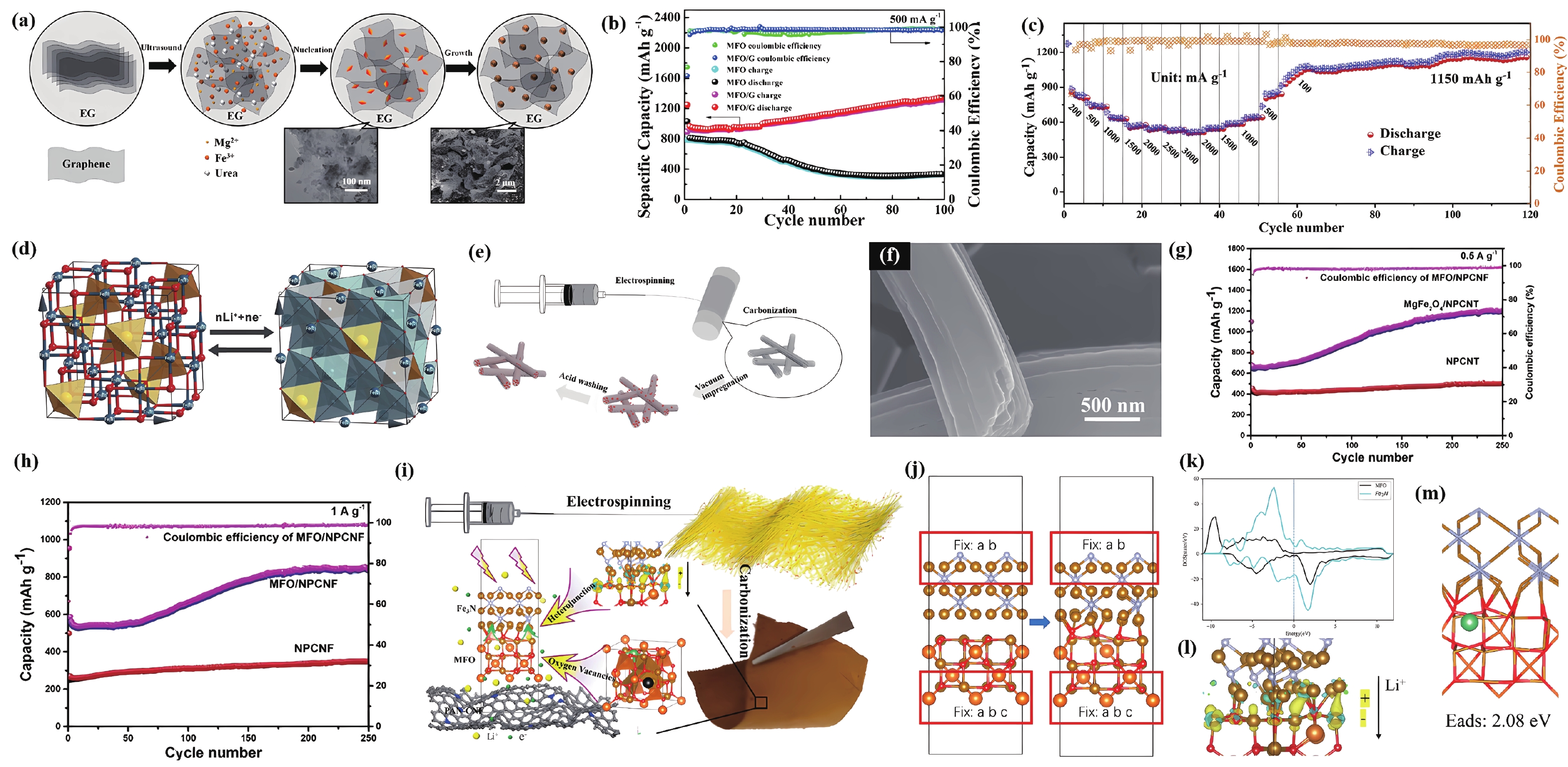
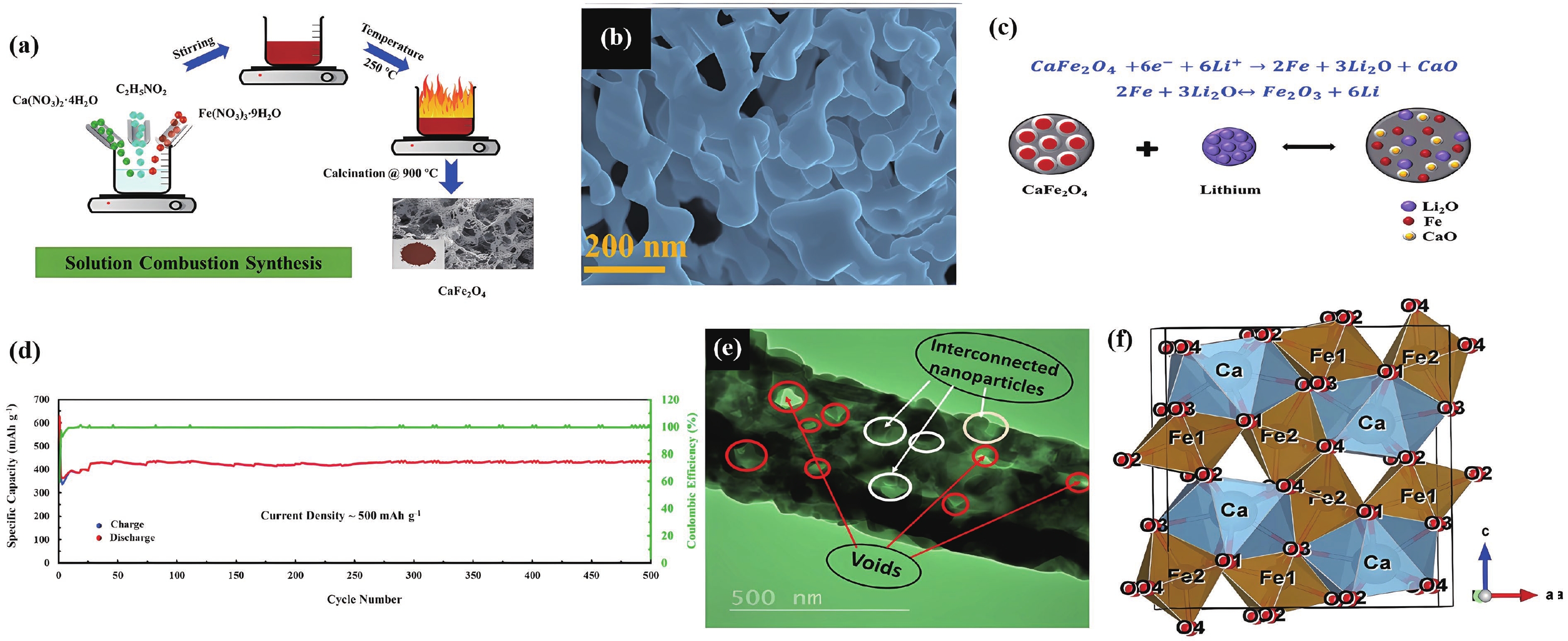
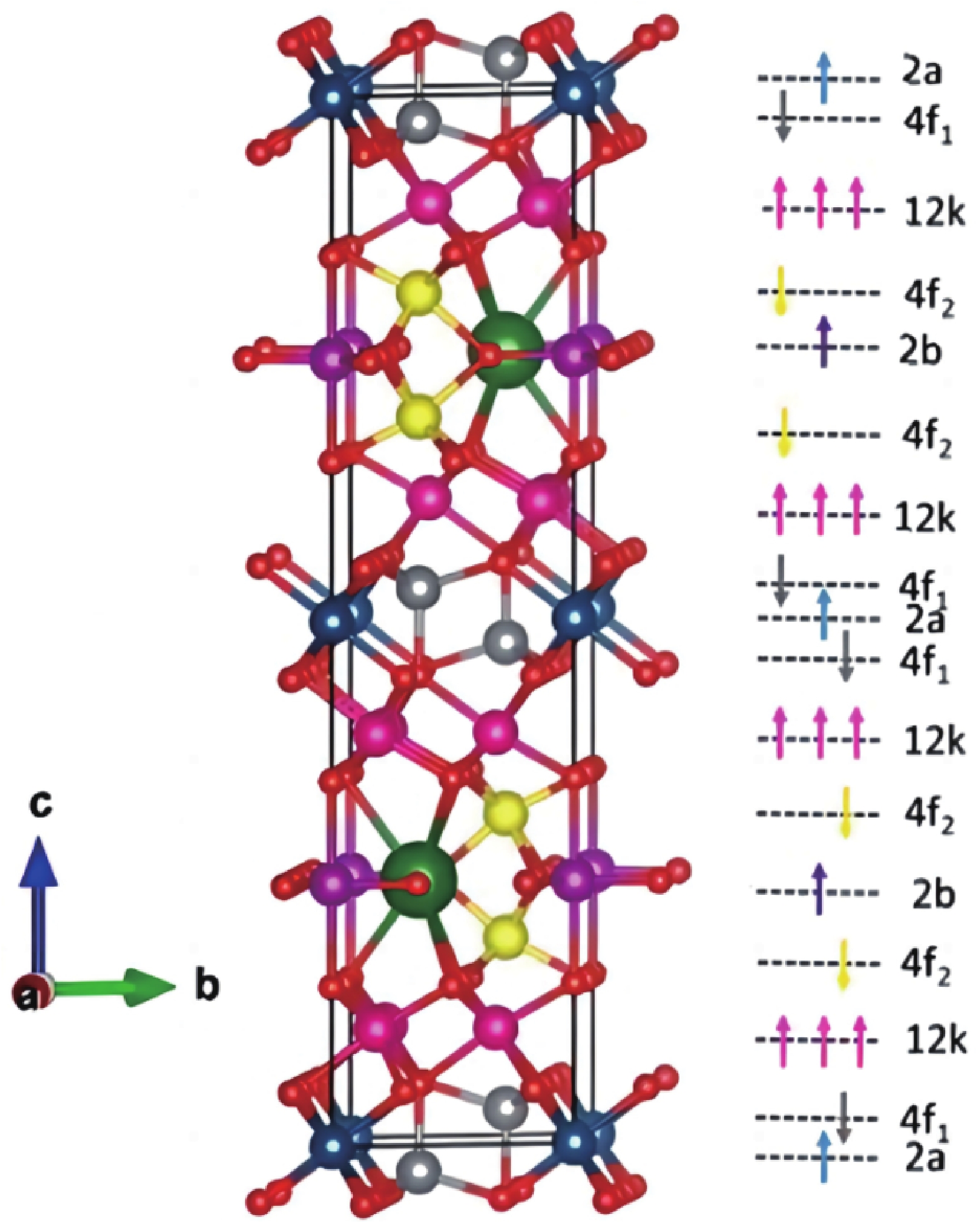
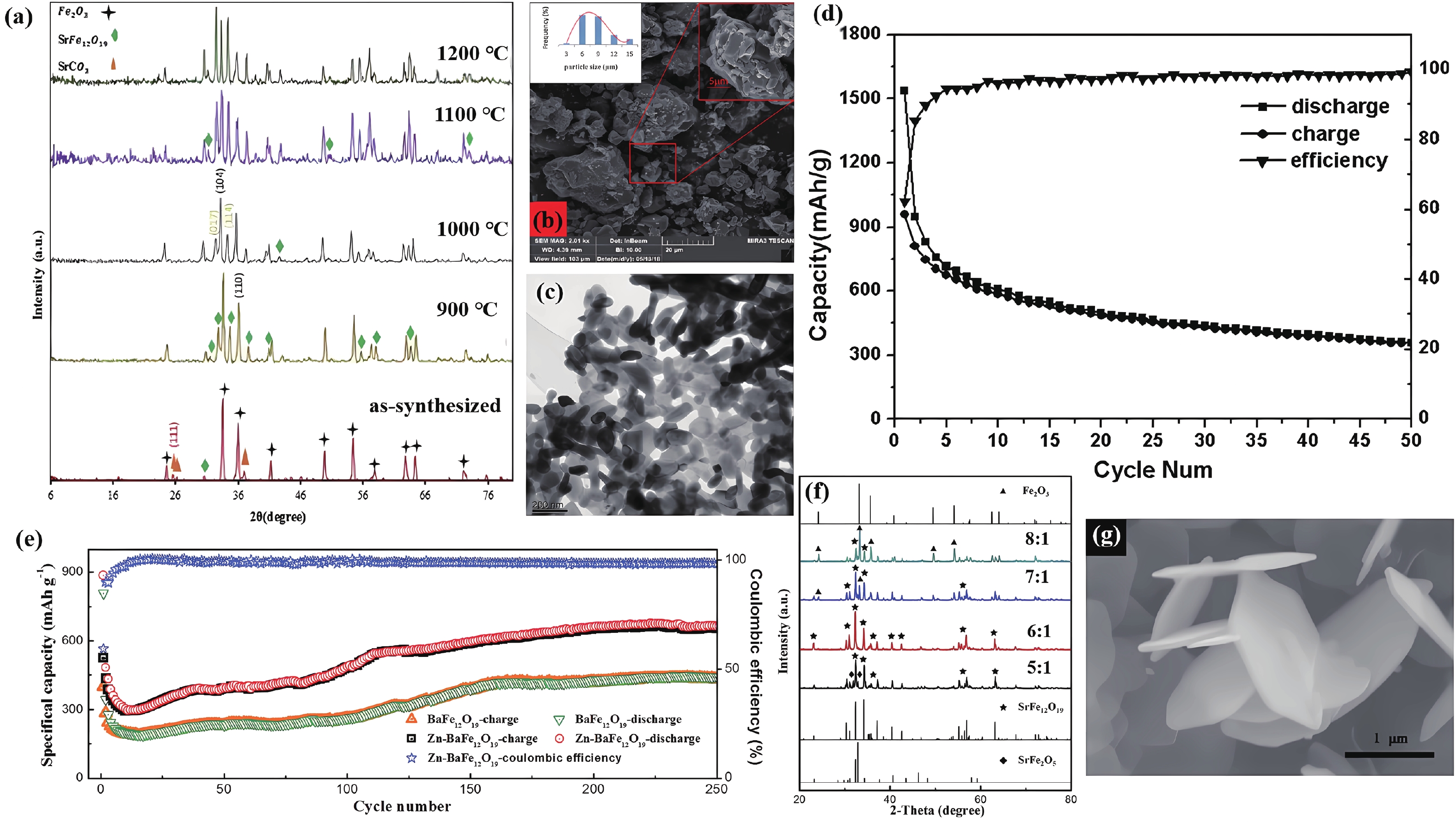
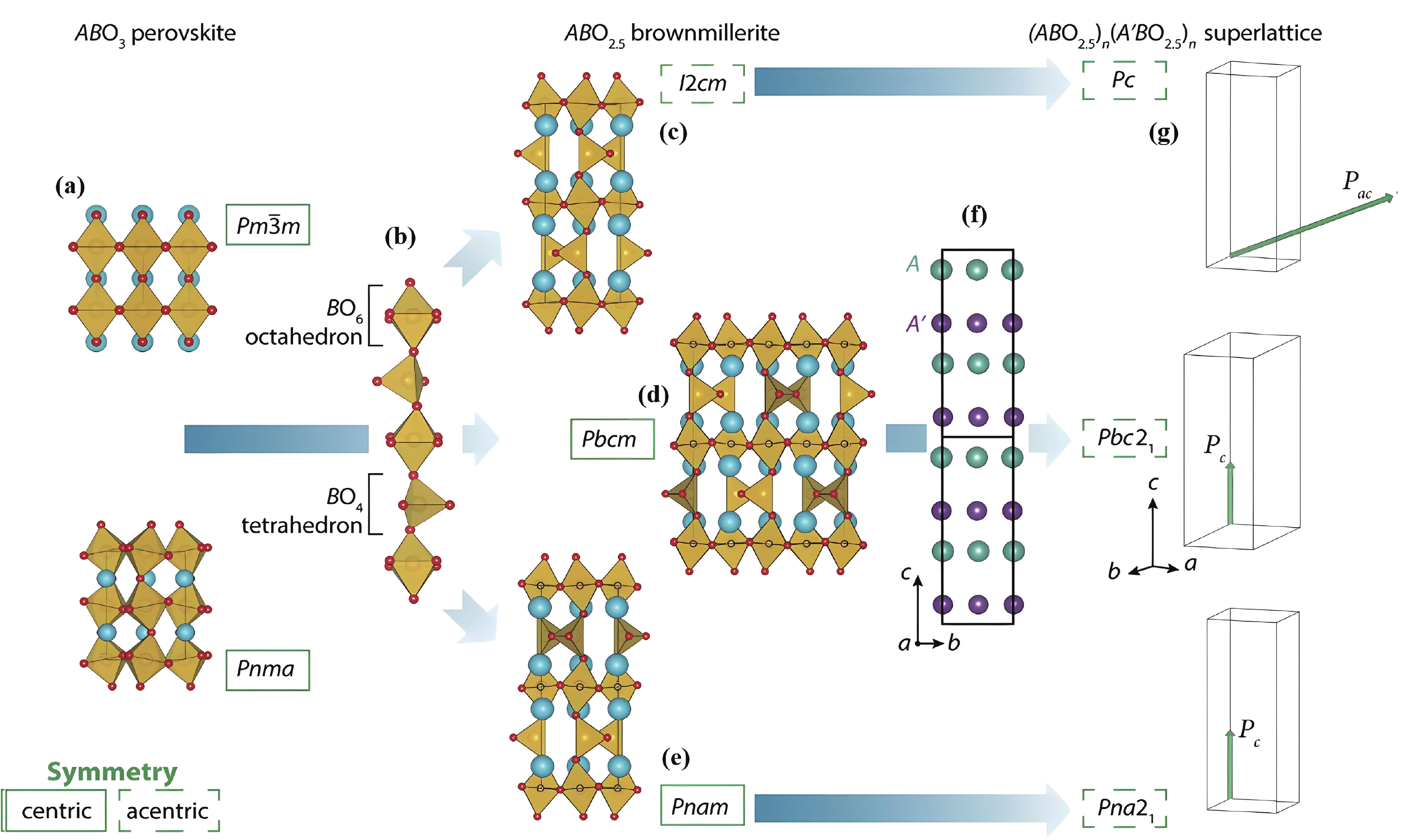
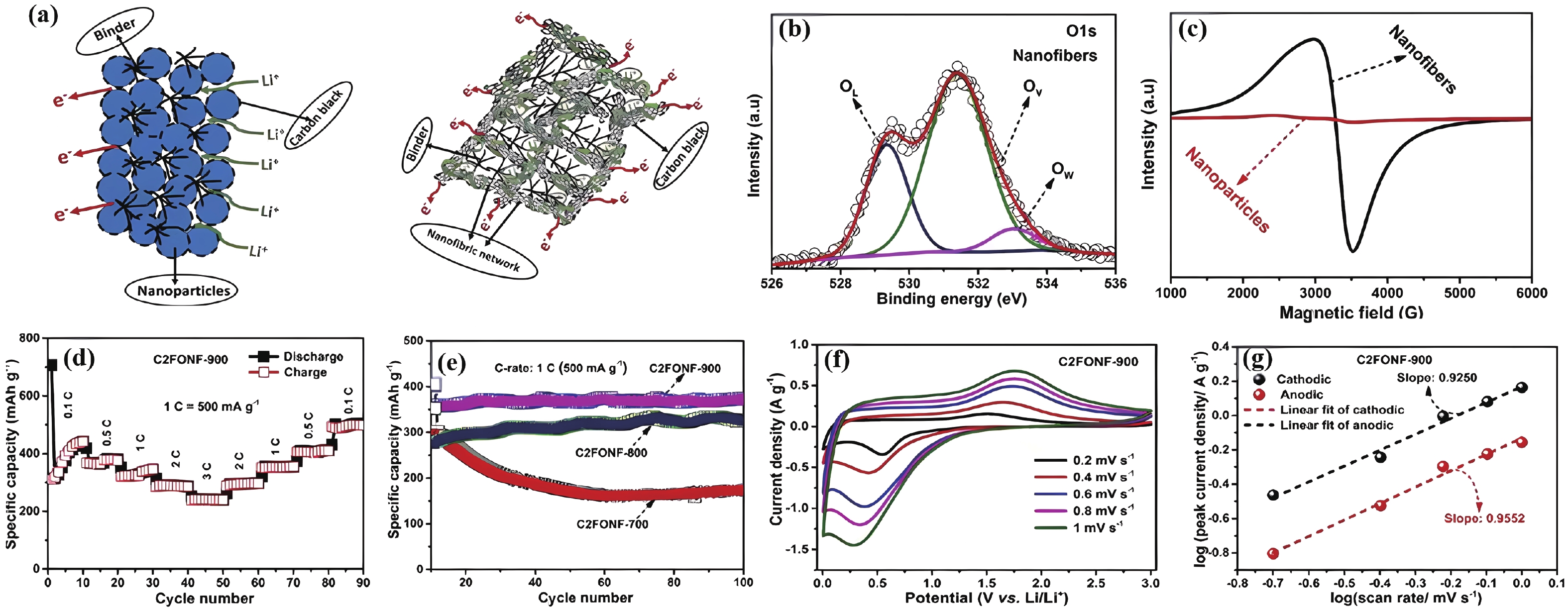
| Citation: |
Mingyuan Ye, Xiaorui Hao, Jinfeng Zeng, Lin Li, Pengfei Wang, Chenglin Zhang, Li Liu, Fanian Shi, Yuhan Wu. Research progress of alkaline earth metal iron-based oxides as anodes for lithium-ion batteries[J]. Journal of Semiconductors, 2024, 45(2): 021801. doi: 10.1088/1674-4926/45/2/021801
****
M Y Ye, X R Hao, J F Zeng, L Li, P F Wang, C L Zhang, L Liu, F N Shi, Y H Wu. Research progress of alkaline earth metal iron-based oxides as anodes for lithium-ion batteries[J]. J. Semicond, 2024, 45(2): 021801. doi: 10.1088/1674-4926/45/2/021801
|
Research progress of alkaline earth metal iron-based oxides as anodes for lithium-ion batteries
DOI: 10.1088/1674-4926/45/2/021801
More Information
-
Abstract
Anode materials are an essential part of lithium-ion batteries (LIBs), which determine the performance and safety of LIBs. Currently, graphite, as the anode material of commercial LIBs, is limited by its low theoretical capacity of 372 mA·h·g−1, thus hindering further development toward high-capacity and large-scale applications. Alkaline earth metal iron-based oxides are considered a promising candidate to replace graphite because of their low preparation cost, good thermal stability, superior stability, and high electrochemical performance. Nonetheless, many issues and challenges remain to be addressed. Herein, we systematically summarize the research progress of alkaline earth metal iron-based oxides as LIB anodes. Meanwhile, the material and structural properties, synthesis methods, electrochemical reaction mechanisms, and improvement strategies are introduced. Finally, existing challenges and future research directions are discussed to accelerate their practical application in commercial LIBs. -
References
[1] Wu Y H, Xu R, Wang Z J, et al. Carbon-free crystal-like Fe1– xS as an anode for potassium-ion batteries. ACS Appl Mater Interfaces, 2021, 13, 55218 doi: 10.1021/acsami.1c17799[2] Wu Y H, Xia W H, Liu Y Z, et al. Tungsten chalcogenides as anodes for potassium-ion batteries. Tungsten, 2023, 1[3] Wu Y H, Zhao Z Q, Hao X R, et al. Cathode materials for calcium-ion batteries: Current status and prospects. Carbon Neutralization, 2023, 2, 551 doi: 10.1002/cnl2.85[4] Luo Y X, Gao X Y, Dong M J, et al. Exploring the structural properties of cathode and anode materials in Li-ion battery via neutron diffraction technique. Chin J Struct Chem, 2023, 42, 100032 doi: 10.1016/j.cjsc.2023.100032[5] Liang Y H, Wu W B, Li D P, et al. Highly stable lithium metal batteries by regulating the lithium nitrate chemistry with a modified eutectic electrolyte. Adv Energy Mater, 2022, 12, 2202493 doi: 10.1002/aenm.202202493[6] Wei Y R, Cheng J, Li D P, et al. A structure self-healing Li-rich cathode achieved by lithium supplement of Li-rich LLZO coating. Adv Funct Mater, 2023, 33, 2214775 doi: 10.1002/adfm.202214775[7] Zeng H H, Xing B L, Zhang C T, et al. In situ synthesis of MnO2/porous graphitic carbon composites as high-capacity anode materials for lithium-ion batteries. Energy Fuels, 2020, 34, 2480 doi: 10.1021/acs.energyfuels.9b04325[8] Zhu L, Li F, Yao T H, et al. Electrospun MnCo2O4 nanotubes as high-performance anode materials for lithium-ion batteries. Energy Fuels, 2020, 34, 11574 doi: 10.1021/acs.energyfuels.0c02302[9] Hadouchi M, Koketsu T, Hu Z W, et al. The origin of fast-charging lithium iron phosphate for batteries. Battery Energy, 2022, 1, 20210010 doi: 10.1002/bte2.20210010[10] Shen S H, Zhang S Z, Deng S J, et al. Bioinspired large-scale production of multidimensional high-rate anodes for both liquid & solid-state lithium ion batteries. J Mater Chem A, 2019, 7, 22958 doi: 10.1039/C9TA08899G[11] Zeng J F, Yang L, Shao R W, et al. Mesoscopic Ti2Nb10O29 cages comprised of nanorod units as high-rate lithium-ion battery anode. J Colloid Interface Sci, 2021, 600, 111 doi: 10.1016/j.jcis.2021.04.136[12] Poizot P, Laruelle S, Grugeon S, et al. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature, 2000, 407, 496 doi: 10.1038/35035045[13] Hong M, Su Y J, Zhou C, et al. Scalable synthesis of γ-Fe2O3/CNT composite as high-performance anode material for lithium-ion batteries. J Alloy Compd, 2019, 770, 116 doi: 10.1016/j.jallcom.2018.08.118[14] Wang J T, Ma C, Tang J W, et al. Facile fabrication of Fe2O3-decorated carbon matrixes with a multidimensional structure as anodes for lithium-ion batteries. Energy Fuels, 2021, 35, 816 doi: 10.1021/acs.energyfuels.0c02947[15] Wang Z Y, Li W L, Pan C F, et al. Carbon coated tetrakaidecahedron tin ferrite (SnFe2O4) with high pseudocapacitance as anode material for lithium-ion batteries. Appl Surf Sci, 2022, 587, 152870 doi: 10.1016/j.apsusc.2022.152870[16] Gu H Y, Zhang Y M, Huang M Q, et al. Hydrolysis-coupled redox reaction to 3D Cu/Fe3O4 nanorod array electrodes for high-performance lithium-ion batteries. Inorg Chem, 2017, 56, 7657 doi: 10.1021/acs.inorgchem.7b00112[17] Mao J Y, Niu D C, Zheng N, et al. Fe3O4-embedded and N-doped hierarchically porous carbon nanospheres as high-performance lithium ion battery anodes. ACS Sustainable Chem Eng, 2019, 7, 3424 doi: 10.1021/acssuschemeng.8b05651[18] Ma J A, Kong Y E, Liu S C, et al. Flexible phosphorus-doped graphene/metal–organic framework-derived porous Fe2O3 anode for lithium-ion battery. ACS Appl Energy Mater, 2020, 3, 11900 doi: 10.1021/acsaem.0c02030[19] Chen X F, Zhu X Y, Cao G P, et al. Fe3O4-based anodes with high conductivity and fast ion diffusivity designed for high-energy lithium-ion batteries. Energy Fuels, 2021, 35, 1810 doi: 10.1021/acs.energyfuels.0c03620[20] Musa N, Woo H J, Teo L P, et al. Optimization of Li2SnO3 synthesis for anode material application in Li-ion batteries. Mater Today:Proc, 2017, 4, 5169 doi: 10.1016/j.matpr.2017.05.023[21] Deng S J, Zhu H, Wang G Z, et al. Boosting fast energy storage by synergistic engineering of carbon and deficiency. Nat Commun, 2020, 11, 132 doi: 10.1038/s41467-019-13945-1[22] Shen S H, Guo W H, Xie D, et al. A synergistic vertical graphene skeleton and S-C shell to construct high-performance TiNb2O7-based core/shell arrays. J Mater Chem A, 2018, 6, 20195 doi: 10.1039/C8TA06858E[23] Yao Z J, Xia X H, Zhang Y, et al. Superior high-rate lithium-ion storage on Ti2Nb10O29 arrays via synergistic TiC/C skeleton and N-doped carbon shell. Nano Energy, 2018, 54, 304 doi: 10.1016/j.nanoen.2018.10.024[24] Wu J B, Pan G X, Zhong W W, et al. Rational synthesis of Cr0.5Nb24.5O62 microspheres as high-rate electrodes for lithium ion batteries. J Colloid Interface Sci, 2020, 562, 511 doi: 10.1016/j.jcis.2019.11.085[25] Li Y Z, Meng Y S, Liu X L, et al. Double-protected zinc ferrite nanospheres as high rate and stable anode materials for lithium ion batteries. J Power Sources, 2019, 442, 227256 doi: 10.1016/j.jpowsour.2019.227256[26] Luo L, Chen Z, Ke H Z, et al. Facile synthesis of three-dimensional MgFe2O4/graphene aerogel composites for high lithium storage performance and its application in full cell. Mater Des, 2019, 182, 108043 doi: 10.1016/j.matdes.2019.108043[27] Bhujun B, Tan M T T, Shanmugam A S. Study of mixed ternary transition metal ferrites as potential electrodes for supercapacitor applications. Results Phys, 2017, 7, 345 doi: 10.1016/j.rinp.2016.04.010[28] Datt G, Kotabage C, Abhyankar A C. Ferromagnetic resonance of NiCoFe2O4 nanoparticles and microwave absorption properties of flexible NiCoFe2O4-carbon black/poly(vinyl alcohol) composites. Phys Chem Chem Phys, 2017, 19, 20699 doi: 10.1039/C7CP03953K[29] Zeng Z X, Lan M S, Zhang Q, et al. Effect of solution concentration on magnetoelectric properties of barium ferrite ceramics. Appl Phys A, 2021, 127, 946 doi: 10.1007/s00339-021-05103-6[30] Bock D C, Tallman K R, Guo H Y, et al. (De)lithiation of spinel ferrites Fe3O4, MgFe2O4, and ZnFe2O4: A combined spectroscopic, diffraction and theory study. Phys Chem Chem Phys, 2020, 22, 26200 doi: 10.1039/D0CP02322A[31] Ortiz-Quiñonez J L, Das S, Pal U. Catalytic and pseudocapacitive energy storage performance of metal (Co, Ni, Cu and Mn) ferrite nanostructures and nanocomposites. Prog Mater Sci, 2022, 130, 100995 doi: 10.1016/j.pmatsci.2022.100995[32] Li J B, Li Z H, Ning F Y, et al. Ultrathin mesoporous Co3O4 nanosheet arrays for high-performance lithium-ion batteries. ACS Omega, 2018, 3, 1675 doi: 10.1021/acsomega.7b01832[33] Feng D Y, Yang H, Guo X Z. 3-Dimensional hierarchically porous ZnFe2O4/C composites with stable performance as anode materials for Li-ion batteries. Chem Eng J, 2019, 355, 687 doi: 10.1016/j.cej.2018.08.202[34] Ma J Y, Guo E Y, Yin L W. Porous hierarchical spinel Mn-doped NiCo2O4 nanosheet architectures as high-performance anodes for lithium-ion batteries and electrochemical reaction mechanism. J Mater Sci, 2019, 30, 8555 doi: 10.1007/s10854-019-01176-5[35] Liu Q, Hu Y H, Yu X R, et al. The pursuit of commercial silicon-based microparticle anodes for advanced lithium-ion batteries: A review. Nano Res Energy, 2022, 1, e9120037 doi: 10.26599/NRE.2022.9120037[36] Sivakumar N, Gnanakan S R P, Karthikeyan K, et al. Nanostructured MgFe2O4 as anode materials for lithium-ion batteries. J Alloys Compd, 2011, 509, 7038 doi: 10.1016/j.jallcom.2011.03.123[37] Rezaie E, Rezanezhad A, Hajalilou A, et al. Electrochemical behavior of SrFe12O19/CoFe2O4 composite nanoparticles synthesized via one-pot hydrothermal method. J Alloys Compd, 2019, 789, 40 doi: 10.1016/j.jallcom.2019.03.079[38] Araújo J C R, Araujo-Barbosa S, Souza A L R, et al. Tuning structural, magnetic, electrical, and dielectric properties of MgFe2O4 synthesized by sol-gel followed by heat treatment. J Phys Chem Solids, 2021, 154, 110051 doi: 10.1016/j.jpcs.2021.110051[39] de Hoyos-Sifuentes D H, Reséndiz-Hernández P J, Díaz-Guillén J A, et al. Synthesis and characterization of MgFe2O4 nanoparticles and PEG-coated MgFe2O4 nanocomposite. J Mater Res Technol, 2022, 18, 3130 doi: 10.1016/j.jmrt.2022.03.117[40] Adewuyi A, Gervasi C A, Mirífico M V. Synthesis of strontium ferrite and its role in the removal of methyl orange, phenolphthalein and bromothymol blue from laboratory wastewater. Surf Interfaces, 2021, 27, 101567 doi: 10.1016/j.surfin.2021.101567[41] Naaz F, Dubey H K, Kumari C, et al. Structural and magnetic properties of MgFe2O4 nanopowder synthesized via co-precipitation route. SN Appl Sci, 2020, 2, 808 doi: 10.1007/s42452-020-2611-9[42] Ghahfarokhi S E M, Shobegar E M, Shoushtari M Z. Preparation and characterization of spinel SrFe2O4 nanoparticles by method sol-gel. J Aust Ceram Soc, 2021, 57, 1359 doi: 10.1007/s41779-021-00633-x[43] Yamaguchi A, Sako H, Miyauchi M. Synthesis of CaFe2O4 nanorod thin film using molten salt method and analysis of its photoelectrochemical properties. Chem Lett, 2020, 49, 1462 doi: 10.1246/cl.200558[44] Guo L, Okinaka N, Zhang L, et al. Molten salt-assisted shape modification of CaFe2O4 nanorods for highly efficient photocatalytic degradation of methylene blue. Opt Mater, 2021, 119, 111295 doi: 10.1016/j.optmat.2021.111295[45] Guo Y, Qin G H, Liang E Q, et al. MOFs-derived MgFe2O4 microboxes as anode material for lithium-ion batteries with superior performance. Ceram Int, 2017, 43, 12519 doi: 10.1016/j.ceramint.2017.06.124[46] Wang H, Yang Q, Zheng N, et al. Roadmap of amorphous metal-organic framework for electrochemical energy conversion and storage. Nano Res, 2023, 16, 4107 doi: 10.1007/s12274-022-5114-8[47] Permien S, Indris S, Scheuermann M, et al. Is there a universal reaction mechanism of Li insertion into oxidic spinels: A case study using MgFe2O4. J Mater Chem A, 2015, 3, 1549 doi: 10.1039/C4TA05054A[48] Guo H Y, Durham J L, Brady A B, et al. Essential role of spinel MgFe2O4 surfaces during discharge. J Electrochem Soc, 2020, 167, 090506 doi: 10.1149/1945-7111/ab7f89[49] Tan Q K, Wang C, Cao Y D, et al. Synthesis of a zinc ferrite effectively encapsulated by reduced graphene oxide composite anode material for high-rate lithium ion storage. J Colloid Interface Sci, 2020, 579, 723 doi: 10.1016/j.jcis.2020.07.004[50] Zhang M Y, Liu Y, Zhu H T, et al. Hierarchical bead chain ZnFe2O4-PEDOT composites with enhanced Li-ion storage properties as anode materials for lithium-ion batteries. Appl Surf Sci, 2020, 529, 147078 doi: 10.1016/j.apsusc.2020.147078[51] Wu Y H, Wu X N, Guan Y Y, et al. Carbon-based flexible electrodes for electrochemical potassium storage devices. New Carbon Mater, 2022, 37, 852 doi: 10.1016/S1872-5805(22)60631-0[52] Zhu H F, Sha M, Zhao H P, et al. Highly-rough surface carbon nanofibers film as an effective interlayer for lithium–sulfur batteries. J Semicond, 2020, 41, 092701 doi: 10.1088/1674-4926/41/9/092701[53] Wu Y H, Chen G B, Wu X N, et al. Research progress on vanadium oxides for potassium-ion batteries. J Semicond, 2023, 44, 041701 doi: 10.1088/1674-4926/44/4/041701[54] He S J, Wang Z D, Wang Z J, et al. Recent progress and future prospect of novel multi-ion storage devices. J Semicond, 2023, 44, 040201 doi: 10.1088/1674-4926/44/4/040201[55] Wang X H, Wang H L. Designing carbon anodes for advanced potassium-ion batteries: Materials, modifications, and mechanisms. Adv Powder Mater, 2022, 1, 100057 doi: 10.1016/j.apmate.2022.100057[56] Ding C Y, Wang L J, Zhou W W, et al. New design on Li-ion battery anode of ternary complex metal/metal oxide@CNT: A case study of hierarchical NiCo-NiCo2O4@CNTs. Chem Eng J, 2018, 353, 340 doi: 10.1016/j.cej.2018.07.125[57] Han W J, Qin X Y, Wu J X, et al. Electrosprayed porous Fe3O4/carbon microspheres as anode materials for high-performance lithium-ion batteries. Nano Res, 2018, 11, 892 doi: 10.1007/s12274-017-1700-6[58] Zhang Y T, Zhang Y L, Zhang K B, et al. Nitrogen-doped graphene nanosheet coated nanospherical Fe3O4 from zeolitic imidazolate frameworks template as anode of lithium ion batteries. Energy Fuels, 2020, 34, 14986 doi: 10.1021/acs.energyfuels.0c02853[59] Zhao C H, Fan J T, Guo J J, et al. Dual carbon-supported ZnO/CuO nanocomposites as an anode with improved performance for Li-ion batteries. Energy Fuels, 2022, 36, 5483 doi: 10.1021/acs.energyfuels.2c00718[60] Tang Y K, Wang H R, Zhang Y, et al. In-situ reduction synthesis of one dimensional hybrid porous TiO@C anode for high-performance Li-ion storage. Ceram Int, 2021, 47, 5832 doi: 10.1016/j.ceramint.2020.10.053[61] Yin Y H, Liu W F, Huo N N, et al. Synthesis of vesicle-like MgFe2O4/graphene 3D network anode material with enhanced lithium storage performance. ACS Sustainable Chem Eng, 2017, 5, 563 doi: 10.1021/acssuschemeng.6b01949[62] Yu Y, Li M T, Li Q W, et al. Core-shell MgFe2O4@C nano-composites derived via thermal decomposition-reduction dual strategy for superior lithium storage. J Alloys Compd, 2020, 834, 155207 doi: 10.1016/j.jallcom.2020.155207[63] Wang B, Ruan T T, Chen Y, et al. Graphene-based composites for electrochemical energy storage. Energy Storage Mater, 2020, 24, 22 doi: 10.1016/j.ensm.2019.08.004[64] Liu W F, Gao R Z, Zhang H S, et al. Improving the cycle performance of MgFe2O4 anode material based on the spatial limiting effect. J Alloys Compd, 2021, 865, 158668 doi: 10.1016/j.jallcom.2021.158668[65] Liu W F, Gao R Z, Yin Y H, et al. 3D hierarchical porous N-doped carbon nanosheets/MgFe2O4 composite as anode material with excellent cycling stability and rate performance. Scr Mater, 2020, 189, 36 doi: 10.1016/j.scriptamat.2020.07.060[66] Gan Q M, Zhao K M, He Z, et al. Zeolitic imidazolate framework-8-derived N-doped porous carbon coated olive-shaped FeO x nanoparticles for lithium storage. J Power Sources, 2018, 384, 187 doi: 10.1016/j.jpowsour.2018.02.073[67] Peng M X, Qiu Y C, Zhang M X, et al. Improved electrochemical performance of SiO-based anode by N, P binary doped carbon coating. Appl Surf Sci, 2020, 507, 145060 doi: 10.1016/j.apsusc.2019.145060[68] Ma C R, Deng C J, Liao X Z, et al. Nitrogen and phosphorus codoped porous carbon framework as anode material for high rate lithium-ion batteries. ACS Appl Mater Interfaces, 2018, 10, 36969 doi: 10.1021/acsami.8b12302[69] Guo L Z, He H Y, Ren Y R, et al. Core-shell SiO@F-doped C composites with interspaces and voids as anodes for high-performance lithium-ion batteries. Chem Eng J, 2018, 335, 32 doi: 10.1016/j.cej.2017.10.145[70] Liu Y, Yang H H, Zheng H Y, et al. Structural characteristics and electrochemical performance of N, P-codoped porous carbon as a lithium-ion battery anode electrode. ACS Omega, 2022, 7, 34109 doi: 10.1021/acsomega.2c03400[71] Sun Y Q, Fu W, Hu Y X, et al. The role of tungsten-related elements for improving the electrochemical performances of cathode materials in lithium ion batteries. Tungsten, 2021, 3, 245 doi: 10.1007/s42864-021-00083-9[72] Liu W F, Pang Y D, Shi Z P, et al. Ultrafast kinetics in a PAN/MgFe2O4 flexible free-standing anode induced by heterojunction and oxygen vacancies. ACS Appl Mater Interfaces, 2022, 14, 11575 doi: 10.1021/acsami.2c01159[73] Yin X X, Shao X, Liang S J, et al. Preparation of well-dispersed MgFe2O4 nanospheres and their electrochemical performance for lithium-ion batteries. Bull Mater Sci, 2021, 44, 21 doi: 10.1007/s12034-020-02303-3[74] Yin Y H, Huo N N, Liu W F, et al. Hollow spheres of MgFe2O4 as anode material for lithium-ion batteries. Scr Mater, 2016, 110, 92 doi: 10.1016/j.scriptamat.2015.08.009[75] Han C H, Zhang X, Xu X M, et al. Porous CaFe2O4 as a promising lithium ion battery anode: A trade-off between high capacity and long-term stability. Nanoscale, 2018, 10, 12963 doi: 10.1039/C8NR03840F[76] Gong C, Bai Y J, Qi Y X, et al. Preparation of carbon-coated MgFe2O4 with excellent cycling and rate performance. Electrochim Acta, 2013, 90, 119 doi: 10.1016/j.electacta.2012.11.128[77] Luo L, Li D W, Zang J, et al. Carbon‐coated magnesium ferrite nanofibers for lithium‐ion battery anodes with enhanced cycling performance. Energy Technol, 2017, 5, 1364 doi: 10.1002/ente.201600686[78] Yin Y H, Liu W F, Huo N N, et al. High rate capability and long cycle stability of Fe2O3/MgFe2O4 anode material synthesized by gel-cast processing. Chem Eng J, 2017, 307, 999 doi: 10.1016/j.cej.2016.09.025[79] Lee S B, Balasubramaniam R. Double-shelled hybrid MgFe2O4/Fe2O3 hollow microspheres as a high-capacity anode for lithium-ion batteries. J Ind Eng Chem, 2022, 110, 262 doi: 10.1016/j.jiec.2022.03.001[80] Yin Y H, Liu W F, Gao R Z, et al. A 3D porous MgFe2O4 integrative electrode as a binder-free anode with high rate capability and long cycle lifetime. ChemElectroChem, 2019, 6, 757 doi: 10.1002/celc.201801374[81] Wang D, Wu J L, Bai D X, et al. Mesoporous spinel ferrite composite derived from a ternary MgZnFe-layered double hydroxide precursor for lithium storage. J Alloys Compd, 2017, 726, 306 doi: 10.1016/j.jallcom.2017.08.005[82] Xu C X, Manukyan K V, Adams R A, et al. One-step solution combustion synthesis of CuO/Cu2O/C anode for long cycle life Li-ion batteries. Carbon, 2019, 142, 51 doi: 10.1016/j.carbon.2018.10.016[83] Adams R A, Pol V G, Varma A. Tailored solution combustion synthesis of high performance ZnCo2O4 anode materials for lithium-ion batteries. Ind Eng Chem Res, 2017, 56, 7173 doi: 10.1021/acs.iecr.7b00295[84] Shaji N, Santhoshkumar P, Nanthagopal M, et al. Electrochemical performance of porous CaFe2O4 as a promising anode material for lithium-ion batteries. Appl Surf Sci, 2019, 491, 757 doi: 10.1016/j.apsusc.2019.06.136[85] Sundriyal S K, Sharma Y. Revealing the effect of oxygen defects and morphology on Li-storage performance of calcium iron oxide. J Electrochem Soc, 2020, 167, 110526 doi: 10.1149/1945-7111/aba36c[86] Mousavi Ghahfarokhi S E, Shobegar E M. Influence of pH on the structural, magnetic and optical properties of SrFe2O4 nanoparticles. J Mater Res Technol, 2020, 9, 12177 doi: 10.1016/j.jmrt.2020.08.063[87] Mousavi Ghahfarokhi S E, Shobegar E M, Shoushtari M Z. Effects of sintering temperature on structural, morphological and magnetic properties of strontium ferrite nanoparticles. J Supercond Nov Magn, 2019, 32, 1067 doi: 10.1007/s10948-018-4799-0[88] Zhu S M, Fu J C, Li H L, et al. Direct observation of magnetocrystalline anisotropy tuning magnetization configurations in uniaxial magnetic nanomaterials. ACS Nano, 2018, 12, 3442 doi: 10.1021/acsnano.8b00058[89] Marouani Y, Massoudi J, Noumi M, et al. Electrical conductivity and dielectric properties of Sr doped M-type Barium hexaferrite BaFe12O19. RSC Adv, 2021, 11, 1531 doi: 10.1039/D0RA09465J[90] Wang Z, Zha X H, Wu Z, et al. First-principles study on electronic and magnetic properties of Mn-doped strontium ferrite SrFe12O19. J Inorg Mater, 2019, 34, 1047 doi: 10.15541/jim20190003[91] Lamouri R, Daoudi K, Absike H, et al. Structural, electronic and magnetic properties of Co-substituted SrFe12O19: A DFT study. Mater Today Commun, 2021, 28, 102589 doi: 10.1016/j.mtcomm.2021.102589[92] Topal U, Ozkan H, Dorosinskii L. Finding optimal Fe/Ba ratio to obtain single phase BaFe12O19 prepared by ammonium nitrate melt technique. J Alloys Compd, 2007, 428, 17 doi: 10.1016/j.jallcom.2006.03.047[93] Rezaie E, Rezanezhad A, Ghadimi L S, et al. Effect of calcination on structural and supercapacitance properties of hydrothermally synthesized plate-like SrFe12O19 hexaferrite nanoparticles. Ceram Int, 2018, 44, 20285 doi: 10.1016/j.ceramint.2018.08.014[94] Zhao Y, Huang Y, Wang Q F, et al. Preparation of BaFe12O19 as anode material for lithium-ion batteries through sol-gel method. J Sol-Gel Sci Technol, 2013, 66, 238 doi: 10.1007/s10971-013-2999-4[95] Hu C X, Qiu S, Lu G X, et al. Enhanced electrochemical performance of Barium hexaferrite nanoplates by Zn2+ doping serving as anode materials. RSC Adv, 2015, 5, 70749 doi: 10.1039/C5RA12127B[96] Hu C X, Cao H L, Wang S Y, et al. Synthesis of strontium hexaferrite nanoplates and the enhancement of their electrochemical performance by Zn2+ doping for high-rate and long-life lithium-ion batteries. New J Chem, 2017, 41, 6427 doi: 10.1039/C7NJ01036B[97] Young J, Moon E J, Mukherjee D, et al. Polar oxides without inversion symmetry through vacancy and chemical order. J Am Chem Soc, 2017, 139, 2833 doi: 10.1021/jacs.6b10697[98] Sundriyal S K, Sharma Y. Role of oxygen deficiency and microstructural voids/gaps in nanostructures of Ca2Fe2O5 as an anode toward next-generation high-performance Li-ion batteries. ACS Appl Energy Mater, 2020, 3, 6360 doi: 10.1021/acsaem.0c00578[99] Wang W X, Xiong F Y, Zhu S H, et al. Defect engineering in molybdenum-based electrode materials for energy storage. eScience, 2022, 2, 278 doi: 10.1016/j.esci.2022.04.005[100] Yang L, Zeng J F, Zhou L, et al. Orderly defective superstructure for enhanced pseudocapacitive storage in titanium niobium oxide. Nano Res, 2022, 15, 1570 doi: 10.1007/s12274-021-3703-6[101] Zhang S M, Wang X H, Li Y, et al. Moderate oxygen-deficient Fe(III) oxide nanoplates for high performance symmetric supercapacitors. J Colloid Interface Sci, 2020, 565, 458 doi: 10.1016/j.jcis.2020.01.060[102] Zhang Y P, Zhang M Q, Liu Y Y, et al. Oxygen vacancy regulated TiNb2O7 compound with enhanced electrochemical performance used as anode material in Li-ion batteries. Electrochim Acta, 2020, 330, 135299 doi: 10.1016/j.electacta.2019.135299[103] Sundriyal S K, Sharma Y. Controlled generation and tuning the oxygen defects in nanofibers of Ca2Fe2O5 toward high and stable Li-ion battery anode. Appl Surf Sci, 2021, 560, 150055 doi: 10.1016/j.apsusc.2021.150055[104] Zhang K, Liang S J, Yin X X, et al. Mesoporous Ca2Fe2O5/α-Fe2O3 nanocomposite anode for lithium-ion batteries. J Solid State Chem, 2020, 286, 121300 doi: 10.1016/j.jssc.2020.121300 -
Proportional views





 Mingyuan Ye is currently a graduate student at Shenyang University of Technology. Her research interest is nanostructural metal oxide-based anode materials for lithium-ion batteries.
Mingyuan Ye is currently a graduate student at Shenyang University of Technology. Her research interest is nanostructural metal oxide-based anode materials for lithium-ion batteries. Xiaorui Hao is currently a graduate student at Nanjing Tech University. Her research focuses on zinc-ion battery electrolytes and the interphase chemistry of electrodes/electrolytes.
Xiaorui Hao is currently a graduate student at Nanjing Tech University. Her research focuses on zinc-ion battery electrolytes and the interphase chemistry of electrodes/electrolytes. Lin Li is an Oujiang Distinguished Professor and associate dean of the Institute for Carbon Neutralization, College of Chemistry and Materials Engineering, Wenzhou University. He received his B.S. degree in materials science and engineering from Nanchang University (2016). He obtained his Ph.D. from Nankai University in 2021. His research focuses on advanced electrode materials and electrolytes for lithium-ion batteries, sodium-ion batteries, and potassium-ion batteries.
Lin Li is an Oujiang Distinguished Professor and associate dean of the Institute for Carbon Neutralization, College of Chemistry and Materials Engineering, Wenzhou University. He received his B.S. degree in materials science and engineering from Nanchang University (2016). He obtained his Ph.D. from Nankai University in 2021. His research focuses on advanced electrode materials and electrolytes for lithium-ion batteries, sodium-ion batteries, and potassium-ion batteries. Chenglin Zhang is currently a Jinshan Youth Distinguished Professor at Jiangsu University, China. He received his Ph.D. degree in Applied Physics from Institute of Physics, Technische Universität Ilmenau in 2022. His research mainly focuses on new electrochemical energy storage devices, such as sodium- and potassium-ion batteries, and hybrid ion capacitors.
Chenglin Zhang is currently a Jinshan Youth Distinguished Professor at Jiangsu University, China. He received his Ph.D. degree in Applied Physics from Institute of Physics, Technische Universität Ilmenau in 2022. His research mainly focuses on new electrochemical energy storage devices, such as sodium- and potassium-ion batteries, and hybrid ion capacitors. Fanian Shi received his Ph.D. in applied chemistry in 1996 at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. He is currently a full professor at the Shenyang University of Technology and a member of the committee on Energy and Environment, China Energy Society. He specializes in the research of MOF composites for energy materials, photocatalysts, etc.
Fanian Shi received his Ph.D. in applied chemistry in 1996 at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. He is currently a full professor at the Shenyang University of Technology and a member of the committee on Energy and Environment, China Energy Society. He specializes in the research of MOF composites for energy materials, photocatalysts, etc. Yuhan Wu received his Ph.D. degree in material physics at the Ilmenau University of Technology in 2021. Since 2022, he has been an associate professor at the Shenyang University of Technology. His research interests focus on designing and synthesizing multiscale materials for energy conversion and storage.
Yuhan Wu received his Ph.D. degree in material physics at the Ilmenau University of Technology in 2021. Since 2022, he has been an associate professor at the Shenyang University of Technology. His research interests focus on designing and synthesizing multiscale materials for energy conversion and storage.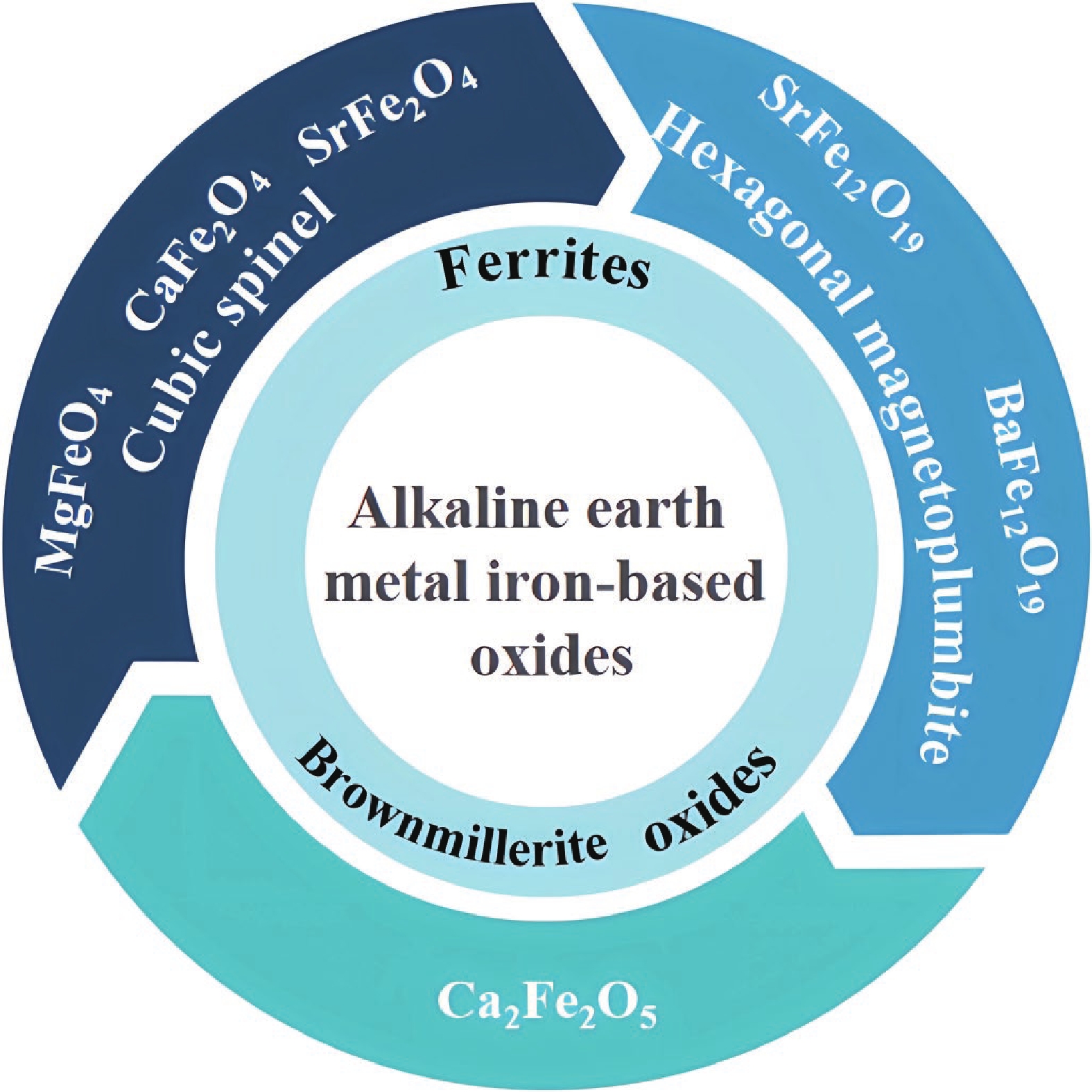
 DownLoad:
DownLoad: