| Citation: |
Danrui Wan, Jianping Zhou, Guoyun Meng, Ning Su, Dongdong Zhang, Lian Duan, Junqiao Ding. Peripheral carbazole units-decorated MR emitter containing B−N covalent bond for highly efficient green OLEDs with low roll-off[J]. Journal of Semiconductors, 2024, 45(8): 082402. doi: 10.1088/1674-4926/24040008
****
D R Wan, J P Zhou, G Y Meng, N Su, D D Zhang, L Duan, and J Q Ding, Peripheral carbazole units-decorated MR emitter containing B−N covalent bond for highly efficient green OLEDs with low roll-off[J]. J. Semicond., 2024, 45(8), 082402 doi: 10.1088/1674-4926/24040008
|
Peripheral carbazole units-decorated MR emitter containing B−N covalent bond for highly efficient green OLEDs with low roll-off
DOI: 10.1088/1674-4926/24040008
More Information
-
Abstract
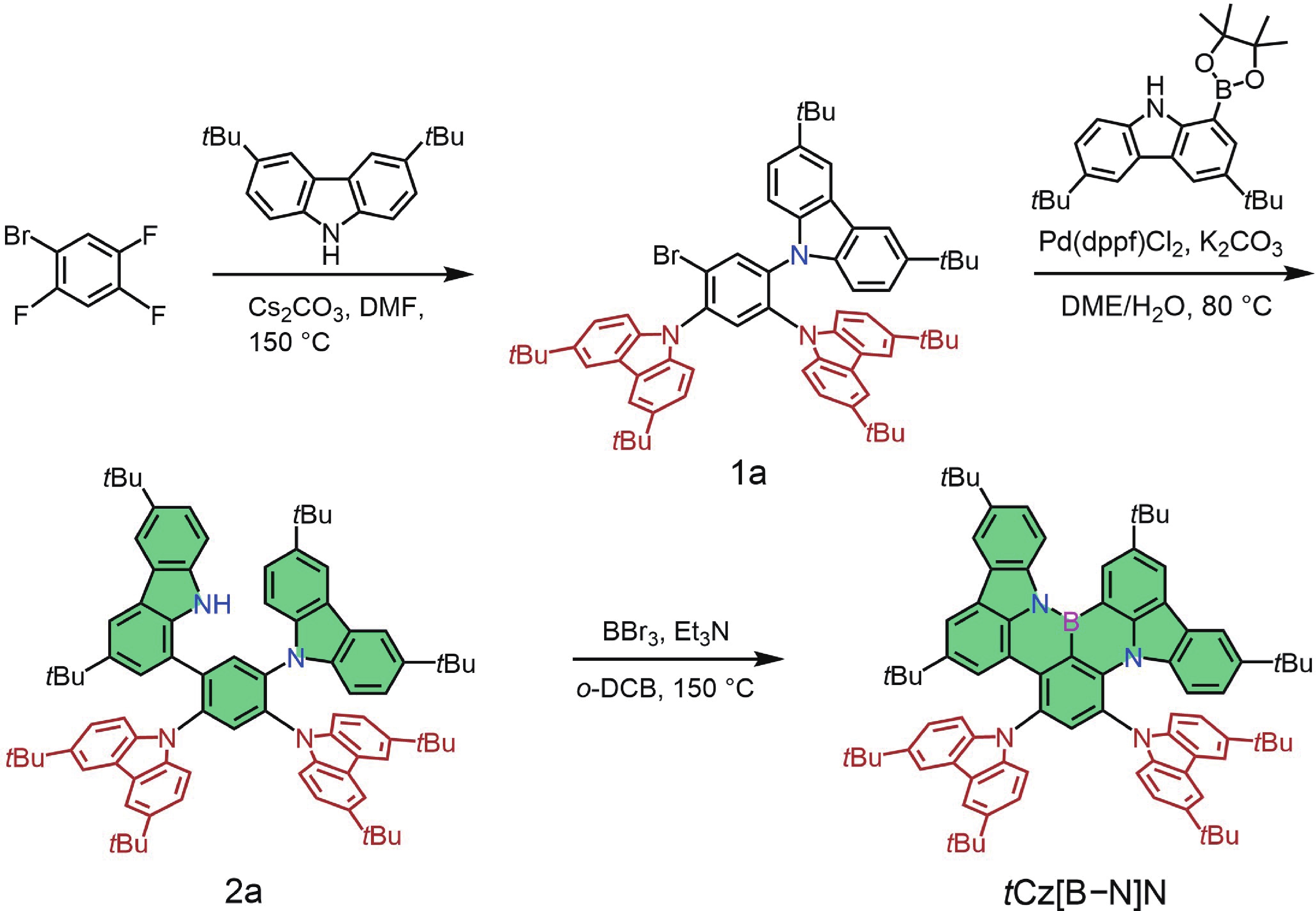
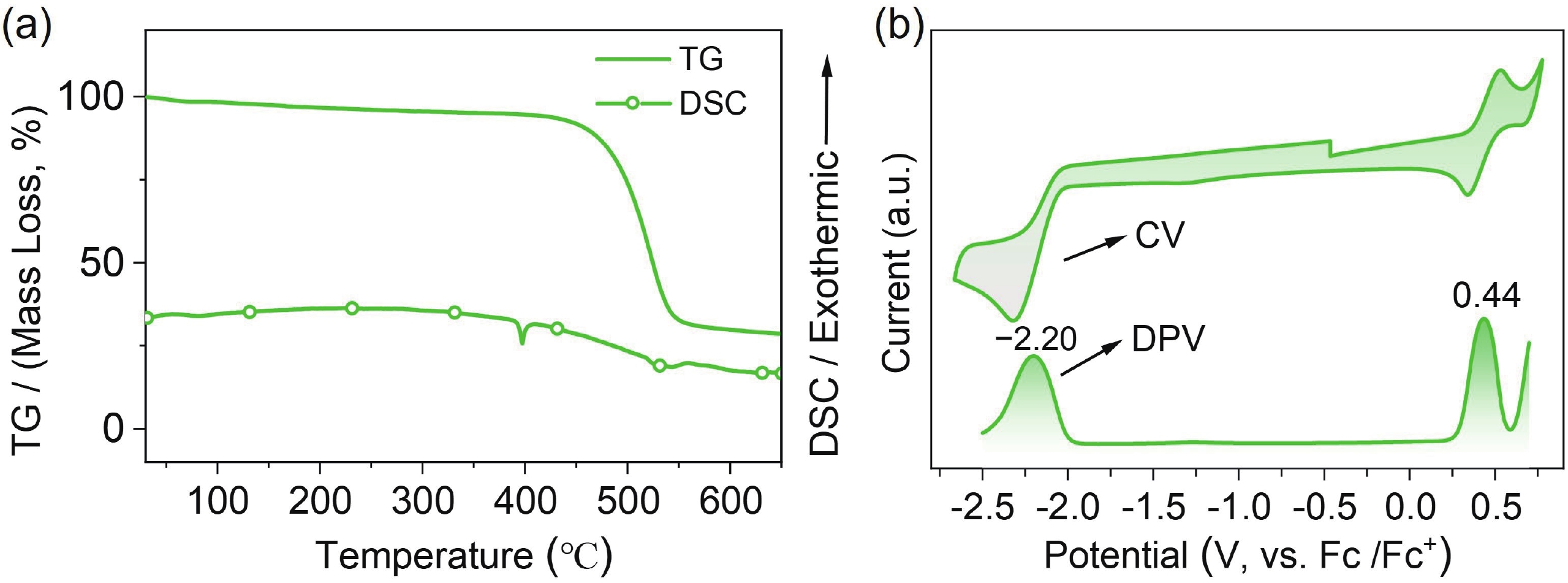
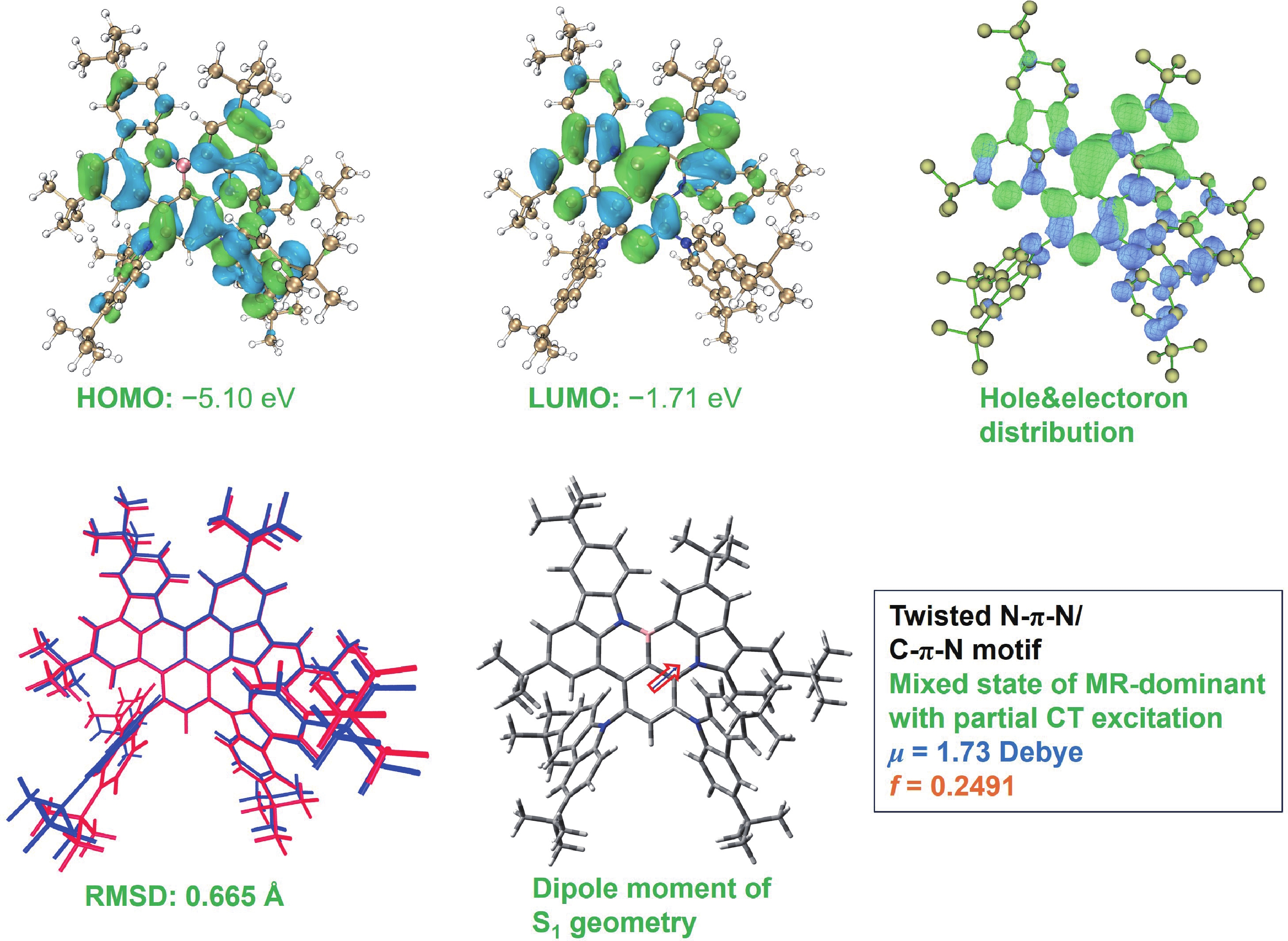
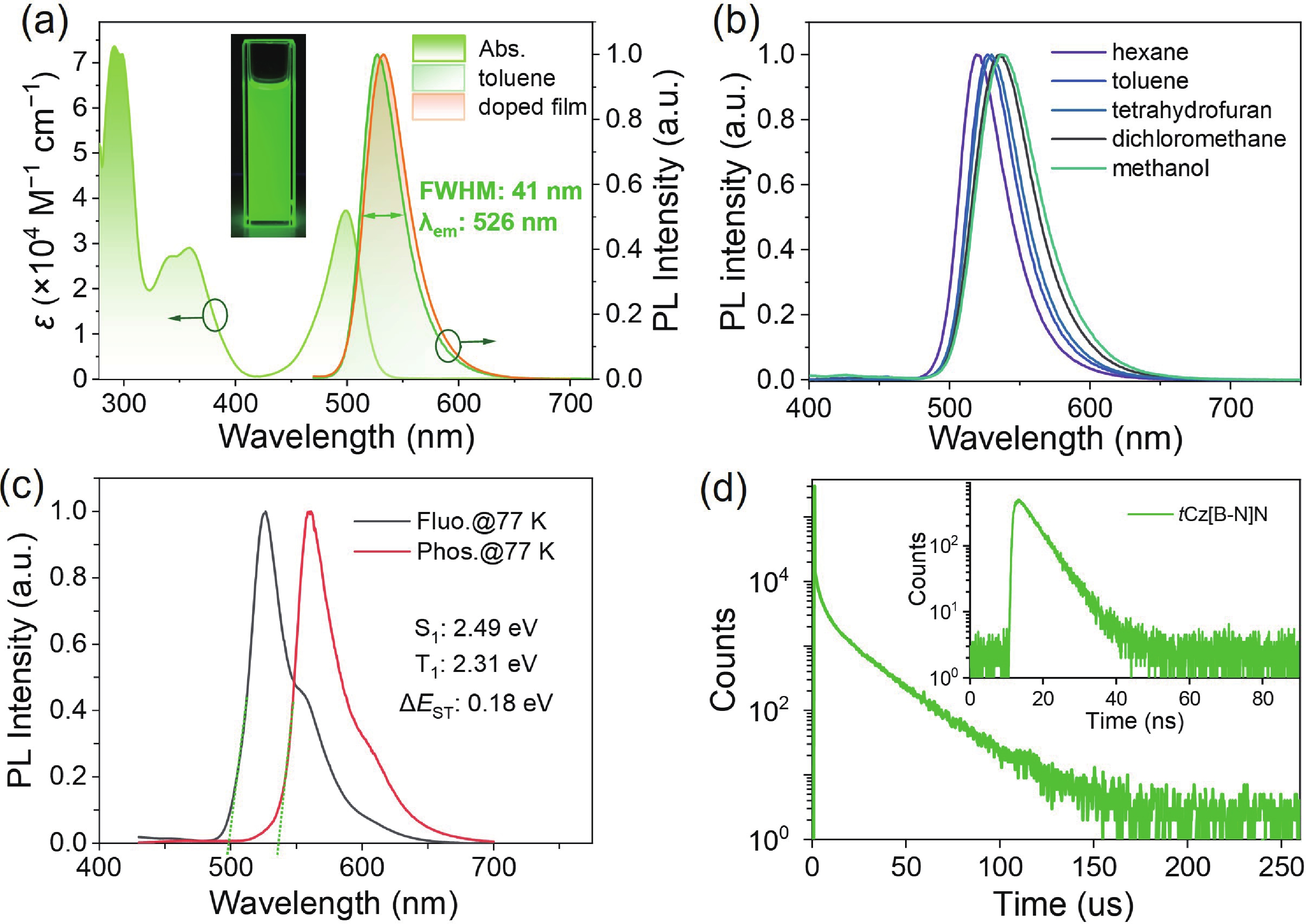
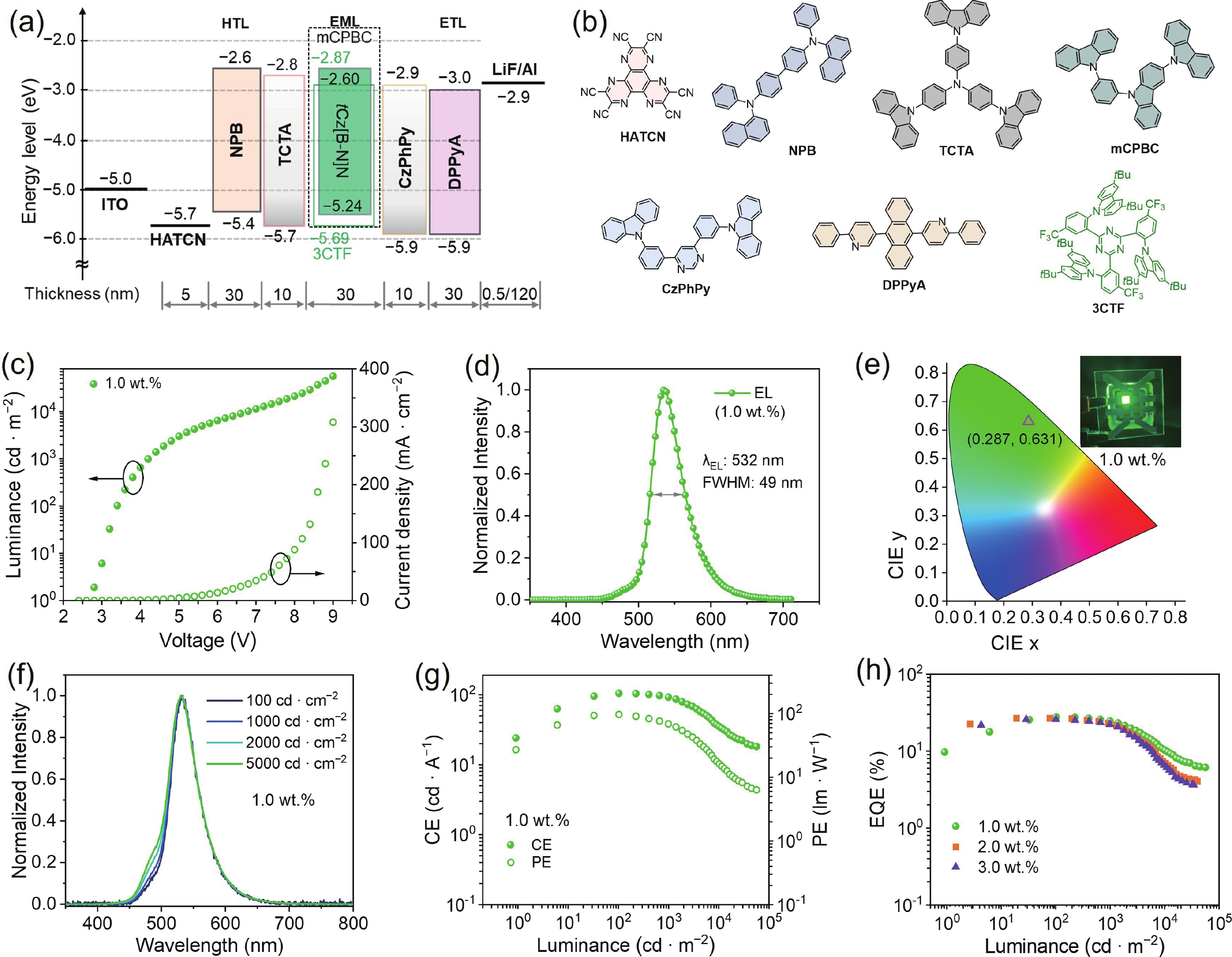
Boron−nitrogen doped multiple resonance (BN-MR) emitters, characterized by B−N covalent bonds, offer distinctive advantages as pivotal building blocks for facile access to novel MR emitters featuring narrowband spectra and high efficiency. However, there remains a scarcity of exploration concerning synthetic methods and structural derivations to expand the library of novel BN-MR emitters. Herein, we present the synthesis of a BN-MR emitter, tCz[B−N]N, through a one-pot borylation reaction directed by the amine group, achieving an impressive yield of 94%. The emitter is decorated by incorporating two 3,6-di-t-butylcarbazole (tCz) units into a B−N covalent bond doped BN-MR parent molecule via para-C−π−D and para-N−π−D conjugations. This peripheral decoration strategy enhances the reverse intersystem crossing process and shifts the emission band towards the pure green region, peaking at 526 nm with a narrowband full-width at half maximum (FWHM) of 41 nm. Consequently, organic light emitting diodes (OLEDs) employing this emitter achieved a maximum external quantum efficiency (EQEmax) value of 27.7%, with minimal efficiency roll-off. Even at a practical luminance of 1000 cd∙m−2, the device maintains a high EQE value of 24.6%. -
References
[1] Hatakeyama T, Shiren K, Nakajima K, et al. Ultrapure blue thermally activated delayed fluorescence molecules: Efficient HOMO-LUMO separation by the multiple resonance effect. Adv Mater, 2016, 28, 2777 doi: 10.1002/adma.201505491[2] Mamada M, Hayakawa M, Ochi J, et al. Organoboron-based multiple-resonance emitters: Synthesis, structure–property correlations, and prospects. Chem Soc Rev, 2024, 53, 1624 doi: 10.1039/D3CS00837A[3] Kim H J, Yasuda T. Narrowband emissive thermally activated delayed fluorescence materials. Adv Opt Mater, 2022, 10, 2201714 doi: 10.1002/adom.202201714[4] Madayanad Suresh S, Hall D, Beljonne D, et al. Multiresonant thermally activated delayed fluorescence emitters based on heteroatom-doped nanographenes: Recent advances and prospects for organic light-emitting diodes. Adv Funct Mater, 2020, 30, 1908677 doi: 10.1002/adfm.201908677[5] Han J M, Chen Y Y, Li N Q, et al. Versatile boron-based thermally activated delayed fluorescence materials for organic light-emitting diodes. Aggregate, 2022, 3, e182 doi: 10.1002/agt2.182[6] Chen C, Du C Z, Wang X Y. The rise of 1, 4-BN-heteroarenes: Synthesis, properties, and applications. Adv Sci, 2022, 9, 2200707 doi: 10.1002/advs.202200707[7] Yu Y J, Liu F M, Meng X Y, et al. Carbonyl-containing thermally activated delayed fluorescence emitters for narrow-band electroluminescence. Chem Eur J, 2023, 29, e202202628 doi: 10.1002/chem.202202628[8] Kang J, Jeon S O, Kim I, et al. Color stable deep blue multi-resonance organic emitters with narrow emission and high efficiency. Adv Sci, 2023, 10, 2302619 doi: 10.1002/advs.202302619[9] Wei J B, Zhang C, Zhang D D, et al. Indolo[3, 2, 1-jk]carbazole embedded multiple-resonance fluorophors for narrowband deep-blue electroluminescence with EQE ≈ 34.7 % and CIEy ≈ 0.085. Angew Chem Int Ed, 2021, 60, 12269 doi: 10.1002/anie.202017328[10] Hirai H, Nakajima K, Nakatsuka S, et al. One-step borylation of 1, 3-diaryloxybenzenes towards efficient materials for organic light-emitting diodes. Angew Chem Int Ed, 2015, 54, 13581 doi: 10.1002/anie.201506335[11] Kondo Y, Yoshiura K, Kitera S, et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat Photonics, 2019, 13, 678 doi: 10.1038/s41566-019-0476-5[12] Yang M L, Park I S, Yasuda T. Full-color, narrowband, and high-efficiency electroluminescence from boron and carbazole embedded polycyclic heteroaromatics. J Am Chem Soc, 2020, 142, 19468 doi: 10.1021/jacs.0c10081[13] Liu Y, Xiao X, Ran Y, et al. Molecular design of thermally activated delayed fluorescent emitters for narrowband orange-red OLEDs boosted by a cyano-functionalization strategy. Chem Sci, 2021, 12, 9408 doi: 10.1039/D1SC02042K[14] Zhang Y W, Zhang D D, Huang T Y, et al. Multi-resonance deep-red emitters with shallow potential-energy surfaces to surpass energy-gap law. Angew Chem Int Ed, 2021, 60, 20498 doi: 10.1002/anie.202107848[15] Park I S, Min H, Yasuda T. Ultrafast triplet–singlet exciton interconversion in narrowband blue organoboron emitters doped with heavy chalcogens. Angew Chem Int Ed, 2022, 61, e202205684 doi: 10.1002/anie.202205684[16] Xu Y C, Wang Q Y, Cai X L, et al. Frontier molecular orbital engineering: Constructing highly efficient narrowband organic electroluminescent materials. Angew Chem Int Ed, 2023, 62, e202312451 doi: 10.1002/anie.202312451[17] Hu Y X, Miao J S, Hua T, et al. Efficient selenium-integrated TADF OLEDs with reduced roll-off. Nat Photonics, 2022, 16, 803 doi: 10.1038/s41566-022-01083-y[18] Wu X G, Huang J W, Su B K, et al. Fabrication of circularly polarized MR-TADF emitters with asymmetrical peripheral-lock enhancing helical B/N-doped nanographenes. Adv Mater, 2022, 34, e2105080 doi: 10.1002/adma.202105080[19] Luo X F, Zhang L X, Zheng Y X, et al. Improving reverse intersystem crossing of MR-TADF emitters for OLEDs. J Semicond, 2022, 43, 110202 doi: 10.1088/1674-4926/43/11/110202[20] Zhang Y W, Zhang D D, Wei J B, et al. Multi-resonance induced thermally activated delayed fluorophores for narrowband green OLEDs. Angew Chem Int Ed, 2019, 58, 16912 doi: 10.1002/anie.201911266[21] Xu Y C, Cheng Z, Li Z Q, et al. Molecular-structure and device-configuration optimizations toward highly efficient green electroluminescence with narrowband emission and high color purity. Adv Opt Mater, 2020, 8, 1902142 doi: 10.1002/adom.201902142[22] Xu Y C, Li C L, Li Z Q, et al. Constructing charge-transfer excited states based on frontier molecular orbital engineering: Narrowband green electroluminescence with high color purity and efficiency. Angew Chem Int Ed, 2020, 59, 17442 doi: 10.1002/anie.202007210[23] Knöller J A, Meng G Y, Wang X, et al. Intramolecular borylation via sequential B-mes bond cleavage for the divergent synthesis of B, N, B-doped benzo[4]helicenes. Angew Chem Int Ed, 2020, 59, 3156 doi: 10.1002/anie.201912340[24] Matsui K, Oda S, Yoshiura K, et al. One-shot multiple borylation toward BN-doped nanographenes. J Am Chem Soc, 2018, 140, 1195 doi: 10.1021/jacs.7b10578[25] Lv X L, Miao J S, Liu M H, et al. Extending the π-skeleton of multi-resonance TADF materials towards high-efficiency narrowband deep-blue emission. Angew Chem Int Ed, 2022, 61, e202201588 doi: 10.1002/anie.202201588[26] Wu L, Huang Z Y, Miao J S, et al. Orienting group directed cascade borylation for efficient one-shot synthesis of 1, 4-BN-doped polycyclic aromatic hydrocarbons as narrowband organic emitters. Angew Chem Int Ed, 2024, 63, e202402020 doi: 10.1002/anie.202402020[27] Fan X C, Huang F, Wu H, et al. A quadruple-borylated multiple-resonance emitter with para/meta heteroatomic patterns for narrowband orange-red emission. Angew Chem Int Ed, 2023, 62, e202305580 doi: 10.1002/anie.202305580[28] Meng G Y, Dai H Y, Huang T Y, et al. Amine-directed formation of B‒N bonds for BN-fused polycyclic aromatic multiple resonance emitters with narrowband emission. Angew Chem Int Ed, 2022, 61, e202207293 doi: 10.1002/anie.202207293[29] Meng G Y, Dai H Y, Zhou J P, et al. Wide-range color-tunable polycyclo-heteraborin multi-resonance emitters containing B-N covalent bonds. Chem Sci, 2023, 14, 979 doi: 10.1039/D2SC06343C[30] Meng G Y, Zhou J P, Han X S, et al. B‒N covalent bond embedded double hetero-[n]helicenes for pure red narrowband circularly polarized electroluminescence with high efficiency and stability. Adv Mater, 2024, 36, 2307420 doi: 10.1002/adma.202307420[31] Meng G Y, Zhou J P, Huang T Y, et al. B‒N/B‒O contained heterocycles as fusion locker in multi-resonance frameworks towards highly-efficient and stable ultra-narrowband emission. Angew Chem Int Ed, 2023, 62, e202309923 doi: 10.1002/anie.202309923[32] Tam B S T, Dong S C, Tang C W. Low-temperature conformal vacuum deposition of OLED devices using close-space sublimation. J Semicond, 2023, 44, 092602 doi: 10.1088/1674-4926/44/9/092602[33] Lu T, Chen F W. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem, 2012, 33, 580 doi: 10.1002/jcc.22885[34] Liu Z Y, Lu T, Chen Q X. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon, 2020, 165, 461 doi: 10.1016/j.carbon.2020.05.023[35] Meng G Y, Dai H Y, Wang Q, et al. High-efficiency and stable short-delayed fluorescence emitters with hybrid long- and short-range charge-transfer excitations. Nat Commun, 2023, 14, 2394 doi: 10.1038/s41467-023-38086-4[36] Ren B Y, Zuo C T, Sun Y G, et al. Intramolecular spatial charge transfer enhances TADF efficiency. J Semicond, 2021, 42, 050201 doi: 10.1088/1674-4926/42/5/050201[37] He X, Lou J L, Li B X, et al. Rational medium-range charge transfer strategy toward highly efficient violet-blue organic light-emitting diodes with narrowed emission. Adv Mater, 2024, 36, 2310417 doi: 10.1002/adma.202310417[38] Wei P C, Zhang D D, Duan L. Modulation of Förster and dexter interactions in single-emissive-layer all-fluorescent WOLEDs for improved efficiency and extended lifetime. Adv Funct Mater, 2020, 30, 1907083 doi: 10.1002/adfm.201907083[39] Huang T Y, Wang Q, Xiao S, et al. Simultaneously enhanced reverse intersystem crossing and radiative decay in thermally activated delayed fluorophors with multiple through-space charge transfers. Angew Chem Int Ed, 2021, 60, 23771 doi: 10.1002/anie.202109041[40] Jeon S O, Lee K H, Kim J S, et al. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat Photonics, 2021, 15, 208 doi: 10.1038/s41566-021-00763-5[41] Chan C Y, Tanaka M, Lee Y T, et al. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat Photonics, 2021, 15, 203 doi: 10.1038/s41566-020-00745-z[42] Zhang Y W, Wei J B, Zhang D D, et al. Sterically wrapped multiple resonance fluorophors for suppression of concentration quenching and spectrum broadening. Angew Chem Int Ed, 2022, 61, e202113206 doi: 10.1002/anie.202113206[43] Jiang P C, Miao J S, Cao X S, et al. Quenching-resistant multiresonance TADF emitter realizes 40% external quantum efficiency in narrowband electroluminescence at high doping level. Adv Mater, 2022, 34, e2106954 doi: 10.1002/adma.202106954 -
Supplements
 24040008Supplementary information.pdf
24040008Supplementary information.pdf

-
Proportional views





 Danrui Wan got her BS degree from Xiamen University in 2017. Now she is pursuing a master’s degree at Yunnan University and Southwest United Graduate School under the supervision of Prof. Junqiao Ding. Her research focuses on boron−nitrogen covalent bonds doped multi-resonance (MR) materials.
Danrui Wan got her BS degree from Xiamen University in 2017. Now she is pursuing a master’s degree at Yunnan University and Southwest United Graduate School under the supervision of Prof. Junqiao Ding. Her research focuses on boron−nitrogen covalent bonds doped multi-resonance (MR) materials. Guoyun Meng obtained his Ph.D. degree in 2020 from Beijing Institute of Technology. From 2020 to 2022, he conducted postdoctoral research at Tsinghua University. Presently, he serves as an associate professor at the School of Chemical Science and Technology, Yunnan University. His research focuses on boron nitrogen-embedded polycyclic aromatic multiple resonance emitters and devices.
Guoyun Meng obtained his Ph.D. degree in 2020 from Beijing Institute of Technology. From 2020 to 2022, he conducted postdoctoral research at Tsinghua University. Presently, he serves as an associate professor at the School of Chemical Science and Technology, Yunnan University. His research focuses on boron nitrogen-embedded polycyclic aromatic multiple resonance emitters and devices. Junqiao Ding received his BS from Wuhan University of Technology in 1999 and gained his PhD from Changchun Institute of Applied Chemistry (CAS) in 2007. After graduation, he joined the same institute as a research fellow and later was promoted to a professor in 2014. During 2010−2011, he performed postdoctoral work at the University of New Mexico. Since 2020, he has been working as a professor at School of Chemical Science and Technology, Yunnan University. His research focuses on organic/polymeric optoelectronic materials and devices.
Junqiao Ding received his BS from Wuhan University of Technology in 1999 and gained his PhD from Changchun Institute of Applied Chemistry (CAS) in 2007. After graduation, he joined the same institute as a research fellow and later was promoted to a professor in 2014. During 2010−2011, he performed postdoctoral work at the University of New Mexico. Since 2020, he has been working as a professor at School of Chemical Science and Technology, Yunnan University. His research focuses on organic/polymeric optoelectronic materials and devices.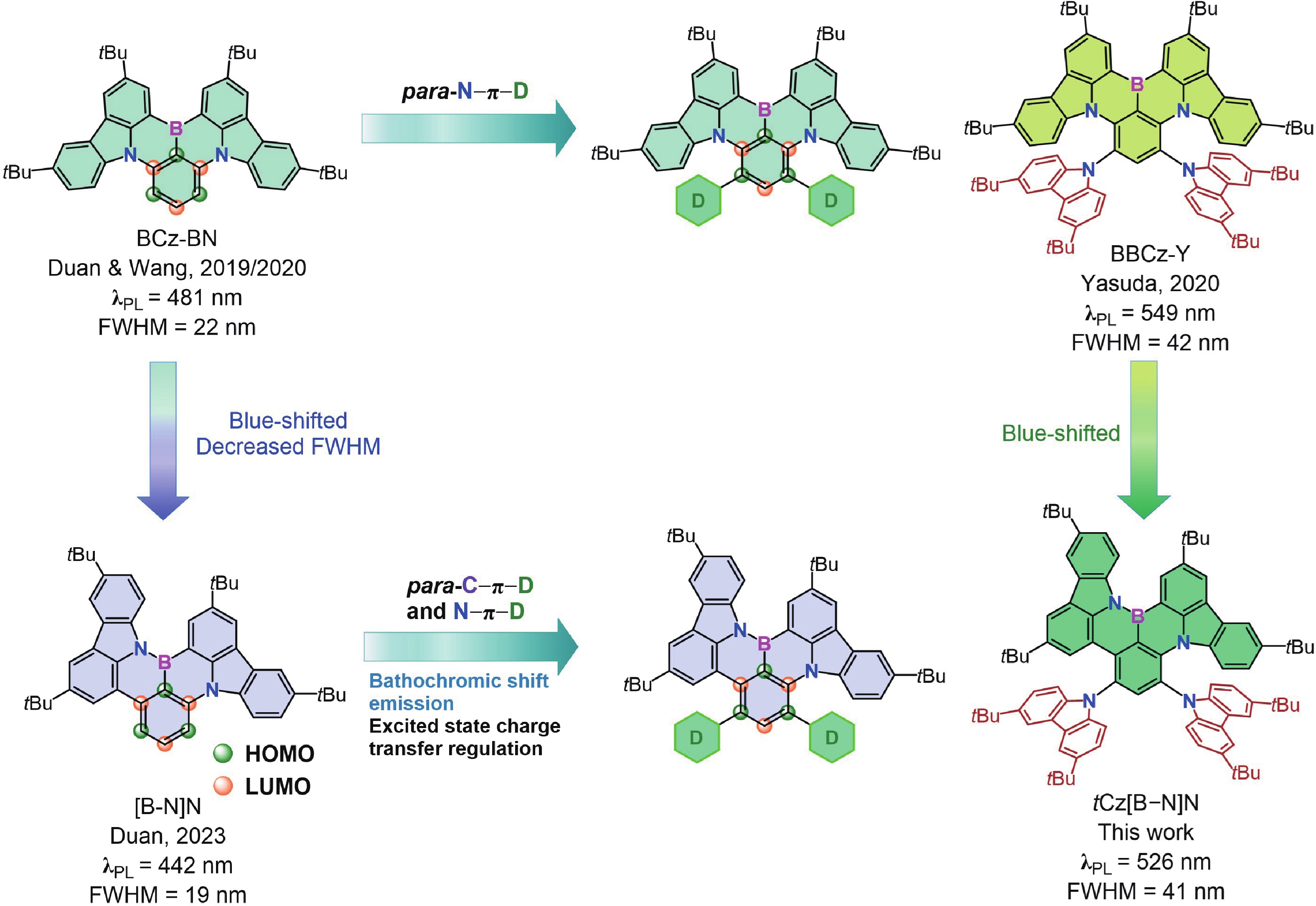
 DownLoad:
DownLoad: