| Citation: |
Kai Xiang, Ning Su, Jianhua Chen, Junqiao Ding. Design and application of all-fused-ring electron acceptors[J]. Journal of Semiconductors, 2024, 45(12): 120201. doi: 10.1088/1674-4926/24090036
****
K Xiang, N Su, J H Chen, and J Q Ding, Design and application of all-fused-ring electron acceptors[J]. J. Semicond., 2024, 45(12), 120201 doi: 10.1088/1674-4926/24090036
|
Design and application of all-fused-ring electron acceptors
DOI: 10.1088/1674-4926/24090036
More Information
-
References
[1] Zhang Y Z, Yu Y J, Liu X Y, et al. An n-type all-fused-ring molecule with photoresponse to 1000 nm for highly sensitive near-infrared photodetector. Adv Mater, 2023, 35, 2211714 doi: 10.1002/adma.202211714[2] Zheng Y Q, Chen Y J, Cao Y H, et al. Design of all-fused-ring nonfullerene acceptor for highly sensitive self-powered near-infrared organic photodetectors. ACS Mater Lett, 2022, 4, 882 doi: 10.1021/acsmaterialslett.2c00191[3] Zhang Y D, Ji Y T, Zhang Y Y, et al. Recent progress of Y6-derived asymmetric fused ring electron acceptors. Adv Funct Mater, 2022, 32, 2205115 doi: 10.1002/adfm.202205115[4] Zhang G C, Lin F R, Qi F, et al. Renewed prospects for organic photovoltaics. Chem Rev, 2022, 122, 14180 doi: 10.1021/acs.chemrev.1c00955[5] Jiang Y Y, Sun S M, Xu R J, et al. Non-fullerene acceptor with asymmetric structure and phenyl-substituted alkyl side chain for 20.2% efficiency organic solar cells. Nat Energy, 2024, 9, 975 doi: 10.1038/s41560-024-01557-z[6] Luke J, Speller E M, Wadsworth A, et al. Twist and degrade—Impact of molecular structure on the photostability of nonfullerene acceptors and their photovoltaic blends. Adv Energy Mater, 2019, 9, 1803755 doi: 10.1002/aenm.201803755[7] Zhu X Y, Hu L, Wang W, et al. Reversible chemical reactivity of non-fullerene acceptors for organic solar cells under acidic and basic environment. ACS Appl Energy Mater, 2019, 2, 7602 doi: 10.1021/acsaem.9b01591[8] Zhu X Z, Liu S J, Yue Q H, et al. Design of all-fused-ring electron acceptors with high thermal, chemical, and photochemical stability for organic photovoltaics. CCS Chem, 2021, 3, 1070 doi: 10.31635/ccschem.021.202100956[9] Yu Y J, Zhang Y Z, Miao J H, et al. An n-type all-fused-ring molecule with narrow bandgap. CCS Chem, 2023, 5, 486 doi: 10.31635/ccschem.022.202101752[10] Zhu X Y, Zhang Y Q, Xie B M, et al. All-fused-ring small molecule acceptors with near-infrared absorption. J Mater Chem C, 2023, 11, 2144 doi: 10.1039/D2TC04917A[11] Feng J, Liu Y, Yang H, et al. Conjugated backbone optimization of an all-fused-ring acceptor for efficient and stable organic solar cells. Chem Commun, 2024, 60, 6206 doi: 10.1039/D4CC00902A[12] Liu W R, Xu S J, Lai H J, et al. Near-infrared all-fused-ring nonfullerene acceptors achieving an optimal efficiency-cost-stability balance in organic solar cells. CCS Chem, 2023, 5, 654 doi: 10.31635/ccschem.022.202201963[13] Liu W, Xu S, Liu W, et al. Large-scale preparation of low-cost nonfullerene acceptors for stable and efficient organic solar cells. ChemRxiv, 2021[14] Sharma G D, Pradhan R, Khandelwal K, et al. All-small-molecule efficient ternary organic solar cells employing a coumarin donor and two fullerene-free acceptors. J Mater Chem C, 2023, 11, 1919 doi: 10.1039/D2TC05318G[15] Zhu X Y, Zhang Y Q, Li H X, et al. A fully-fluorinated all-fused-ring acceptor for highly sensitive near-infrared organic photodetectors. Sci Bull, 2024, 69, 2679 doi: 10.1016/j.scib.2024.06.030[16] Liao Z X, Miao J H, Liu J, et al. All-fused-ring molecules with high photostability for near-infrared security and anti-counterfeiting applications. Sci China Mater, 2023, 66, 4037 doi: 10.1007/s40843-023-2564-0 -
Proportional views





 Kai Xiang received his B.Eng from Qilu University of Technology in 2023. Now he is an M.E. student at Yunnan University under the guidance of Associate Professor Chen Jianhua. His research focuses on the development of Y-series receptor materials for organic solar cells.
Kai Xiang received his B.Eng from Qilu University of Technology in 2023. Now he is an M.E. student at Yunnan University under the guidance of Associate Professor Chen Jianhua. His research focuses on the development of Y-series receptor materials for organic solar cells. Ning Su received her Ph.D. degree in Chemistry from Nanjing University. Afterwards, she worked as a post-doc at Shenzhen University and as a visiting scholar at Northwestern University in USA. She is now working as an assistant professor at Yunnan University. Her current research activities focus on organic luminescent materials and organic light-emitting diodes.
Ning Su received her Ph.D. degree in Chemistry from Nanjing University. Afterwards, she worked as a post-doc at Shenzhen University and as a visiting scholar at Northwestern University in USA. She is now working as an assistant professor at Yunnan University. Her current research activities focus on organic luminescent materials and organic light-emitting diodes. Jianhua Chen obtained his Ph.D. degree from Xiangtan University in 2016. Then he carried out his postdoctoral trainings at the Southern University of Science and Technology and Northwestern University from 2016 before joined Yunnan University as an associate professor in 2021. His recent research focuses on the optoelectronic polymer semiconductors and flexible electronics.
Jianhua Chen obtained his Ph.D. degree from Xiangtan University in 2016. Then he carried out his postdoctoral trainings at the Southern University of Science and Technology and Northwestern University from 2016 before joined Yunnan University as an associate professor in 2021. His recent research focuses on the optoelectronic polymer semiconductors and flexible electronics. Junqiao Ding received his BS degree from Wuhan University of Technology in 1999 and gained his PhD degree from Changchun Institute of Applied Chemistry, CAS, in 2007. After graduation, he joined the same institute as a research fellow and later was promoted as a professor in 2014. During 2010−2011, he performed postdoctoral work at the University of New Mexico. Since 2020, he worked as a professor in School of Chemical Science and Technology, Yunnan University. His research focuses on organic/polymeric optoelectronic materials and devices. He has filed 16 granted Chinese patents, and co-authored 130 papers in refereed journals including Nat. Commun., Angew. Chem. Int. Ed., J. Am. Chem. Soc., Adv. Mater., Macromolecules etc.
Junqiao Ding received his BS degree from Wuhan University of Technology in 1999 and gained his PhD degree from Changchun Institute of Applied Chemistry, CAS, in 2007. After graduation, he joined the same institute as a research fellow and later was promoted as a professor in 2014. During 2010−2011, he performed postdoctoral work at the University of New Mexico. Since 2020, he worked as a professor in School of Chemical Science and Technology, Yunnan University. His research focuses on organic/polymeric optoelectronic materials and devices. He has filed 16 granted Chinese patents, and co-authored 130 papers in refereed journals including Nat. Commun., Angew. Chem. Int. Ed., J. Am. Chem. Soc., Adv. Mater., Macromolecules etc.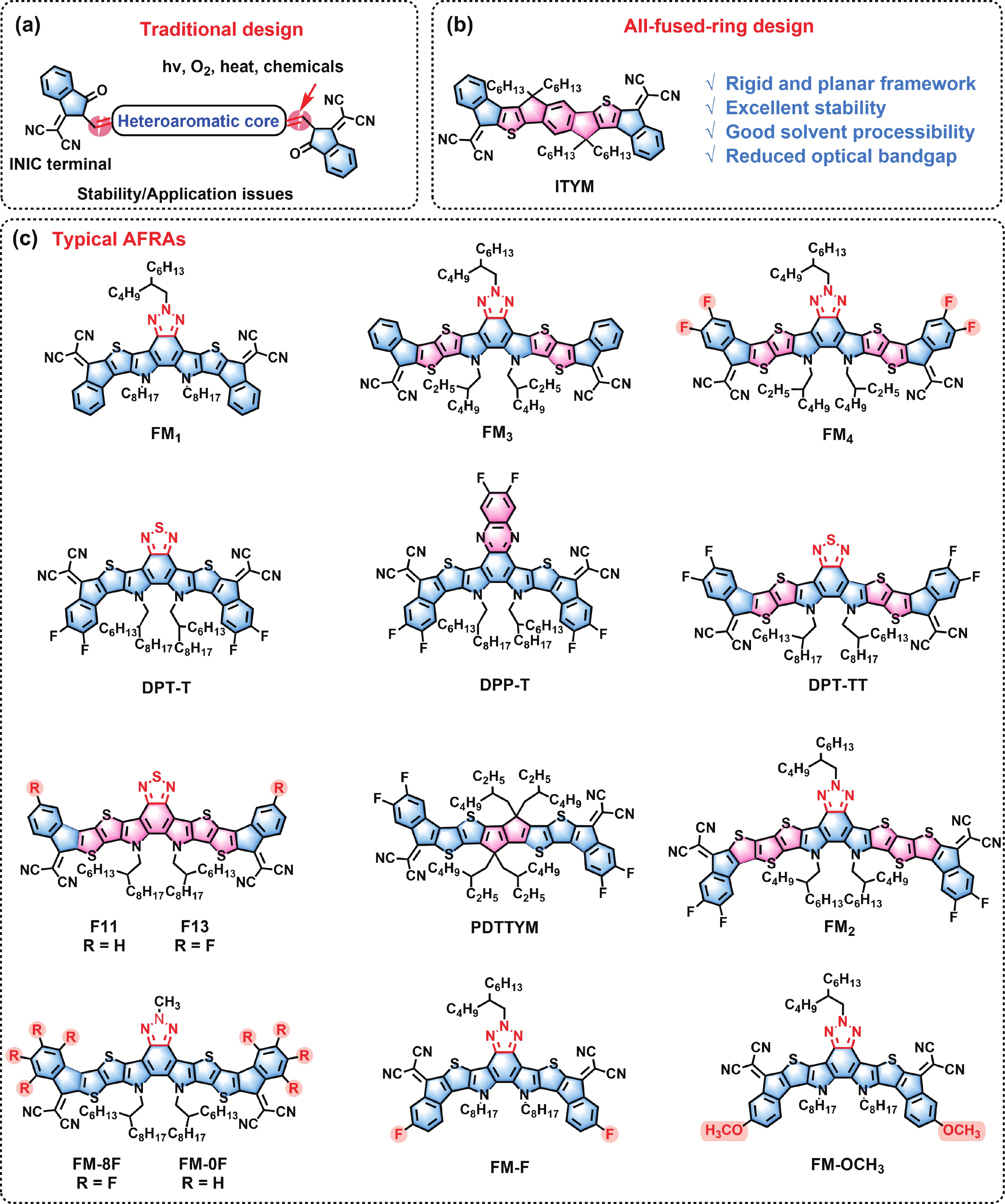
 DownLoad:
DownLoad:














