| Citation: |
Zhengyuan Li, Jiaqi Wei, Yiyuan Liu, Huihui Li, Yang Li, Zhitai Jia, Xutang Tao, Wenxiang Mu. Growth and optical properties of large-sized Co2+: ZnGa2O4 single crystal[J]. Journal of Semiconductors, 2025, 46(7): 072501. doi: 10.1088/1674-4926/25010017
****
Z Y Li, J Q Wei, Y Y Liu, H H Li, Y Li, Z T Jia, X T Tao, and W X Mu, Growth and optical properties of large-sized Co2+: ZnGa2O4 single crystal[J]. J. Semicond., 2025, 46(7), 072501 doi: 10.1088/1674-4926/25010017
|
Growth and optical properties of large-sized Co2+: ZnGa2O4 single crystal
DOI: 10.1088/1674-4926/25010017
CSTR: 32376.14.1674-4926.25010017
More Information-
Abstract
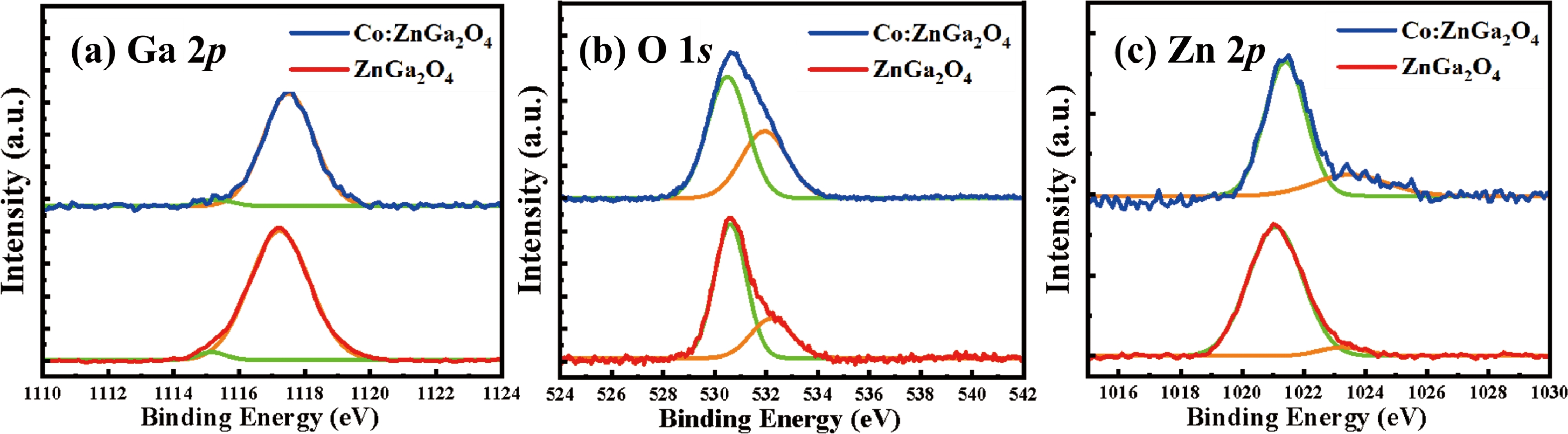
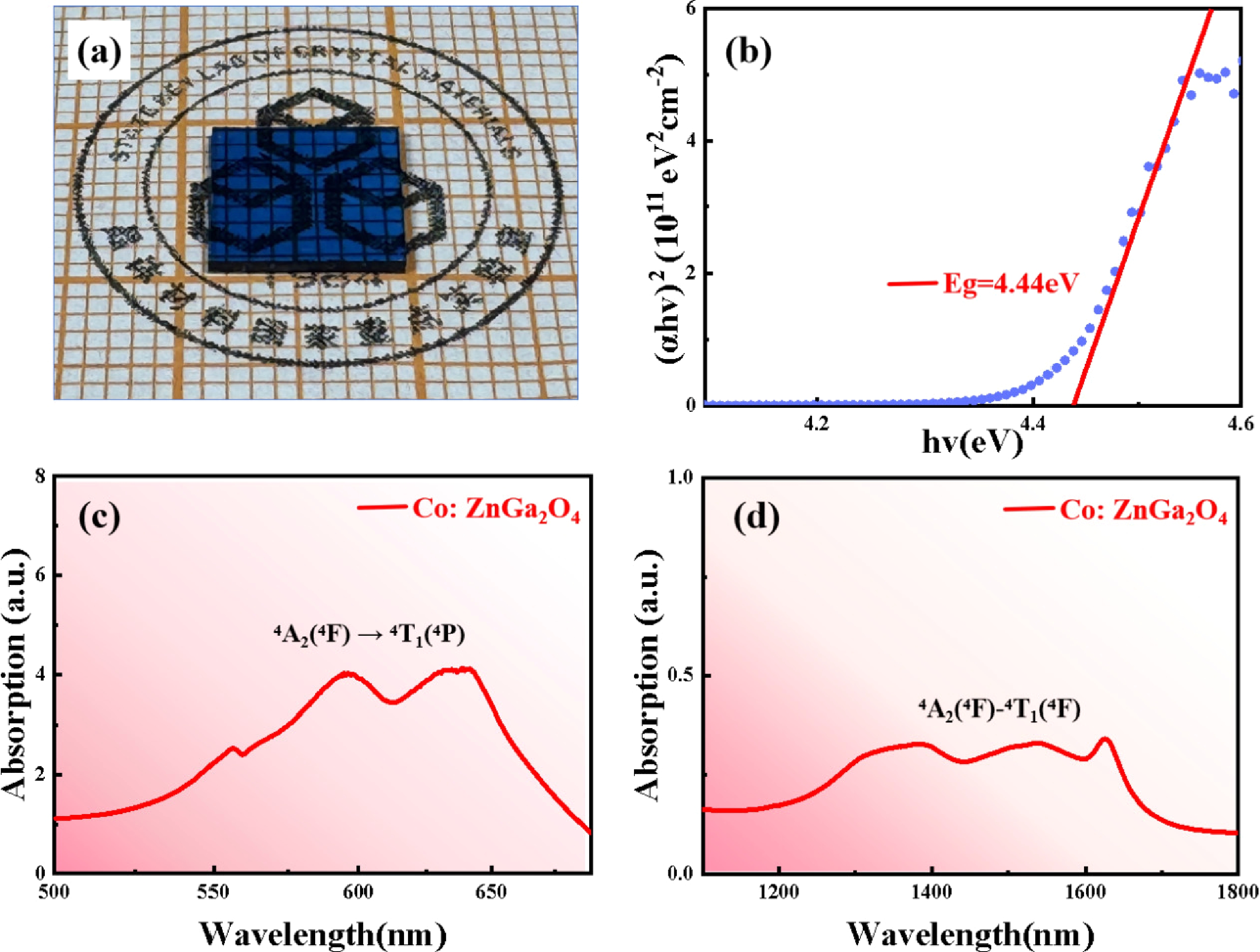
The transition of cobalt ions located at tetrahedral sites will produce strong absorption in the visible and near-infrared regions, and is expected to work in a passively Q-switched solid-state laser at the eye-safe wavelength of 1.5 µm. In this study, Co2+ ions were introduced into the wide bandgap semiconductor material ZnGa2O4, and large-sized and high-quality Co2+-doped ZnGa2O4 crystals with a volume of about 20 cm3 were grown using the vertical gradient freeze (VGF) method. Crystal structure and optical properties were analyzed using X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and absorption spectroscopy. XRD results show that the Co2+-doped ZnGa2O4 crystal has a pure spinel phase without impurity phases and the rocking curve full width at half maximum (FWHM) is only 58 arcsec. The concentration of Co2+ in Co2+-doped ZnGa2O4 crystals was determined to be 0.2 at.% by the energy dispersive X-ray spectroscopy. The optical band gap of Co2+-doped ZnGa2O4 crystals is 4.44 eV. The optical absorption spectrum for Co2+-doped ZnGa2O4 reveals a prominent visible absorption band within 550−670 nm and a wide absorption band spanning from 1100 to 1700 nm. This suggests that the Co2+ ions have substituted the Zn2+ ions, which are typically tetrahedrally coordinated, within the lattice structure of ZnGa2O4. The visible region's absorption peak and the near-infrared broad absorption band are ascribed to the 4A2(4F) → 4T1(4P) and 4A2(4F) →4T1(4F) transitions, respectively. The optimal ground state absorption cross section was determined to be 3.07 × 10−19 cm2 in ZnGa2O4, a value that is comparatively large within the context of similar materials. This finding suggests that ZnGa2O4 is a promising candidate for use in near-infrared passive Q-switched solid-state lasers. -
References
[1] Mlynczak J, Belghachem N, Kopczynski K, et al. Performance analysis of thermally bonded Er3+, Yb3+: Glass/Co2+: MgAl2O4 microchip lasers. Opt Quantum Electron, 2016, 48(4), 247 doi: 10.1007/s11082-016-0508-z[2] Camargo M B, Stultz R D, Birnbaum M, et al. Co(2+): YSGG saturable absorber Q switch for infrared erbium lasers. Opt Lett, 1995, 20(3), 339 doi: 10.1364/OL.20.000339[3] Denisov I A, Demchuk M I, Kuleshov N V, et al. Co2+: LiGa5O8 saturable absorber passive Q switch for 1.34 μm Nd3+: YAlO3 and 1.54 μm Er3+: Glass lasers. Appl Phys Lett, 2000, 77(16), 2455 doi: 10.1063/1.1319179[4] Kuleshov N V, Mikhailov V P, Scherbitsky V G, et al. Absorption and luminescence of tetrahedral Co2+ ion in MgAl2O4. J Lumin, 1993, 55(5/6), 265 doi: 10.1016/0022-2313(93)90021-E[5] Abritta T, Blak F H. Luminescence study of ZnGa2O4: Co24. J Lumin, 1991, 48/49, 558 doi: 10.1016/0022-2313(91)90192-X[6] Yumashev K V. Saturable absorber Co2+: MgAl2O4 crystal for Q switching of 1.34-µm Nd3+: YAlO3 and 1.54-µm Er3+: Glass lasers. Appl Opt, 1999, 38(30), 6343 doi: 10.1364/AO.38.006343[7] Donegan J F, Anderson F G, Bergin F J, et al. Optical and magnetic-circular-dichroism–optically-detected-magnetic-resonance study of the Co2+ ion in LiGa5O8. Phys Rev B, 1992, 45(2), 563 doi: 10.1103/PhysRevB.45.563[8] Sosman L P, Dias Tavares A Jr, Abritta T. Near infrared spectroscopy of divalent cobalt in polycrystalline magnesium and zinc gallate. J Phys D: Appl Phys, 2000, 33(2), L19 doi: 10.1088/0022-3727/33/2/103[9] Mary Jacintha A, Manikandan A, Chinnaraj K, et al. Comparative studies of spinel MnFe2O4 nanostructures: Structural, morphological, optical, magnetic and catalytic properties. J Nanosci Nanotechnol, 2015, 15(12), 9732 doi: 10.1166/jnn.2015.10343[10] Suguna S, Shankar S, Jaganathan S K, et al. Novel synthesis and characterization studies of spinel NixCo1–xAl2O4 (x = 0.0 to 1.0) nano-catalysts for the catalytic oxidation of benzyl alcohol. J Nanosci Nanotechnol, 2018, 18(2), 1019 doi: 10.1166/jnn.2018.13960[11] Zeb M, Tahir M, Muhammad F, et al. Pyrrol-anthracene: Synthesis, characterization and its application as active material in humidity, temperature and light sensors. Coatings, 2022, 12(6), 848 doi: 10.3390/coatings12060848[12] Ullah F, Qureshi M T, Abbas S K, et al. Dilute magnetic ions mediated magneto-dielectric, optical and ferroelectric response of MgAl2O4 spinels. J Phys: Condens Matter, 2020, 32(36), 365701 doi: 10.1088/1361-648X/ab8aa0[13] Itoh S, Toki H, Sato Y, et al. The ZnGa2O4 phosphor for low-voltage blue cathodoluminescence. J Electrochem Soc, 1991, 138(5), 1509 doi: 10.1149/1.2085816[14] Shea L E, Datta R K, Brown J J. Low voltage cathodoluminescence of Mn2+-activated ZnGa2O4. J Electrochem Soc, 1994, 141(8), 2198 doi: 10.1149/1.2055086[15] Minami T, Kuroi Y, Miyata T, et al. ZnGa2O4 as host material for multicolor-emitting phosphor layer of electroluminescent devices. J Lumin, 1997, 72, 997 doi: 10.1016/S0022-2313(97)80796-X[16] Kim J S, Lee S G, Park H L, et al. Optical and electrical properties of ZnGa2O4/Mn2+ powder electroluminescent device. Mater Lett, 2004, 58(7/8), 1354 doi: 10.1016/j.matlet.2003.06.017[17] Minami T, Maeno T, Kuroi Y, et al. High-luminance green-emitting thin-film electroluminescent devices using ZnGa2O4: Mn phosphor. Jpn J Appl Phys, 1995, 34(6A), L684 doi: 10.1143/JJAP.34.L684[18] Jović N G, Masadeh A S, Kremenović A S, et al. Effects of thermal annealing on structural and magnetic properties of lithium ferrite nanoparticles. J Phys Chem C, 2009, 113(48), 20559 doi: 10.1021/jp907559y[19] Sreeja V, Smitha T S, Nand D, et al. Size dependent coordination behavior and cation distribution in MgAl2O4 nanoparticles from 27Al solid state NMR studies. J Phys Chem C, 2008, 112(38), 14737 doi: 10.1021/jp800412k[20] Mittal V K, Chandramohan P, Bera S, et al. Cation distribution in NixMg1−xFe2O4 studied by XPS and mössbauer spectroscopy. Solid State Commun, 2006, 137(1/2), 6 doi: 10.1016/j.ssc.2005.10.019[21] Hsieh I J, Chu K T, Yu C F, et al. Cathodoluminescent characteristics of ZnGa2O4 phosphor grown by radio frequency magnetron sputtering. J Appl Phys, 1994, 76(6), 3735 doi: 10.1063/1.358500[22] Omata T, Ueda N, Ueda K, et al. New ultraviolet-transport electroconductive oxide, ZnGa2O4 spinel. Appl Phys Lett, 1994, 64(9), 1077 doi: 10.1063/1.110937[23] Shea L E, Datta R K, Brown J J. Photoluminescence of Mn2+-activated ZnGa2O4. J Electrochem Soc, 1994, 141(7), 1950 doi: 10.1149/1.2055033[24] Jeong I K, Park H L, Mho S I. Two self-activated optical centers of blue emission in zinc gallate. Solid State Commun, 1998, 105(3), 179 doi: 10.1016/S0038-1098(97)10101-6[25] Yu C F, Lin P. Manganese-activated luminescence in ZnGa2O4. J Appl Phys, 1996, 79(9), 7191 doi: 10.1063/1.361435[26] Kim J S, Kim J S, Kim T W, et al. Energy transfer among three luminescent centers in full-color emitting ZnGa2O4: Mn2+, Cr3+ phosphors. Solid State Commun, 2004, 131(8), 493 doi: 10.1016/j.ssc.2004.06.023[27] Yu M, Lin J, Zhou Y H, et al. Citrate–gel synthesis and luminescent properties of ZnGa2O4 doped with Mn2+ and Eu3+. Mater Lett, 2002, 56(6), 1007 doi: 10.1016/S0167-577X(02)00664-X[28] Xu Z H, Li Y X, Liu Z F, et al. UV and X-ray excited luminescence of Tb3+-doped ZnGa2O4 phosphors. J Alloys Compd, 2005, 391(1/2), 202 doi: 10.1016/j.jallcom.2004.08.088[29] Kim J S, Kim J S, Park H L. Optical and structural properties of nanosized ZnGa2O4: Cr3+ phosphor. Solid State Commun, 2004, 131(12), 735 doi: 10.1016/j.ssc.2004.07.026[30] Kim J S, Kim J S, Kim T W, et al. Correlation between the crystalline environment and optical property of Mn2+ ions in ZnGa2O4: Mn2+ phosphor. Appl Phys Lett, 2005, 86(9), 091912 doi: 10.1063/1.1869550[31] Ohtake T, Sonoyama N, Sakata T. Electrochemical luminescence of ZnGa2O4 semiconductor electrodes activated with Cr and Co. Chem Phys Lett, 2000, 318(6), 517 doi: 10.1016/S0009-2614(00)00082-8[32] Zhuang Y X, Ueda J, Tanabe S. Enhancement of red persistent luminescence in Cr3+-doped ZnGa2O4 phosphors by Bi2O3 codoping. Appl Phys Express, 2013, 6, 052602 doi: 10.7567/APEX.6.052602[33] Rack P D, Peterson J J, Potter M D, et al. Eu+3 and Cr+3 doping for red cathodoluminescence in ZnGa2O4. J Mater Res, 2001, 16(5), 1429 doi: 10.1557/JMR.2001.0199[34] Balda R, Fernández J, De Pablos A, et al. Cr3+→Nd3+ energy transfer in fluorophosphate glass investigated by time-resolved laser spectroscopy. Physical Review B, 1993, 48, 294 doi: 10.1103/PhysRevB.48.2941[35] Wu B T, Zhou S F, Ruan J, et al. Energy transfer between Cr3+ and Ni2+ in transparent silicate glass ceramics containing Cr3+/Ni2+ Co-doped ZnAl2O4 nanocrystals. Opt Express, 2008, 16(4), 2508 doi: 10.1364/OE.16.002508[36] Hsu K H, Chen K S. Photoluminescence of ZnGa2O4 phosphor prepared by a microencapsulation method. Ceram Int, 2000, 26(5), 469 doi: 10.1016/S0272-8842(99)00081-4[37] Jung H K, Park D S, Park Y C. Preparation and characterization of ZnGa2O4: Mn phosphors by multistage precipitation method. Mater Res Bull, 1999, 34(1), 43 doi: 10.1016/S0025-5408(98)00216-5[38] Hirano M, Imai M, Inagaki M. Preparation of ZnGa2O4 spinel fine particles by the hydrothermal method. J Am Ceram Soc, 2000, 83(4), 977 doi: 10.1111/j.1151-2916.2000.tb01310.x[39] Li Y D, Duan X F, Liao H W, et al. Self-regulation synthesis of nanocrystalline ZnGa2O4 by hydrothermal reaction. Chem Mater, 1998, 10(1), 17 doi: 10.1021/cm970557m[40] Pellerin M, Castaing V, Gourier D, et al. Persistent luminescence of transition metal (Co, Ni..)-doped ZnGa2O4 phosphors for applications in the near-infrared range. Oxide-based Materials and Devices IX, 2018, 10533, 196 doi: 10.1117/12.2294986[41] Duan X L, Liu J, Wu Y C, et al. Structure and luminescent properties of Co2+/Cr3+ Co-doped ZnGa2O4 nanoparticles. J Lumin, 2014, 153, 361 doi: 10.1016/j.jlumin.2014.03.027[42] Li X Y, Liu Q, Hu Z W, et al. Influence of ammonium hydrogen carbonate to metal ions molar ratio on co-precipitated nanopowders for TGG transparent ceramics. J Inorganic Mater, 2019, 34, 791 doi: 10.15541/jim20180574[43] Dai Y H, Li J, Zhang Y, et al. Preparation of Er, Yb: (LaLu)2O3 ceramic and its upconversion luminescent properties. Chin J Lumin, 2018, 39(4), 488 doi: 10.3788/fgxb20183904.0488[44] Wei J B, Toci G, Pirri A, et al. Fabrication and property of Yb: CaF2 laser ceramics from co-precipitated nanopowders. J Inorganic Mater, 2019, 34, 1341 doi: 10.15541/jim20190121[45] White W B, DeAngelis B A. Interpretation of the vibrational spectra of spinels. Spectrochim Acta Part A Mol Spectrosc, 1967, 23(4), 985 doi: 10.1016/0584-8539(67)80023-0[46] Bukhari S H, Ahmad J. Infrared active phonons and optical band gap in multiferroic GdMnO3 studied by infrared and UV-visible spectroscopy. Acta Phys Pol A, 2016, 129(1), 43 doi: 10.12693/APhysPolA.129.43[47] Kuleshov N V, Mikhailov V P, Scherbitsky V G. Co-doped spinels: promising materials for solid state lasers . Longer Wavelength Lasers and Applications, 1994, 2138, 175 doi: 10.1117/12.181357[48] Wang B, Wang H, Tu B T, et al. Optical transmission, dispersion, and transition behavior of ZnGa2O4 transparent ceramic. J Am Ceram Soc, 2023, 106(2), 1230 doi: 10.1111/jace.18857[49] Li N N, Duan X L, Yu F P, et al. Effects of preparation method and temperature on the cation distribution of ZnGa2O4 spinel studied by X-ray photoelectron spectroscopy. Vacuum, 2017, 142, 1 doi: 10.1016/j.vacuum.2017.04.035[50] Chi Z, Tarntair F G, Frégnaux M, et al. Bipolar self-doping in ultra-wide bandgap spinel ZnGa2O4. Mater Today Phys, 2021, 20, 100466 doi: 10.1016/j.mtphys.2021.100466[51] Kamal C S, Mishra R K, Rao K R, et al. Influence of Ge4+ doping on photo- and electroluminescence properties of ZnGa2O4. J Alloys Compd, 2021, 852, 156967 doi: 10.1016/j.jallcom.2020.156967[52] Drasovean R, Condurache-Bota S. Structural characterization and optical properties of Co3O4 and CoO films. J Optoelectron Adv Mater, 2009, 11, 2141[53] Ferguson J, Wood D L, Van Uitert L G. Crystal-field spectra of d3, 7 ions. V. tetrahedral Co2+ in ZnAl2O4 spinel. J Chem Phys, 1969, 51(7), 2904 doi: 10.1063/1.1672431[54] Donegan J F, Bergin F J, Imbusch G F, et al. Luminescence from LiGa5O8: Co. J Lumin, 1984, 31(1), 278 doi: 10.1016/0022-2313(84)90272-2[55] Duan X L, Yuan D R, Wang L H, et al. Synthesis and optical properties of Co2+-doped ZnGa2O4 nanocrystals. J Cryst Growth, 2006, 296(2), 234 doi: 10.1016/j.jcrysgro.2006.07.035[56] Luo W, Ma P, Xie T F, et al. Fabrication and spectroscopic properties of Co: MgAl2O4 transparent ceramics by the HIP post-treatment. Opt Mater, 2017, 69, 152 doi: 10.1016/j.optmat.2017.03.036[57] Pappalardo R, Wood D L, Linares R C. Optical absorption spectra of Ni-doped oxide systems. I J ChemPhys, 1961, 35(4), 1460 doi: 10.1063/1.1732066[58] Wood D L, Remeika J P. Optical absorption of tetrahedral Co3+ and Co2+ in garnets. J Chem Phys, 1967, 46(9), 3595 doi: 10.1063/1.1841263[59] Izumi K, Miyazaki S, Yoshida S, et al. Optical properties of 3d transition-metal-doped MgAl2O4 spinels. Phys Rev B, 2006, 76, 075111 doi: 10.1103/PhysRevB.76.075111[60] Goldstein A, Loiko P, Burshtein Z, et al. Development of saturable absorbers for laser passive Q-switching near 1.5 μm based on transparent ceramic Co2+: MgAl2O4. J Am Ceram Soc, 2016, 99(4), 1324 doi: 10.1111/jace.14102[61] Su S, Liu Q, Hu Z W, et al. A simple way to prepare Co: MgAl2O4 transparent ceramics for saturable absorber. J Alloys Compd, 2019, 797, 1288 doi: 10.1016/j.jallcom.2019.04.322[62] Luo W, Pan Y B, Li C Y, et al. Fabrication and spectral properties of hot-pressed Co: MgAl2O4 transparent ceramics for saturable absorber. J Alloys Compd, 2017, 724, 45 doi: 10.1016/j.jallcom.2017.04.292 -
Proportional views





 Zhengyuan Li got his BS degree from Shandong University in 2020. Now he is a PhD student at Shandong University under the supervision of Prof. Zhitai Jia. His research focuses on the crystal growth and investigation of properties of ultrawide-bandgap semiconductor material of β-Ga2O3 and ZnGa2O4.
Zhengyuan Li got his BS degree from Shandong University in 2020. Now he is a PhD student at Shandong University under the supervision of Prof. Zhitai Jia. His research focuses on the crystal growth and investigation of properties of ultrawide-bandgap semiconductor material of β-Ga2O3 and ZnGa2O4. Wenxiang Mu got his BS degree in 2013 and PhD degree in 2018 at Shandong University. Now he is an associate professor at institute of novel semiconductors of Shandong University. His research interests include crystal growth, substrate processing, performance optimization and device design based on ultrawide-bandgap semiconductor material of β-Ga2O3.
Wenxiang Mu got his BS degree in 2013 and PhD degree in 2018 at Shandong University. Now he is an associate professor at institute of novel semiconductors of Shandong University. His research interests include crystal growth, substrate processing, performance optimization and device design based on ultrawide-bandgap semiconductor material of β-Ga2O3.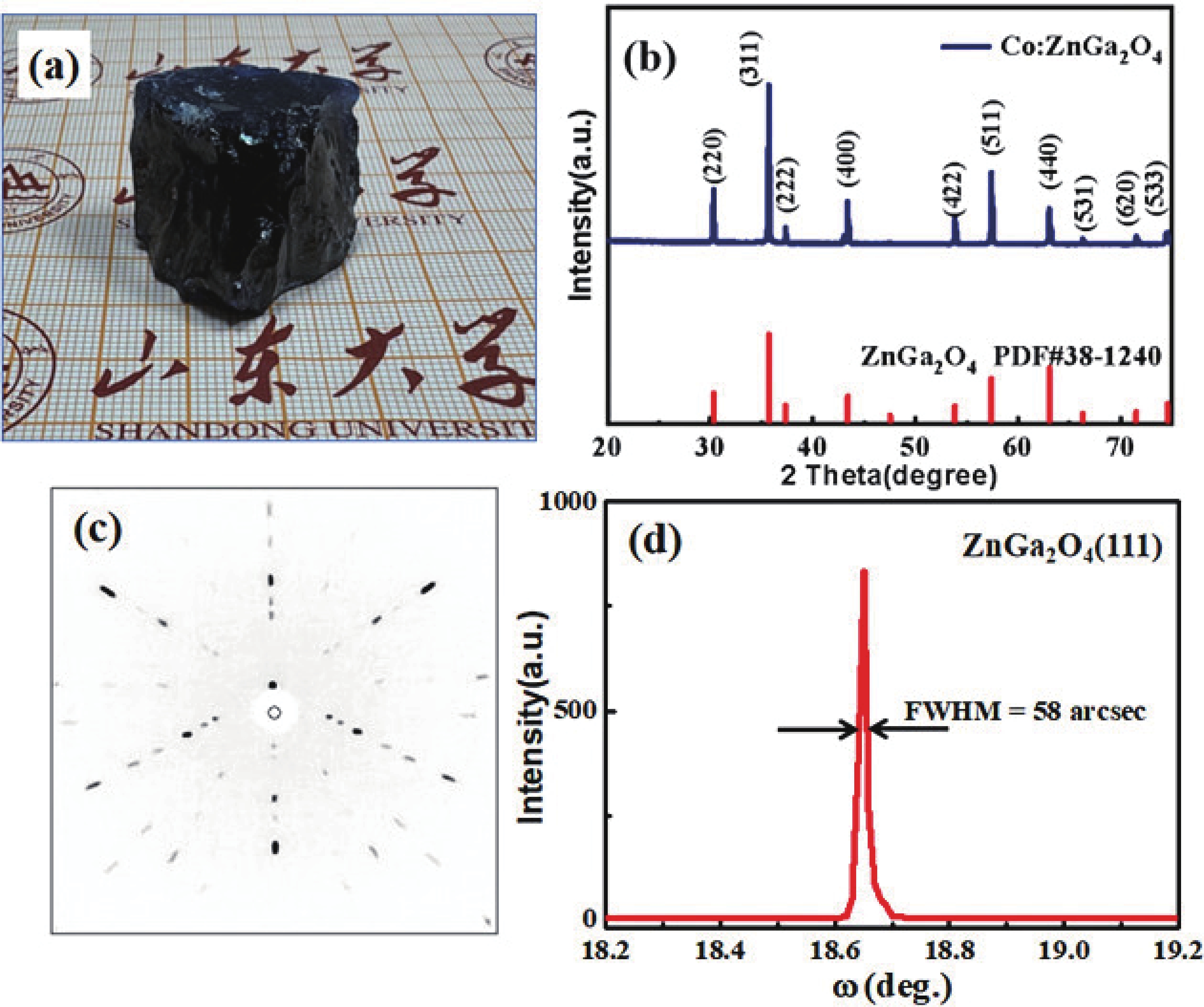
 DownLoad:
DownLoad: