| Citation: |
Heba Hussein, Sobhy Sayed Ibrahim, Sherif Ahmed Khairy. Eco-sustainable biosynthesis of CoFe2O4 nanoparticles using apple extract for multifunctional applications[J]. Journal of Semiconductors, 2025, 46(12): 122101. doi: 10.1088/1674-4926/25040013
****
H Hussein, S S Ibrahim, and S A Khairy, Eco-sustainable biosynthesis of CoFe2O4 nanoparticles using apple extract for multifunctional applications[J]. J. Semicond., 2025, 46(12), 122101 doi: 10.1088/1674-4926/25040013
|
Eco-sustainable biosynthesis of CoFe2O4 nanoparticles using apple extract for multifunctional applications
DOI: 10.1088/1674-4926/25040013
CSTR: 32376.14.1674-4926.25040013
More Information-
Abstract
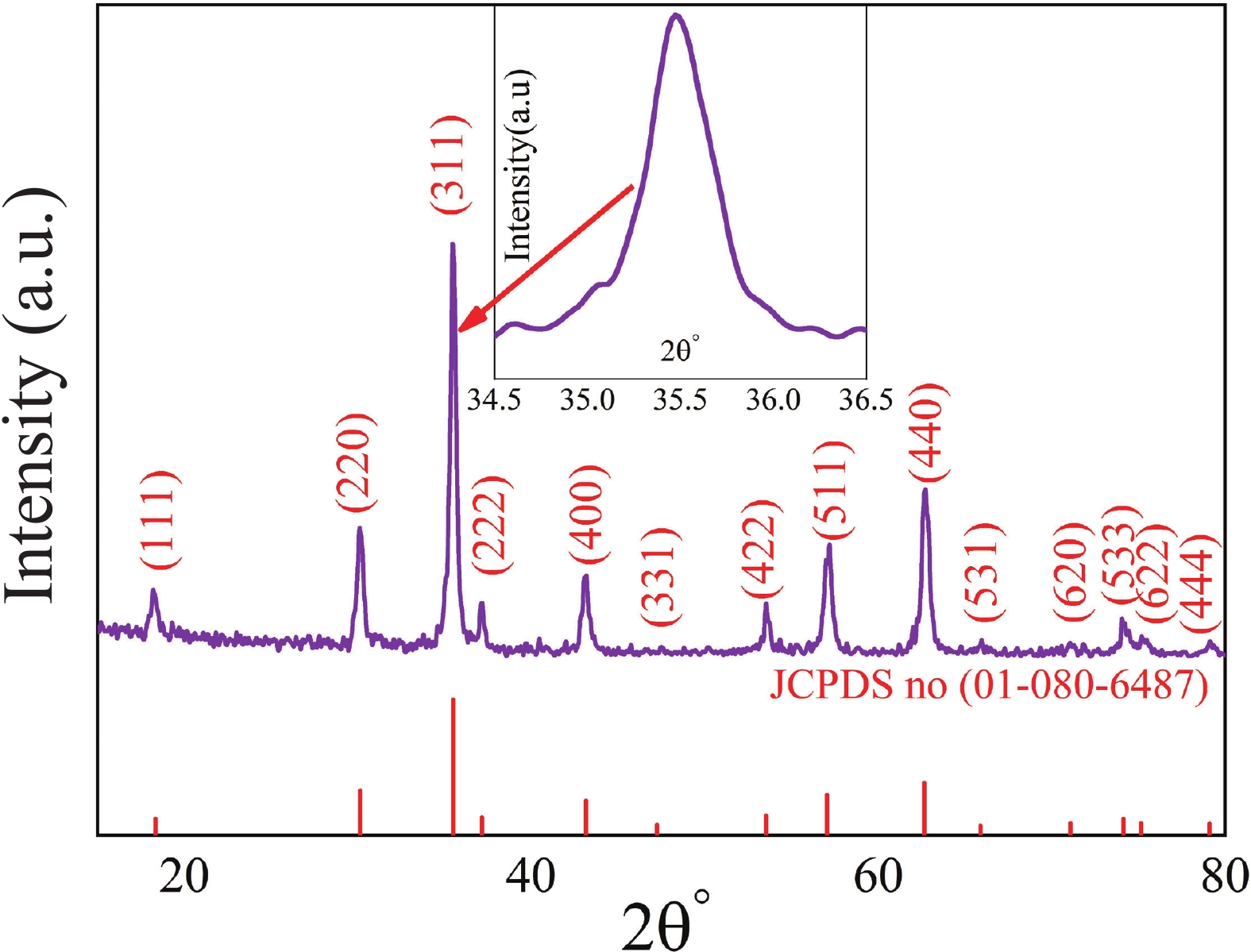
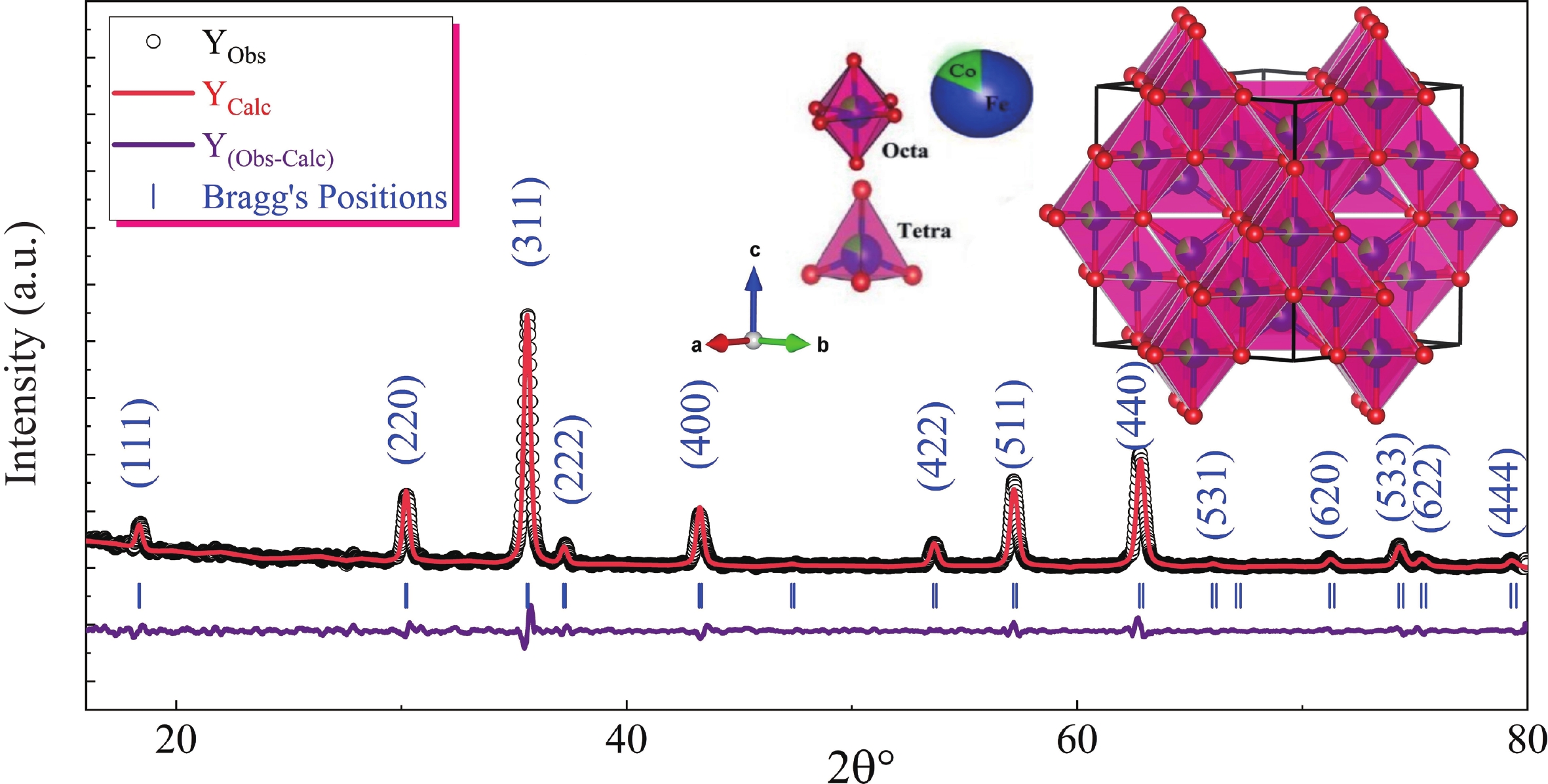
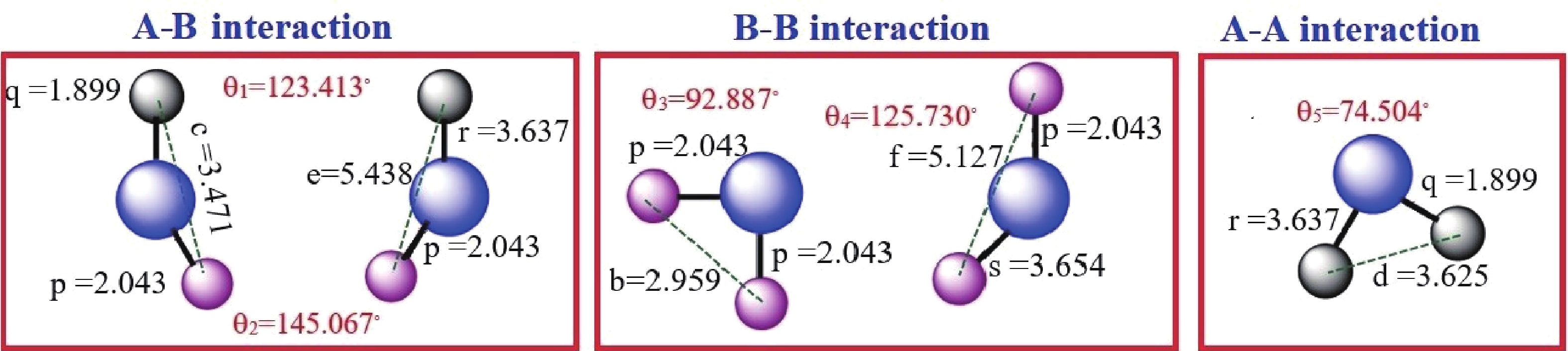
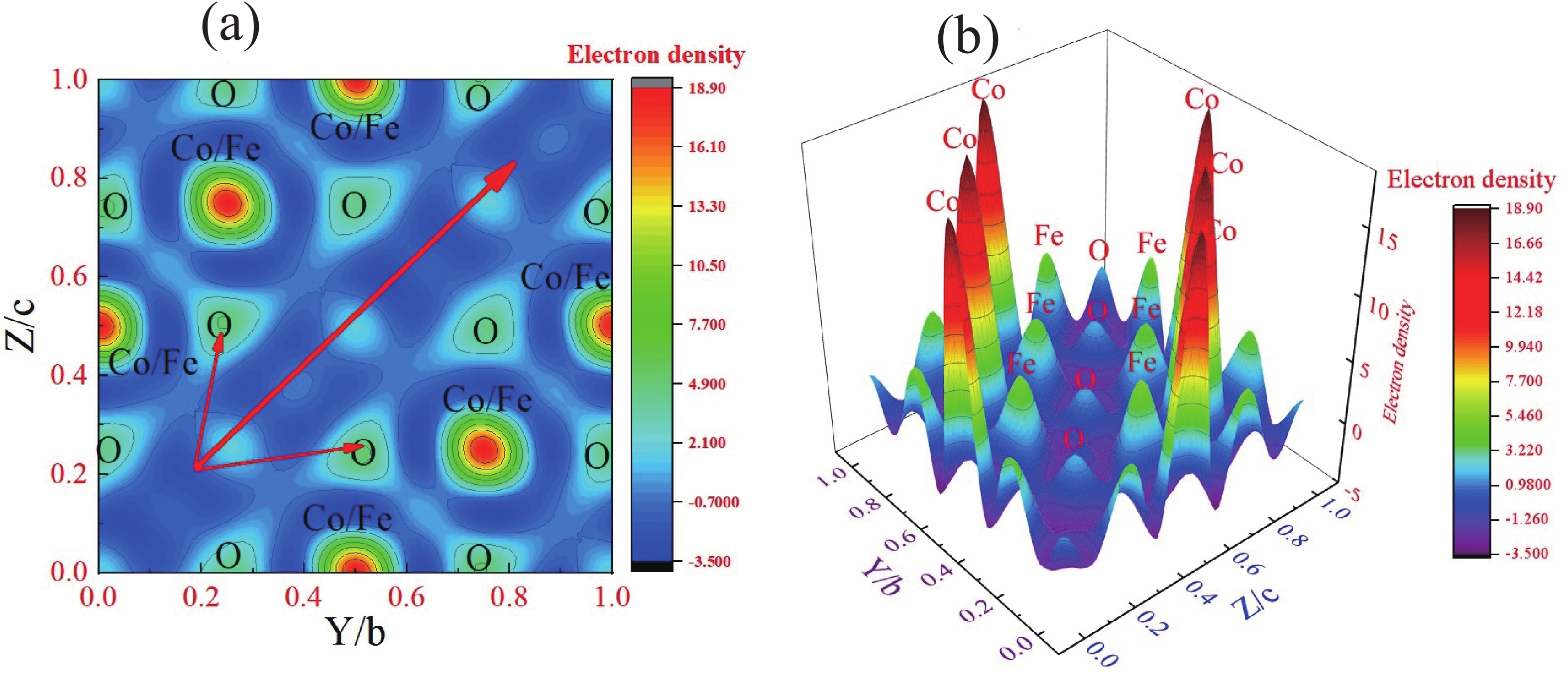
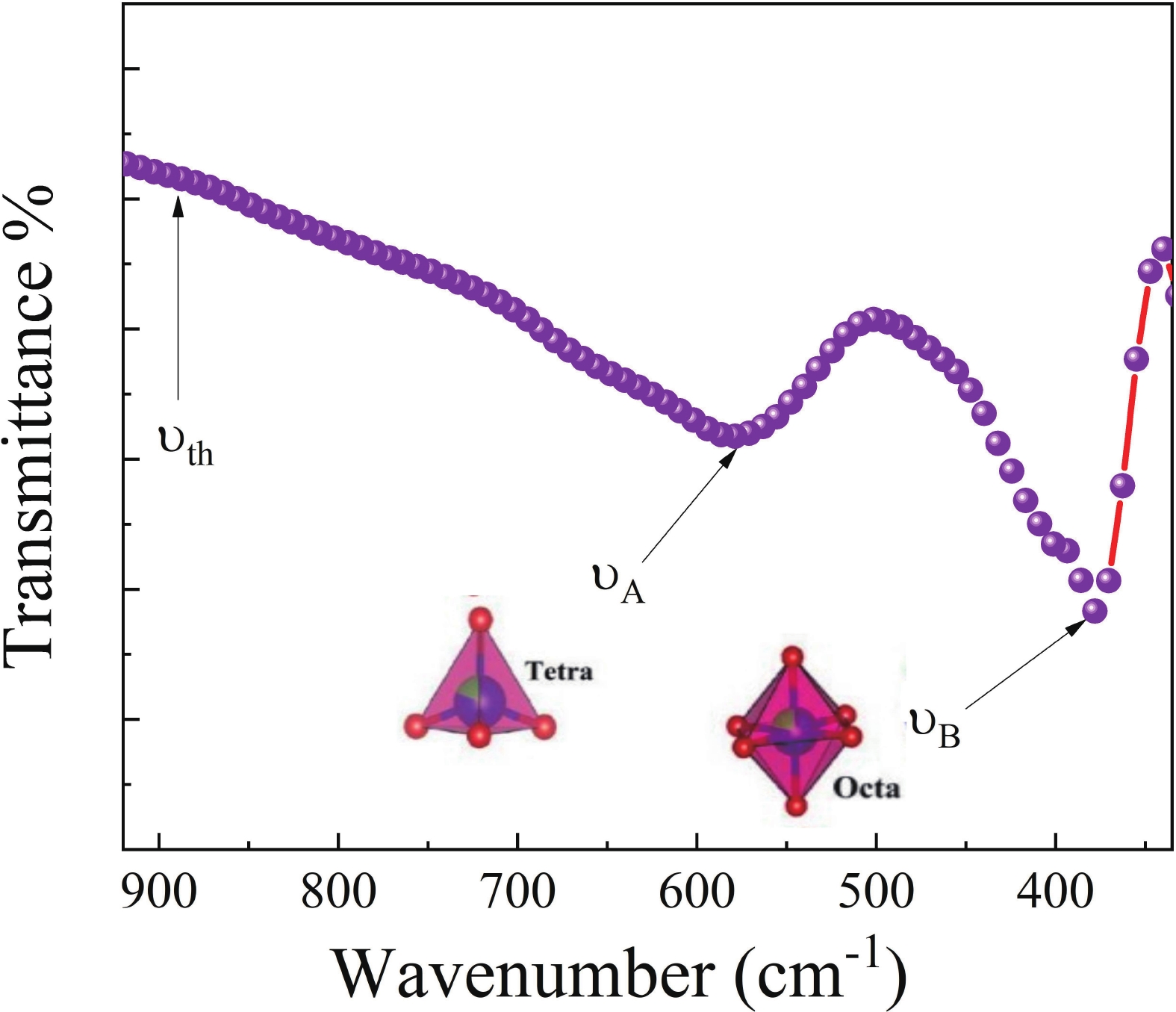
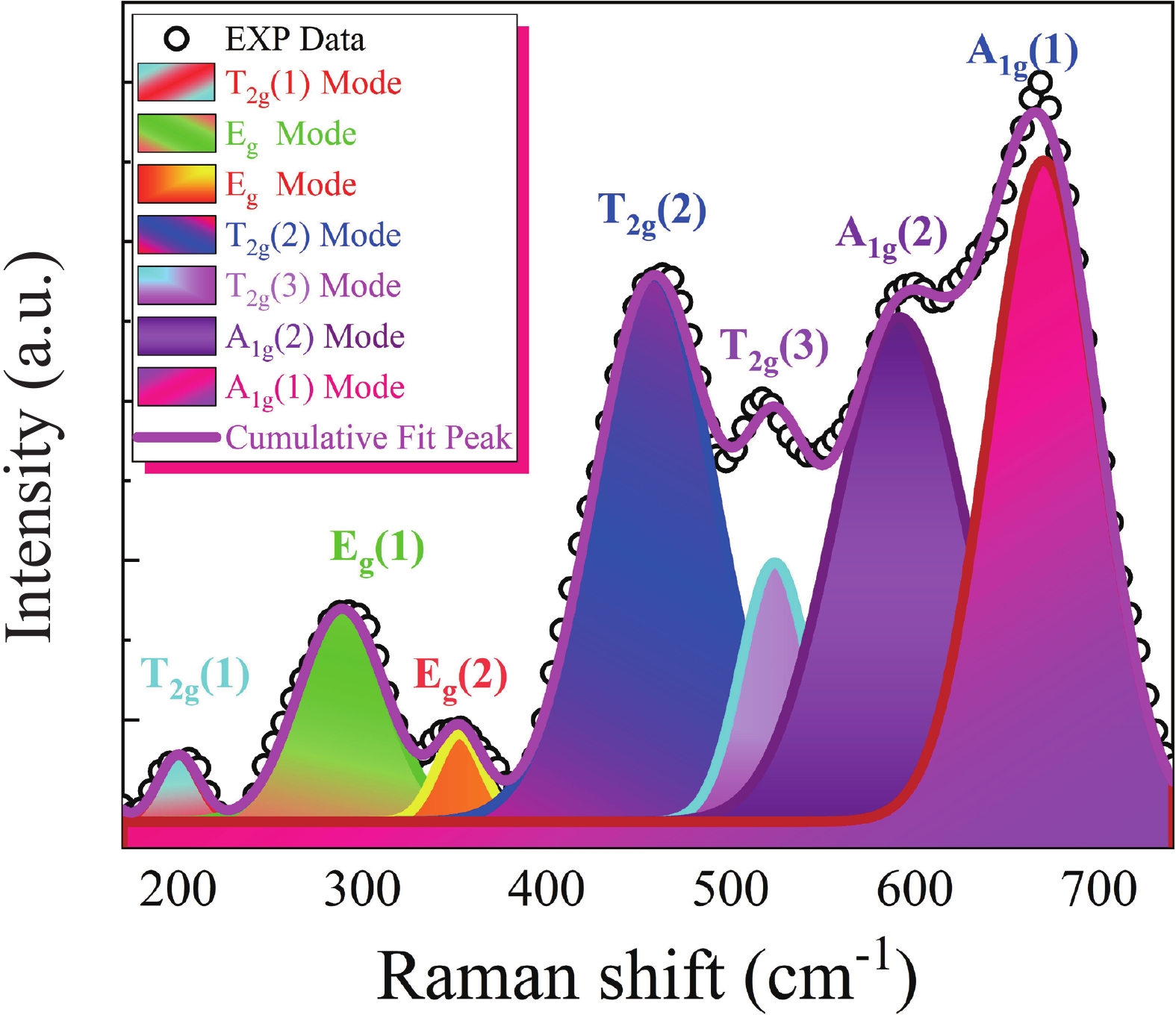
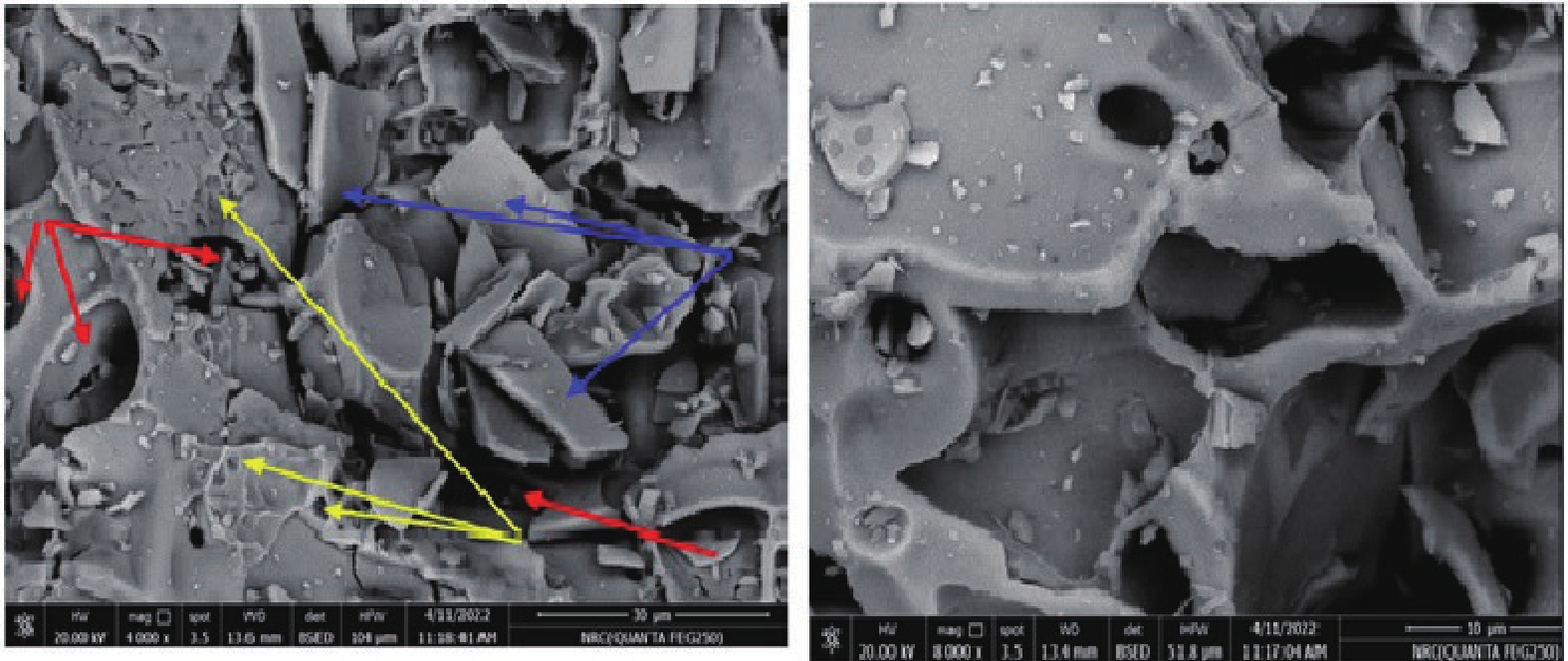
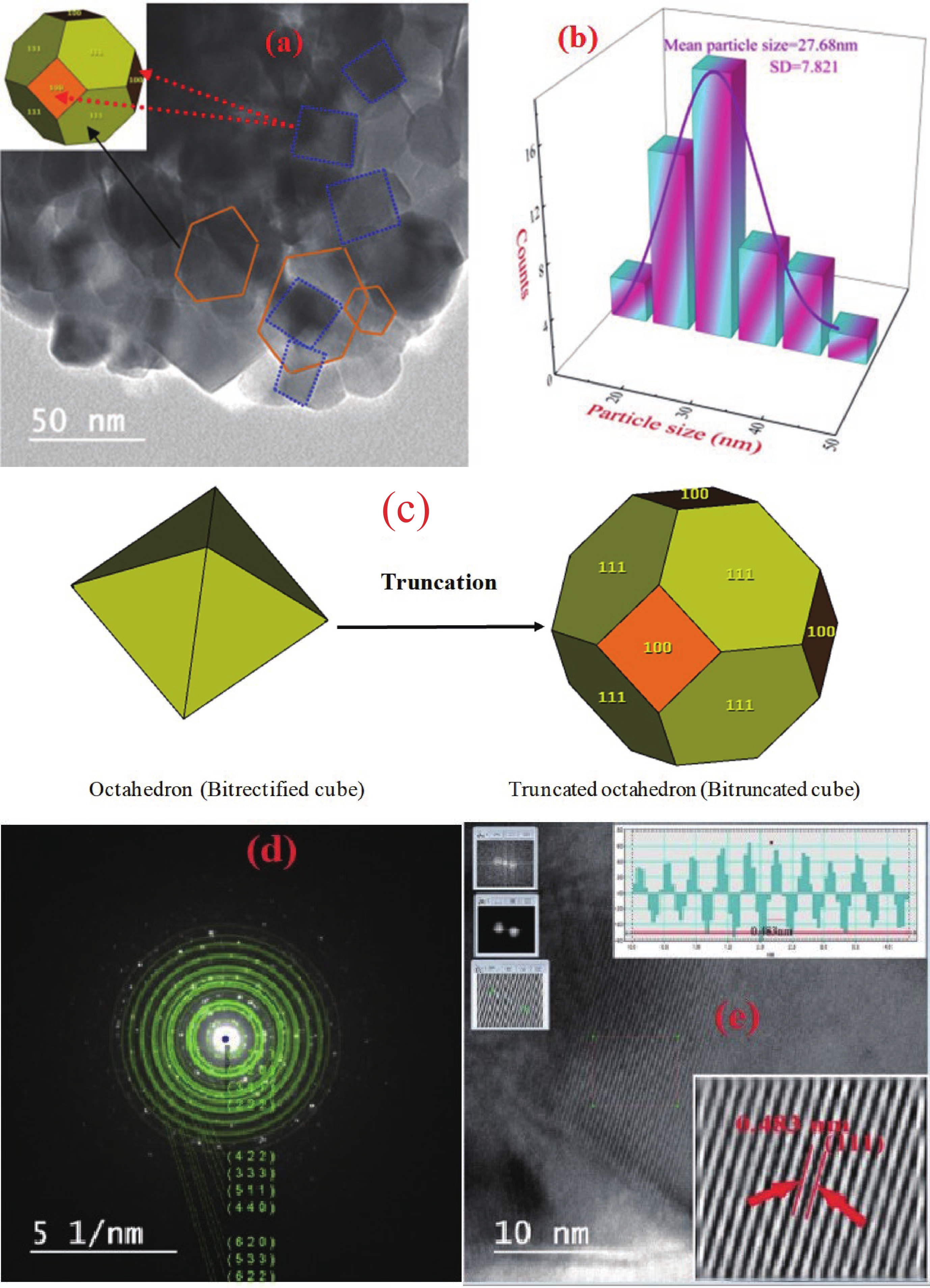
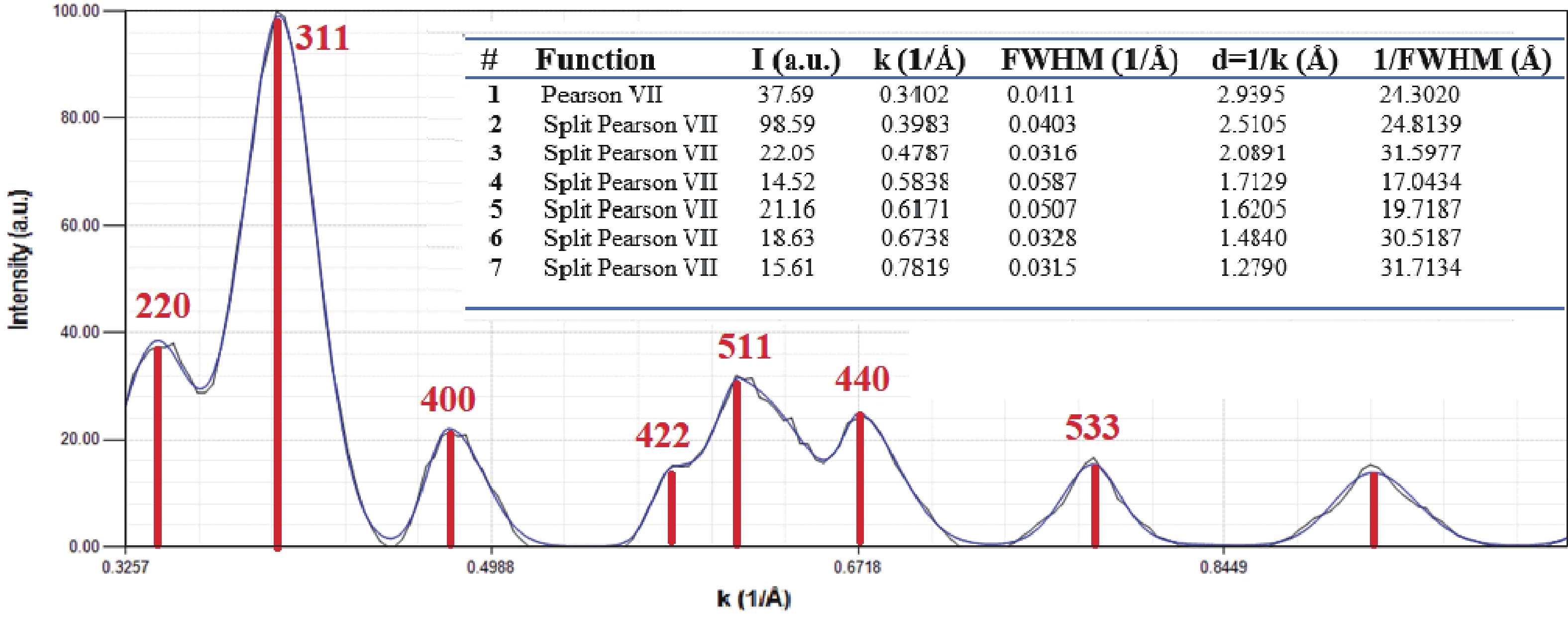
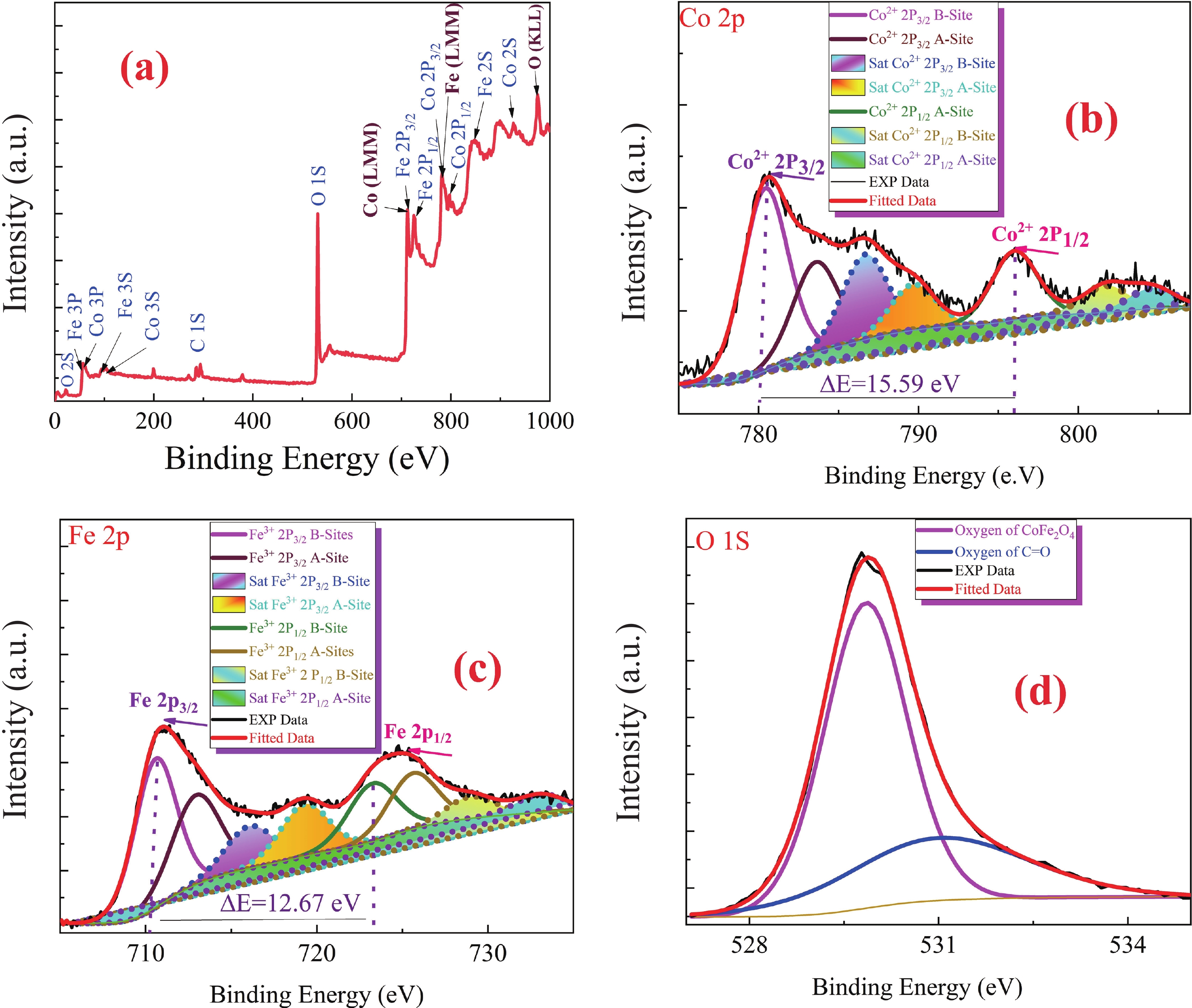
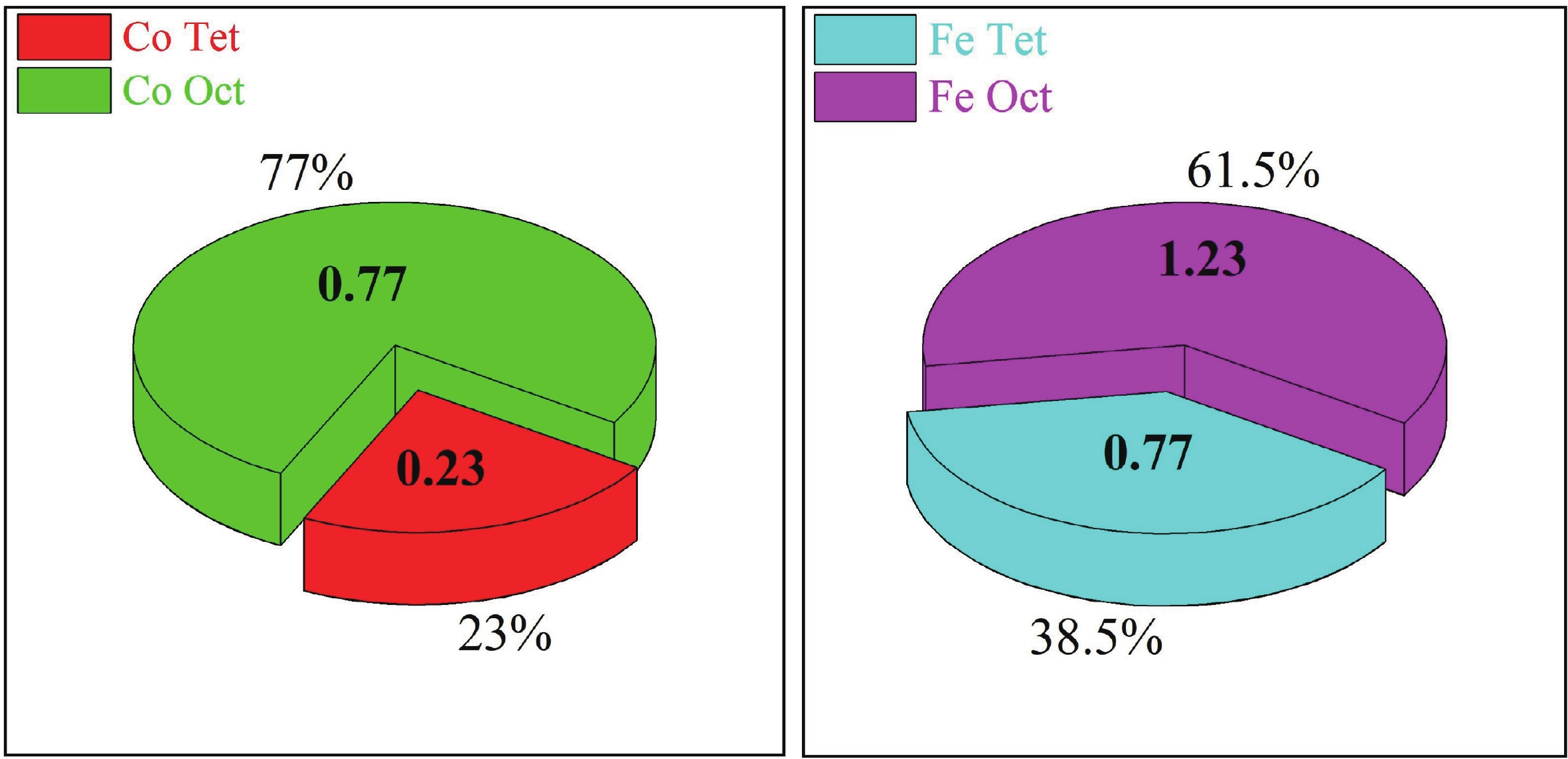
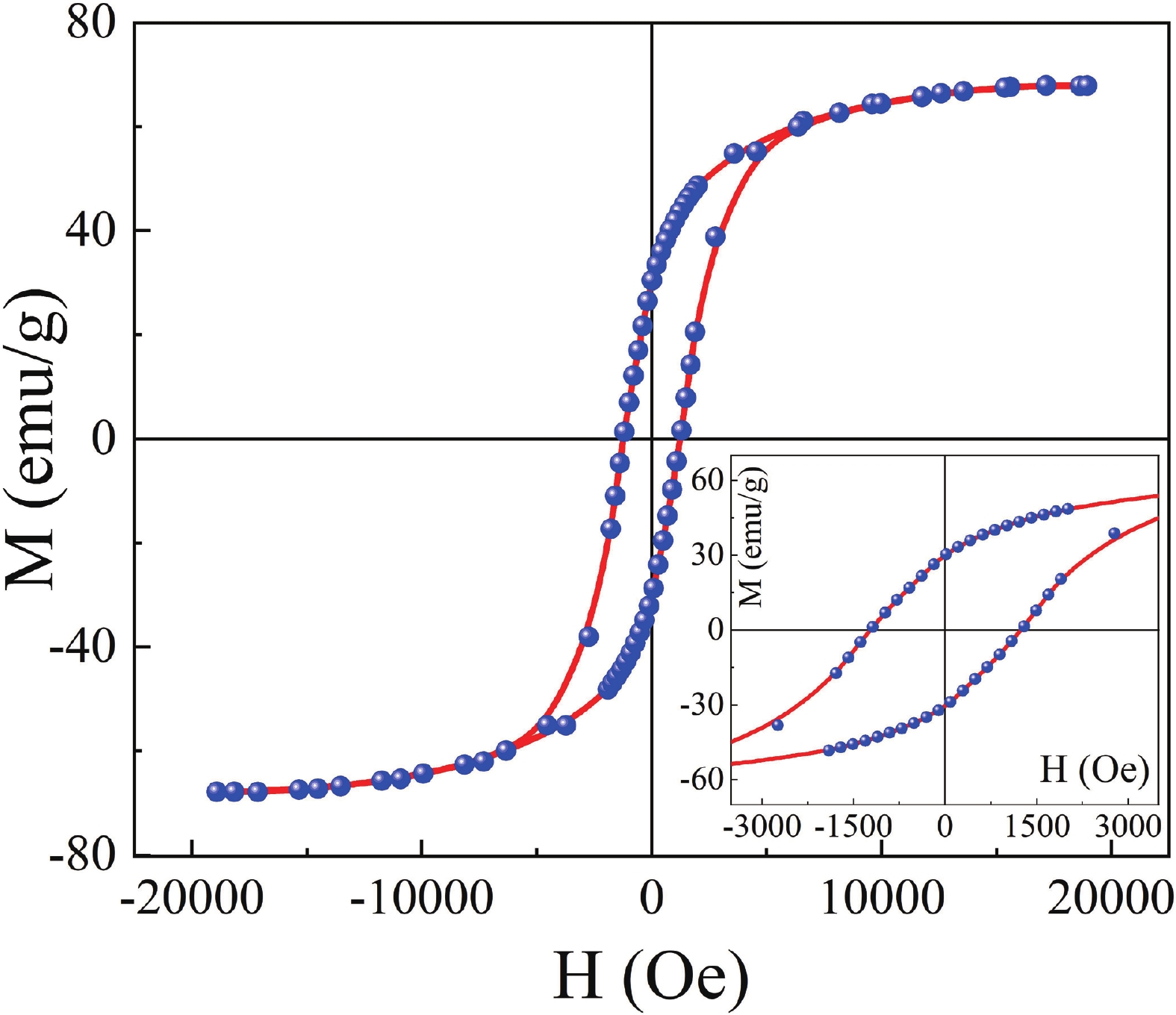
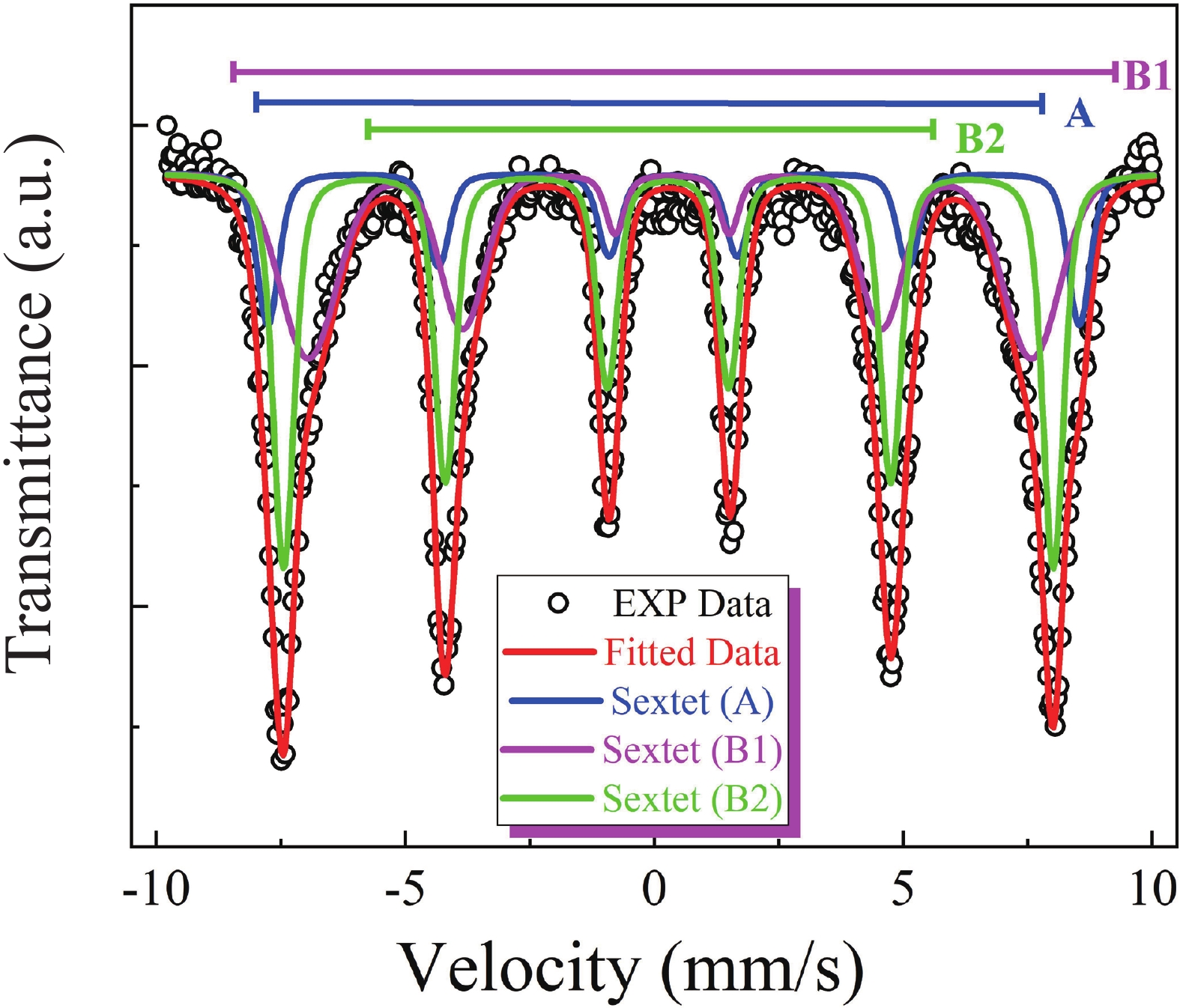
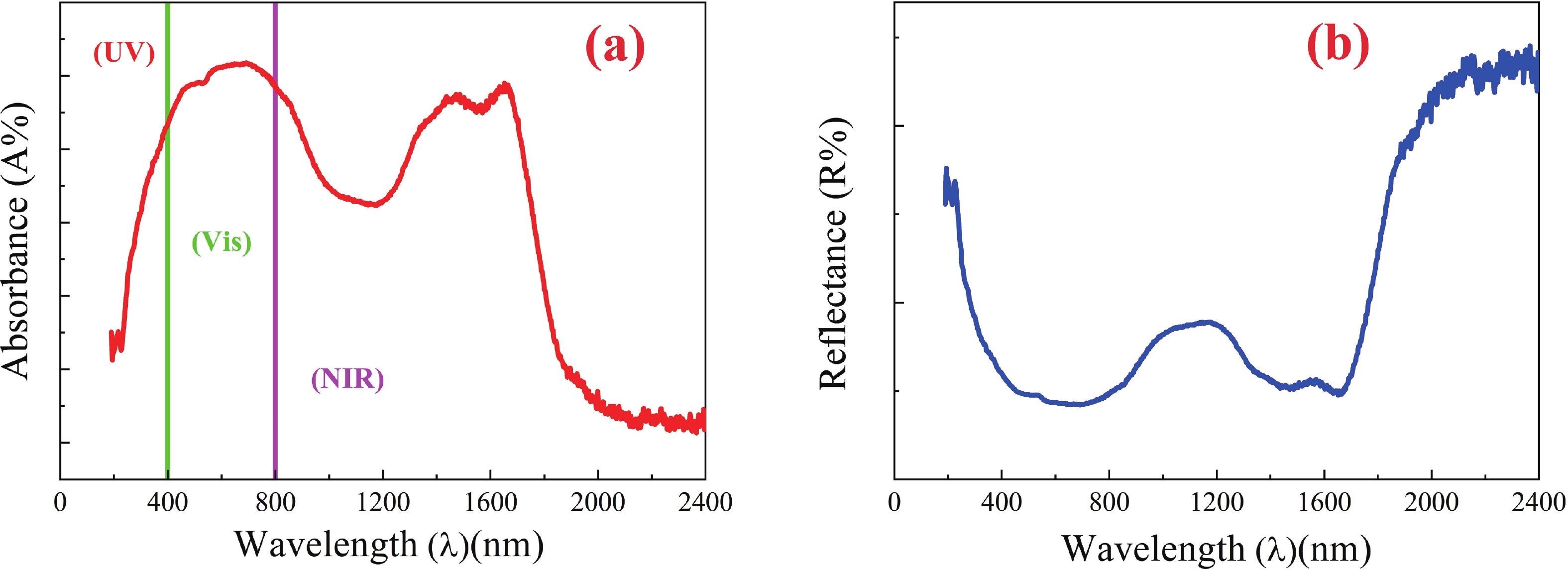
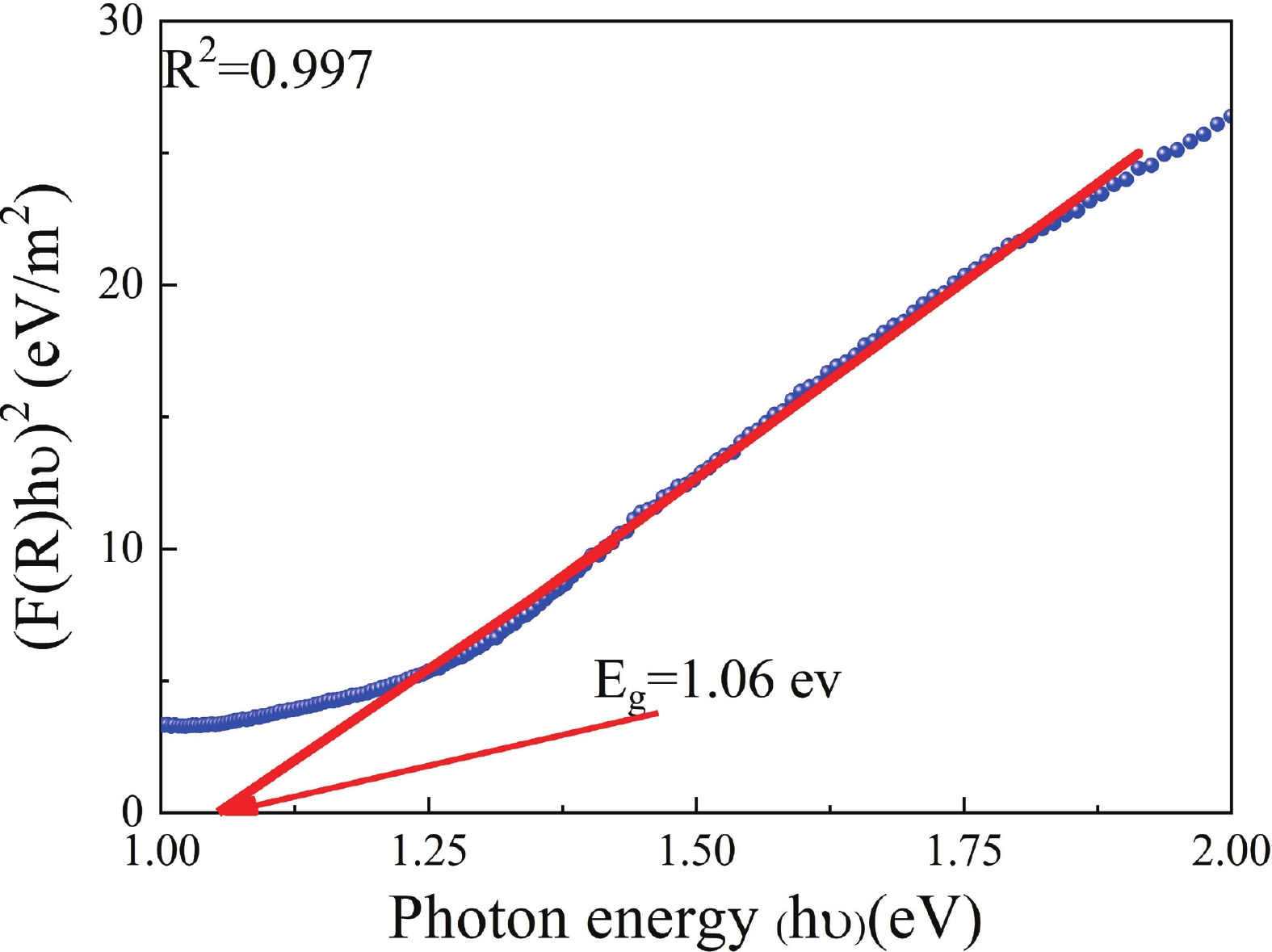
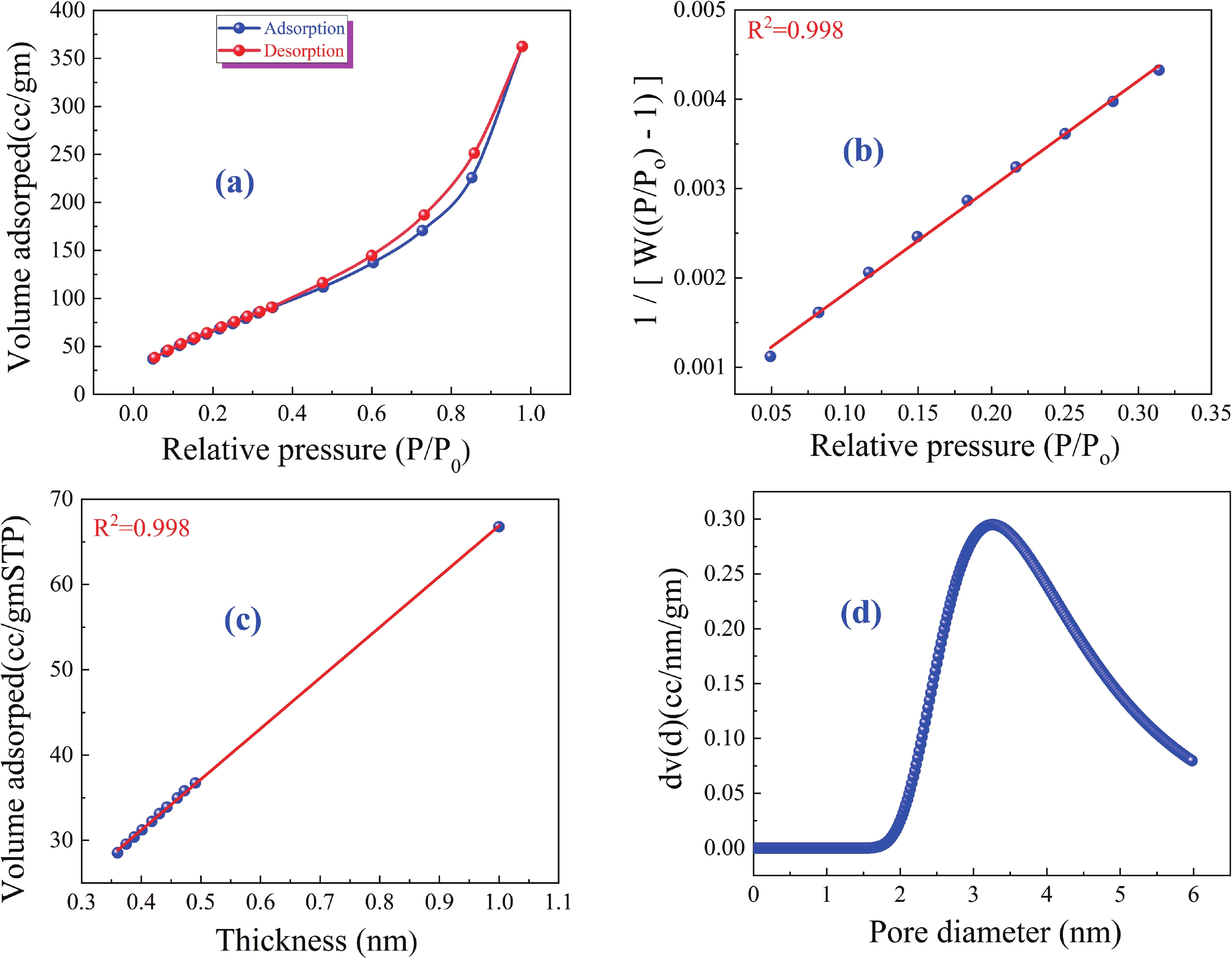
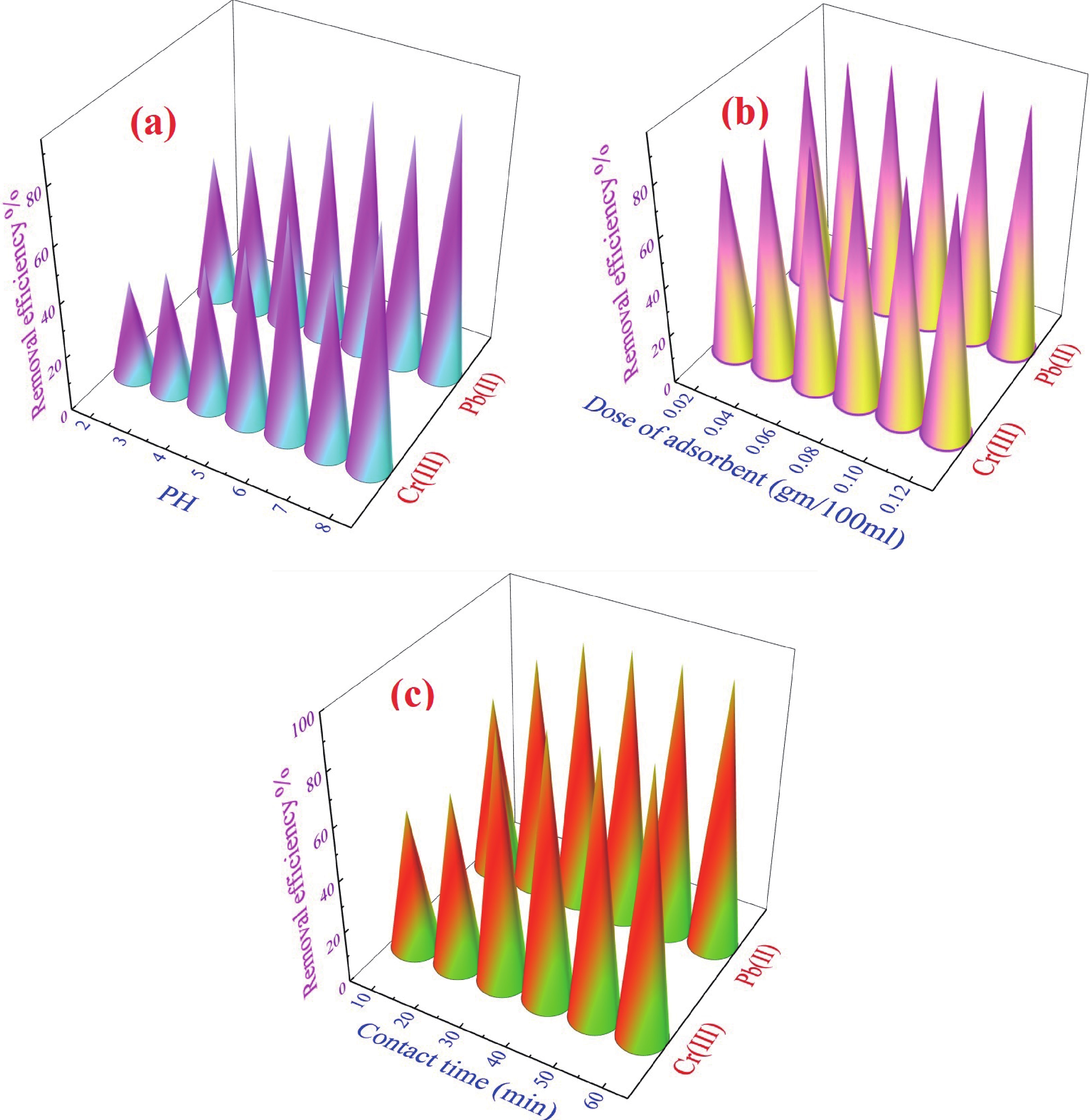
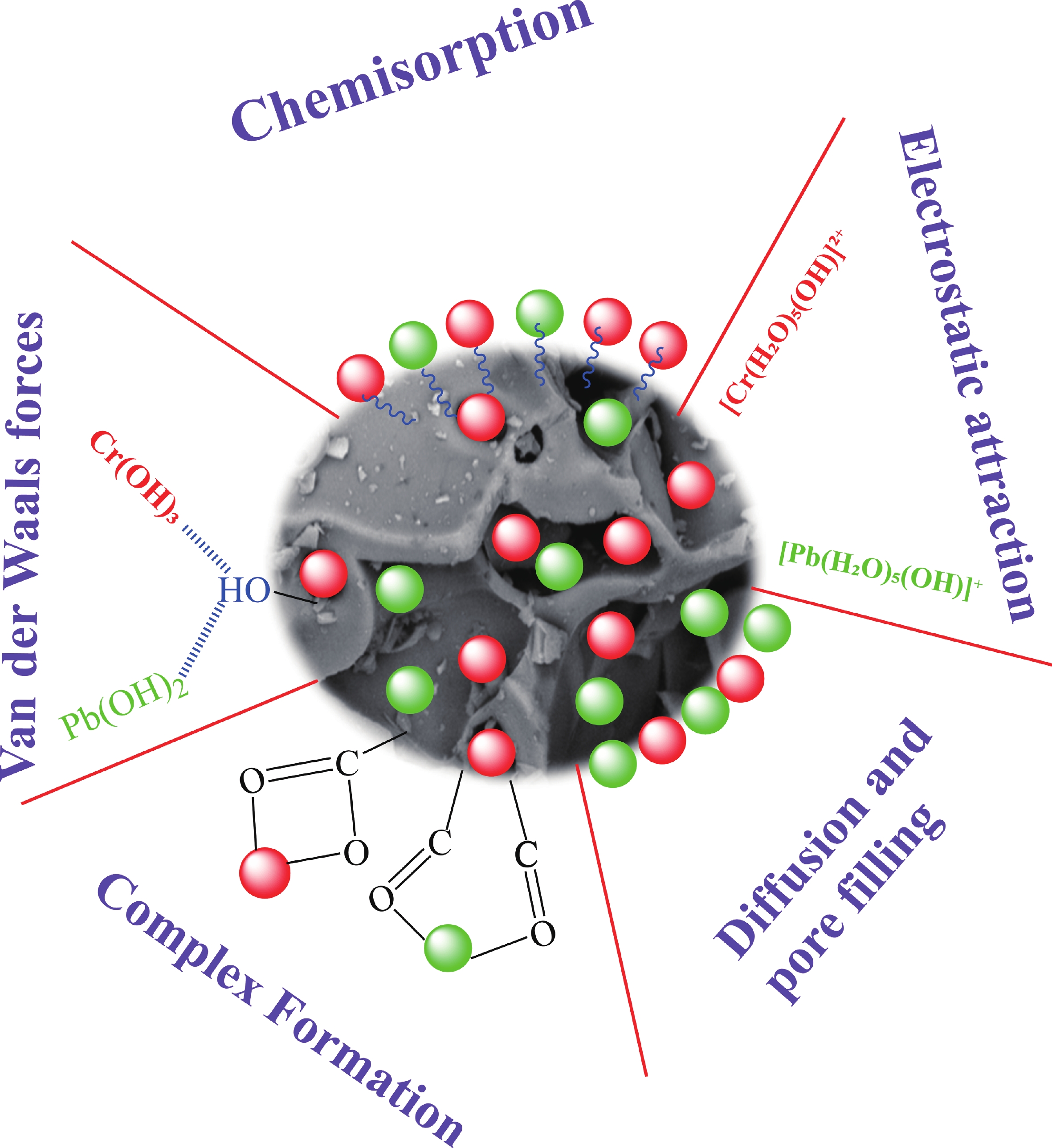
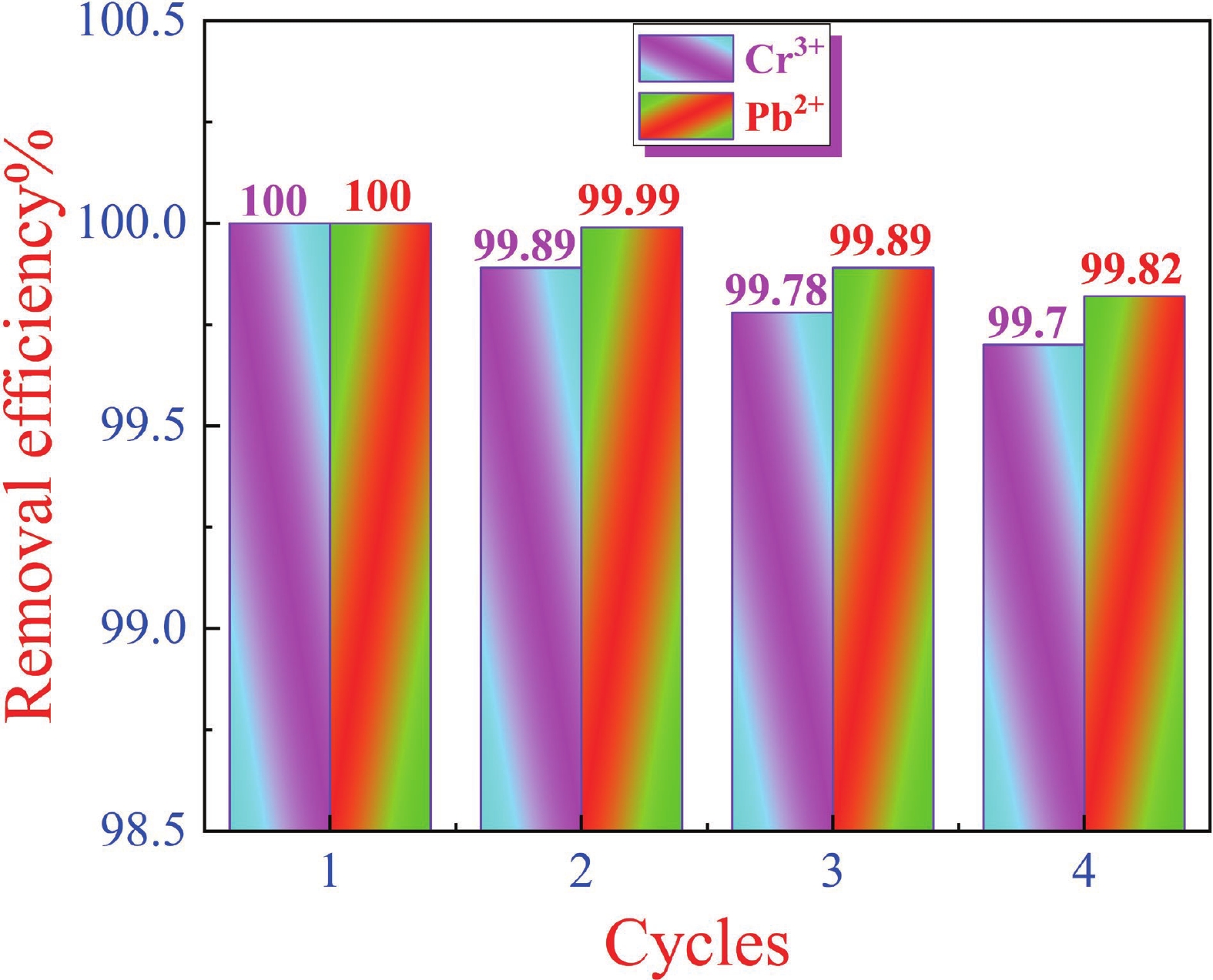
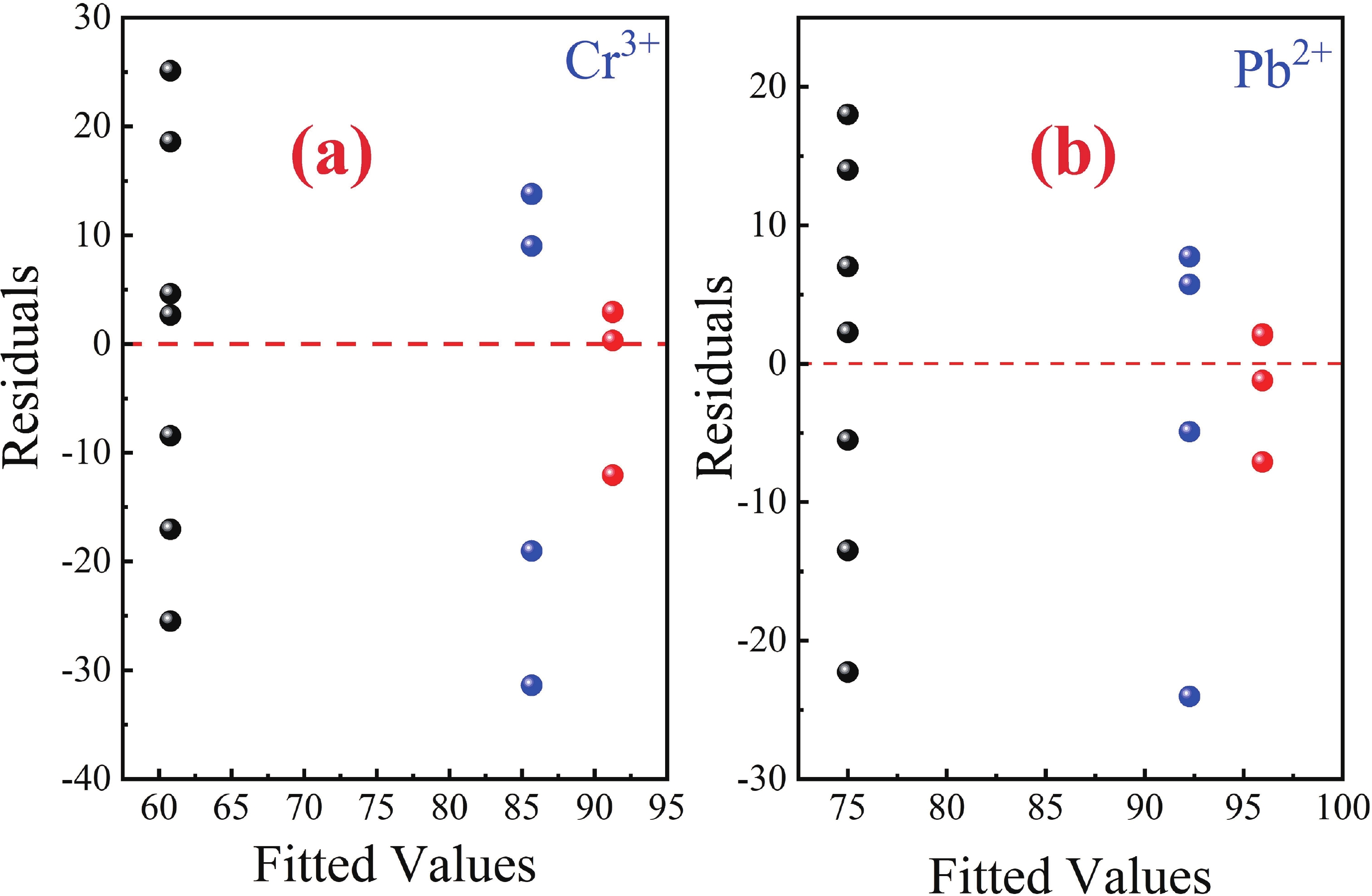
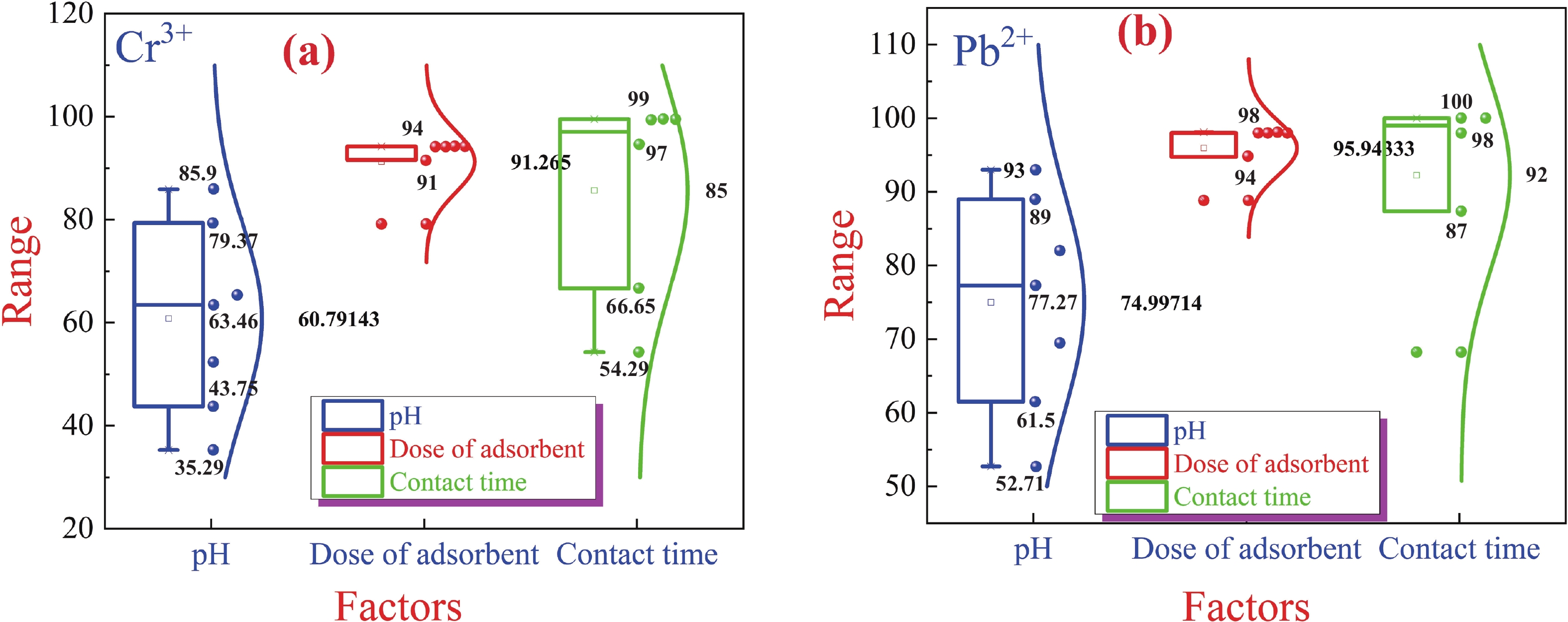
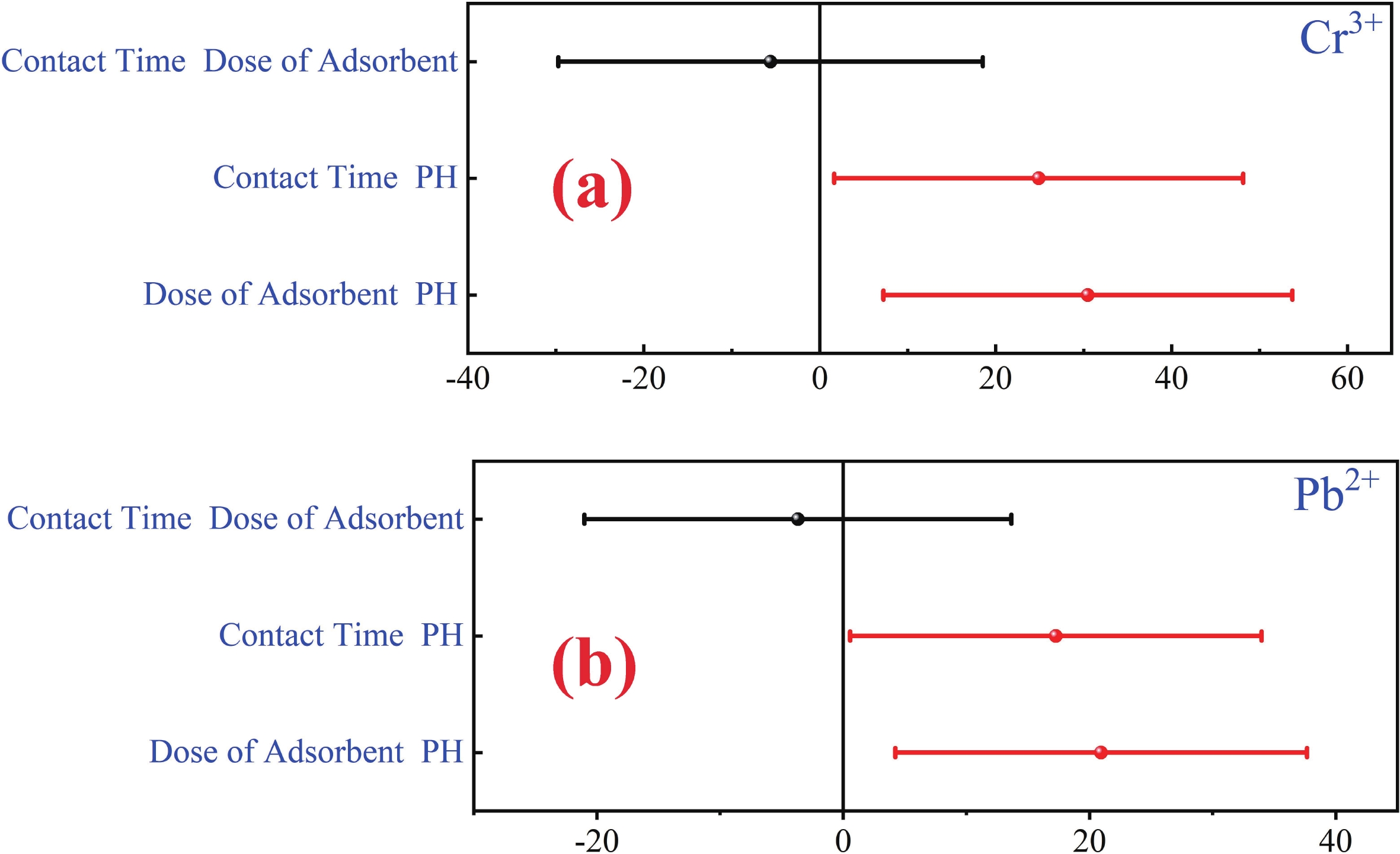
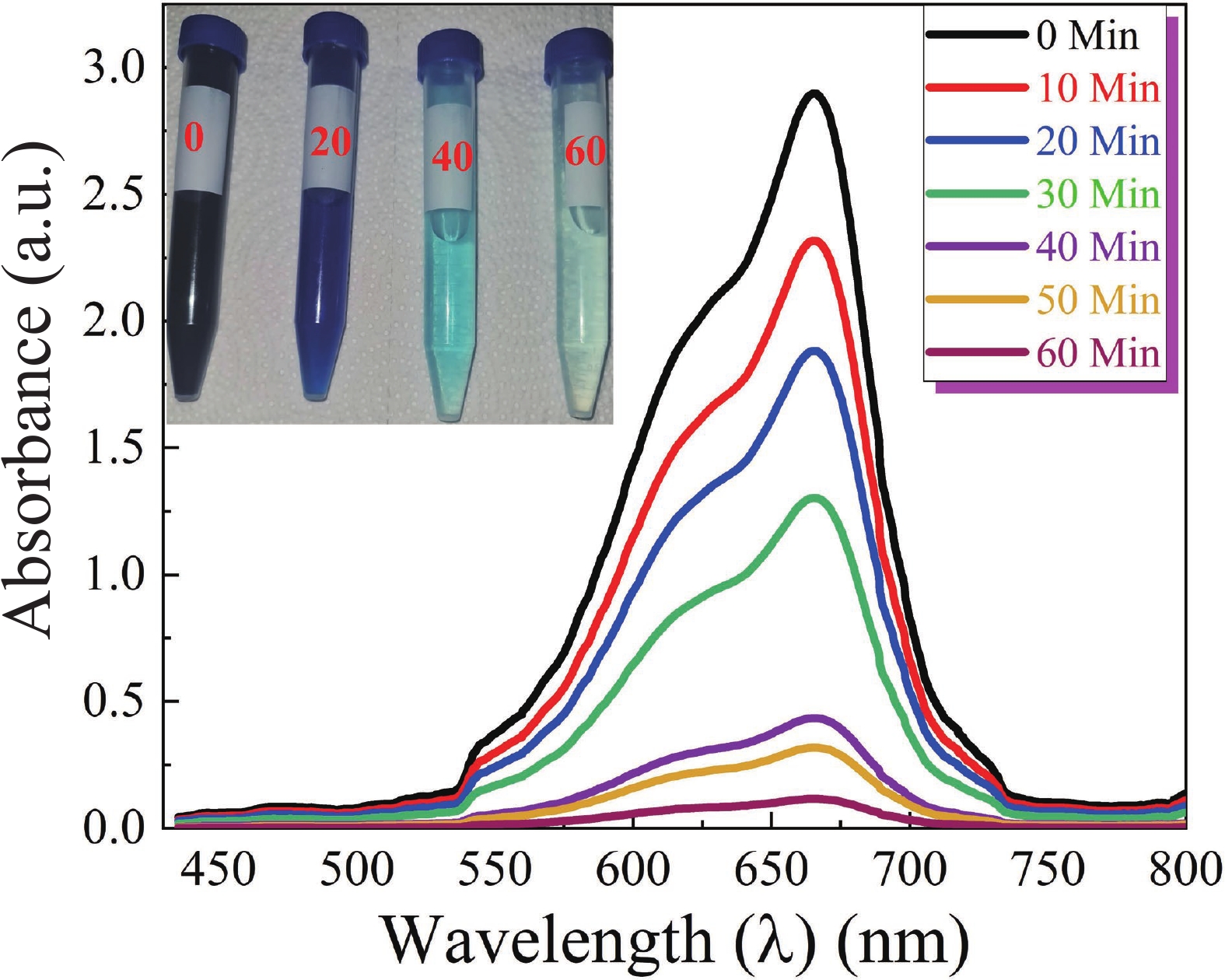
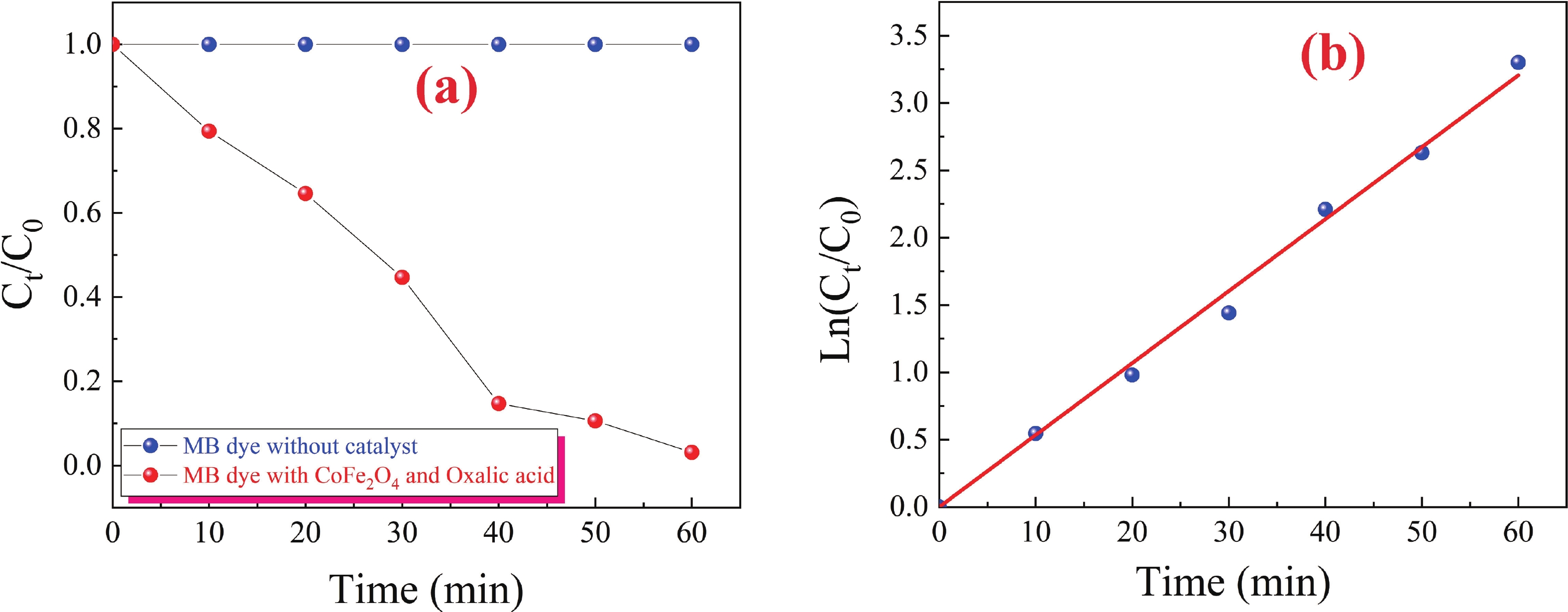
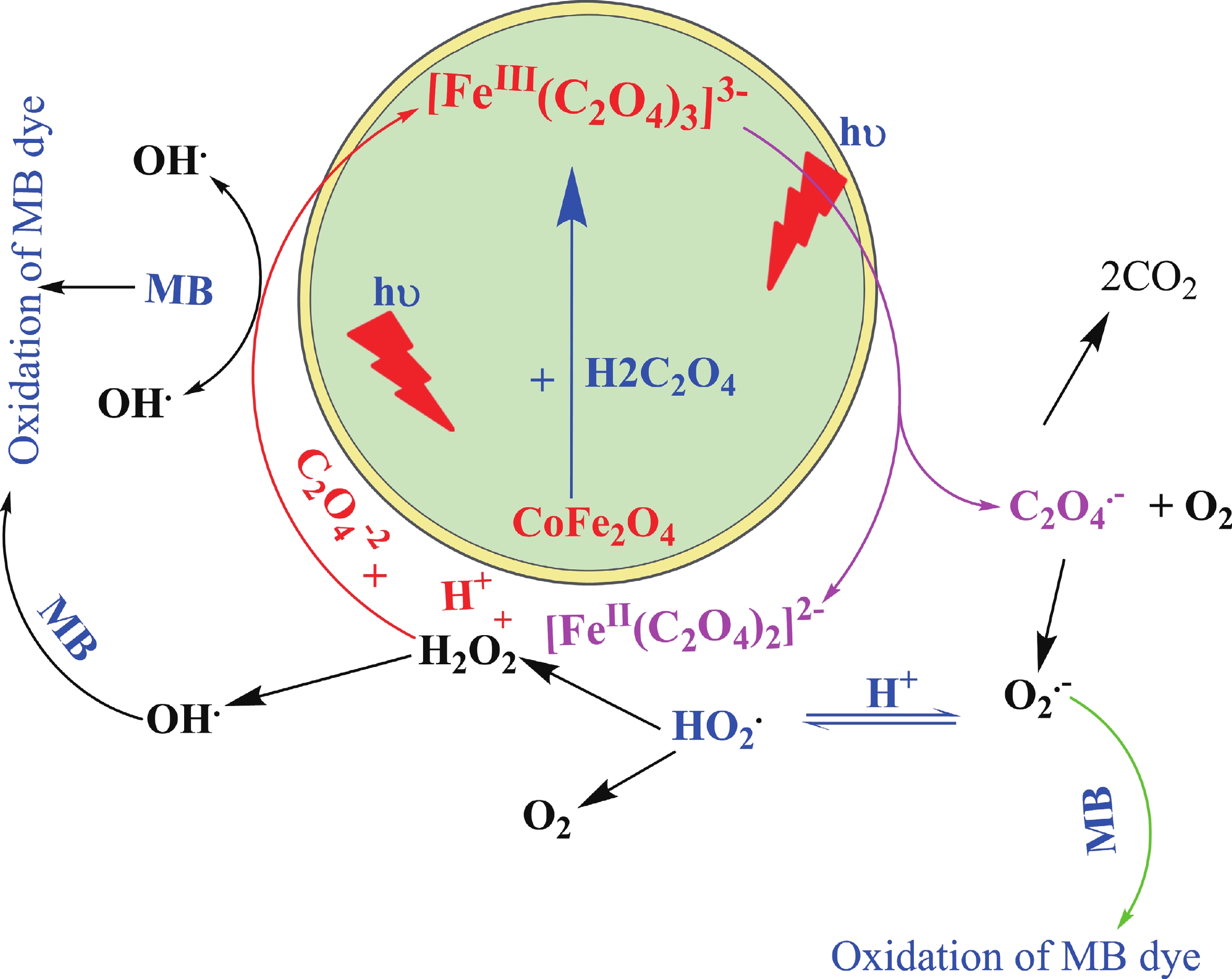
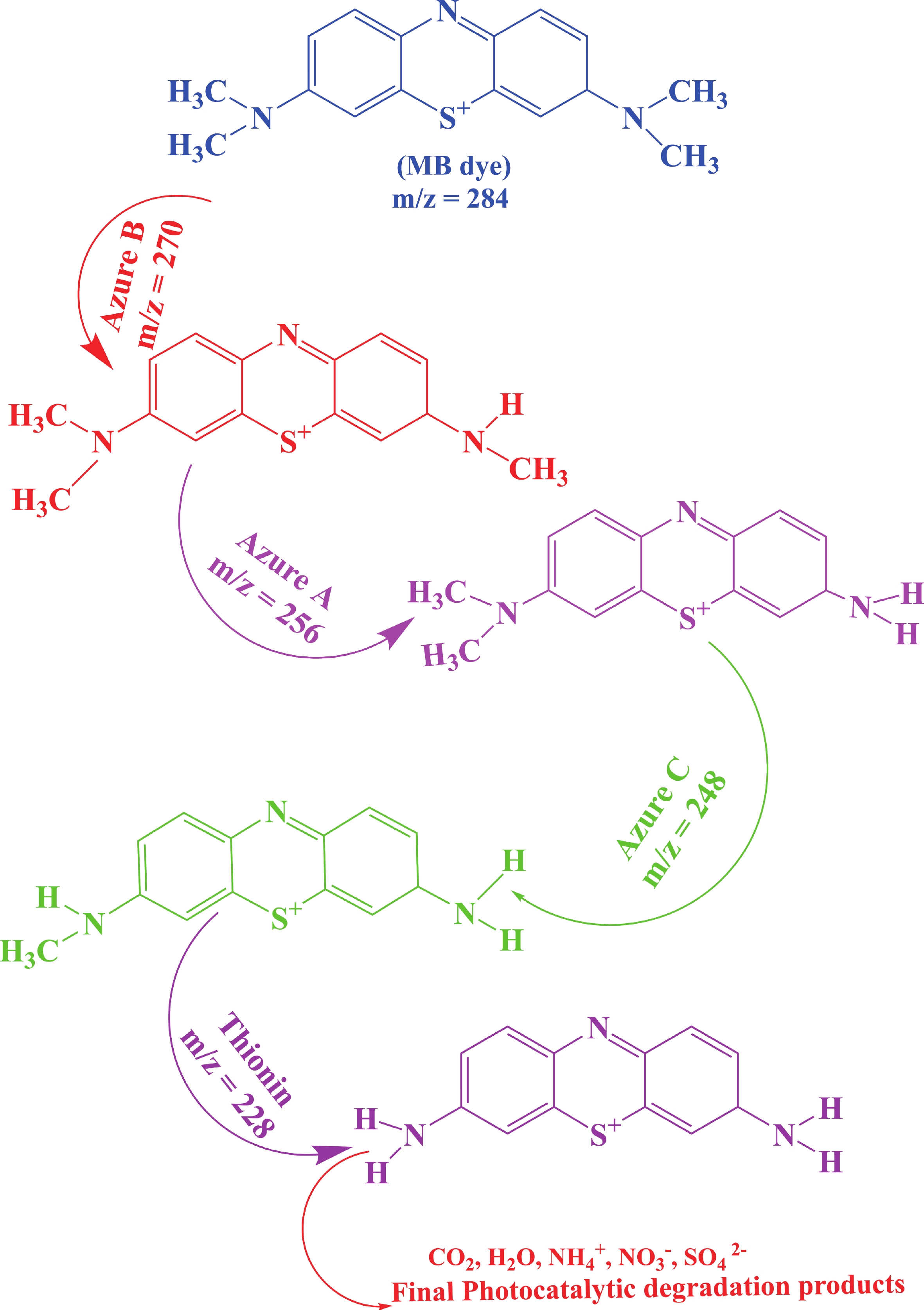
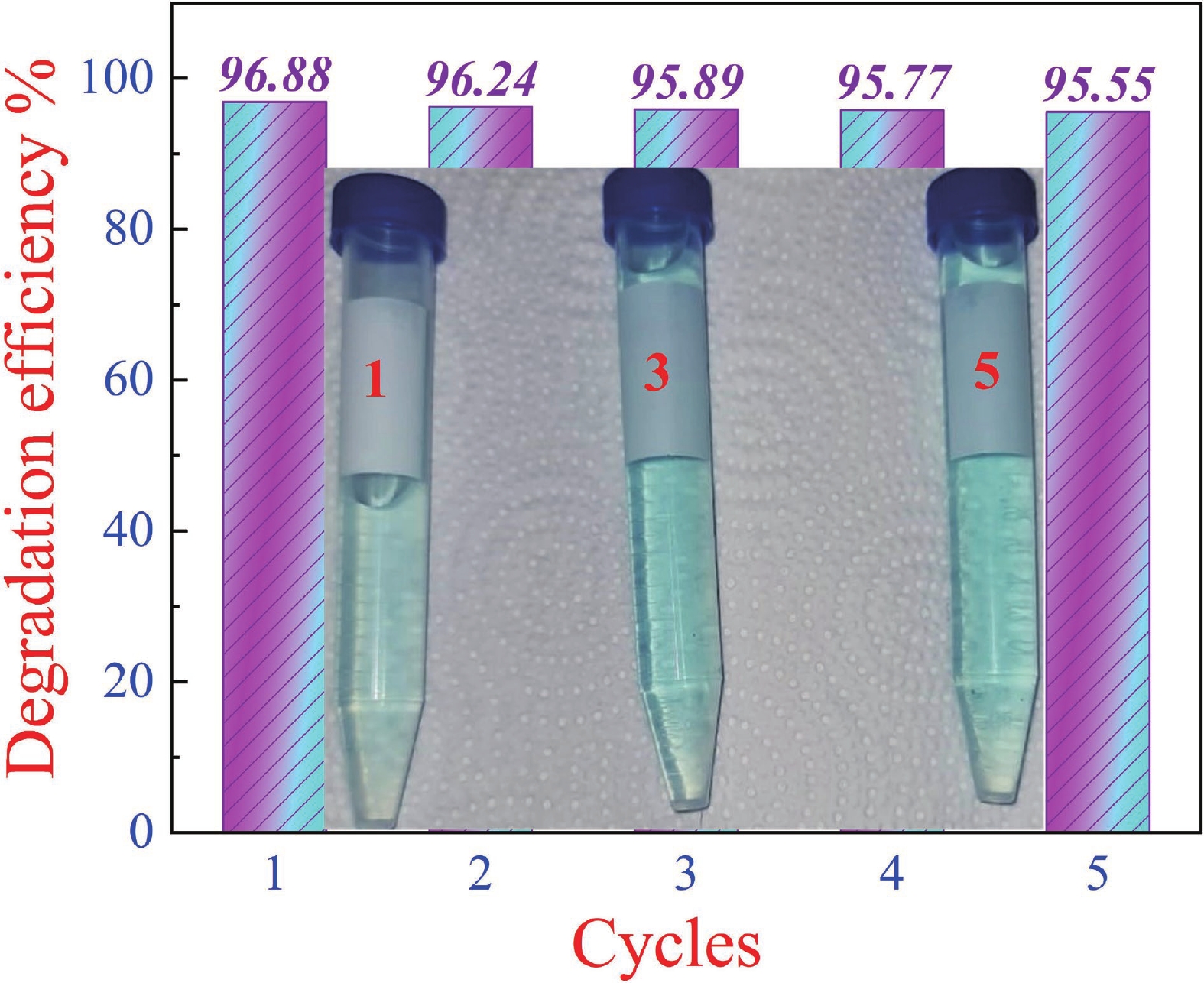
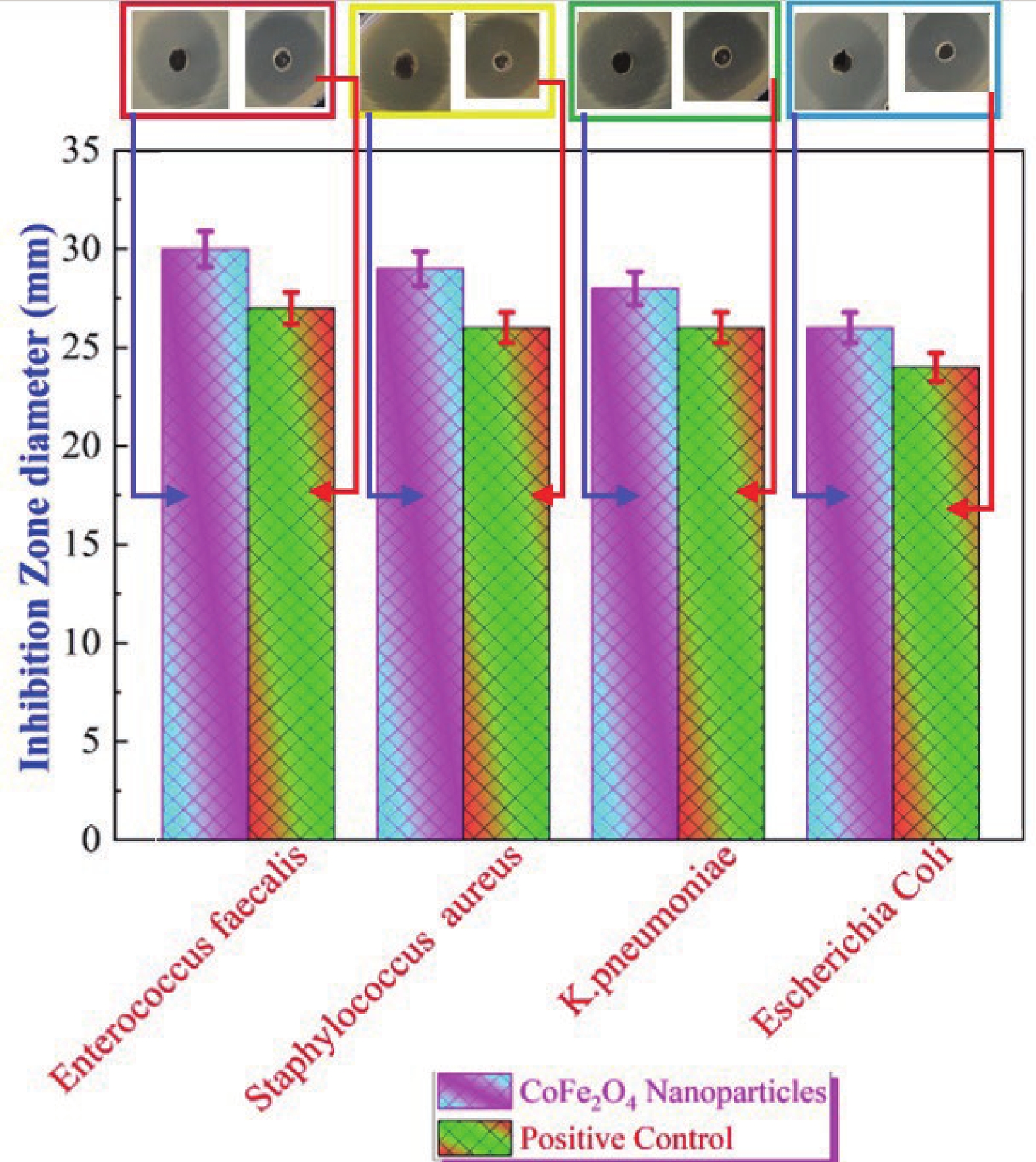
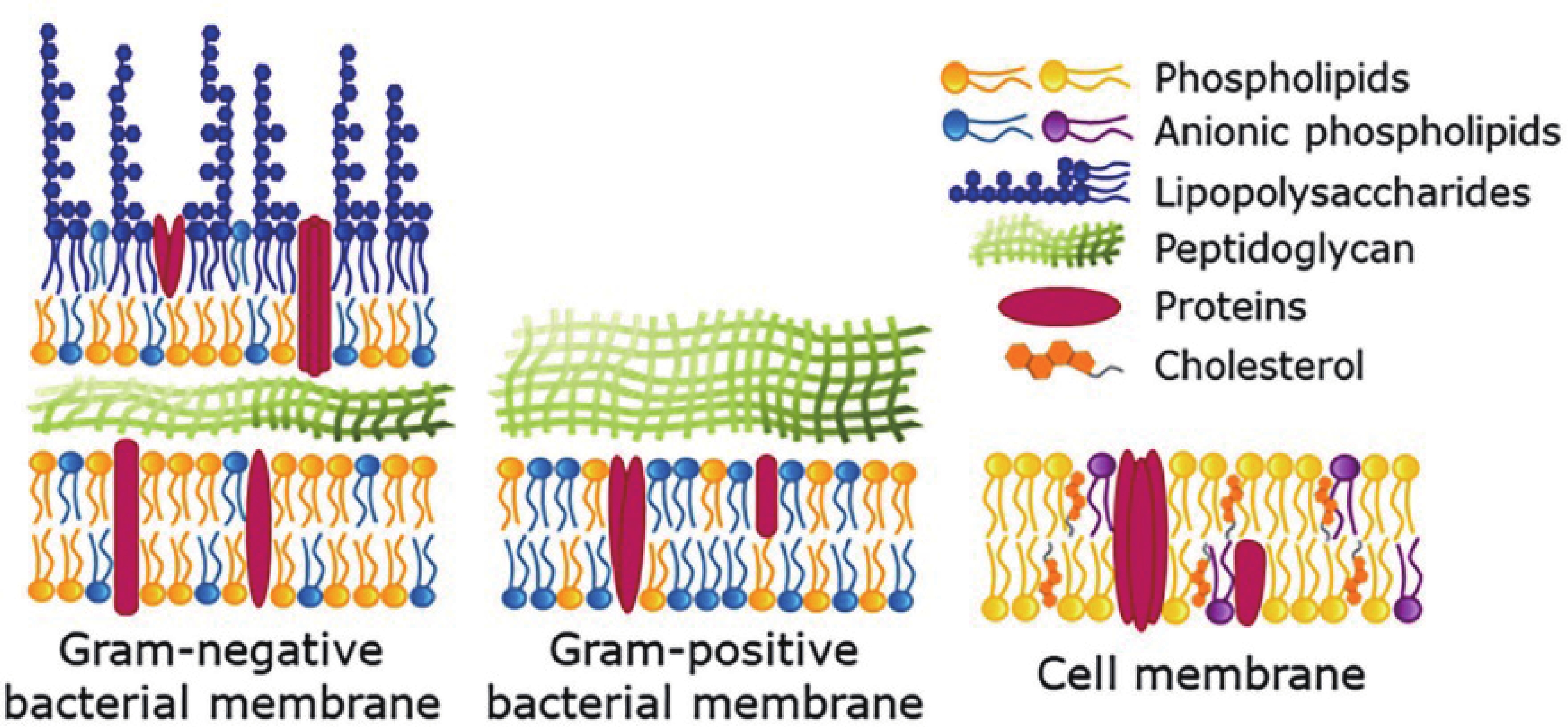
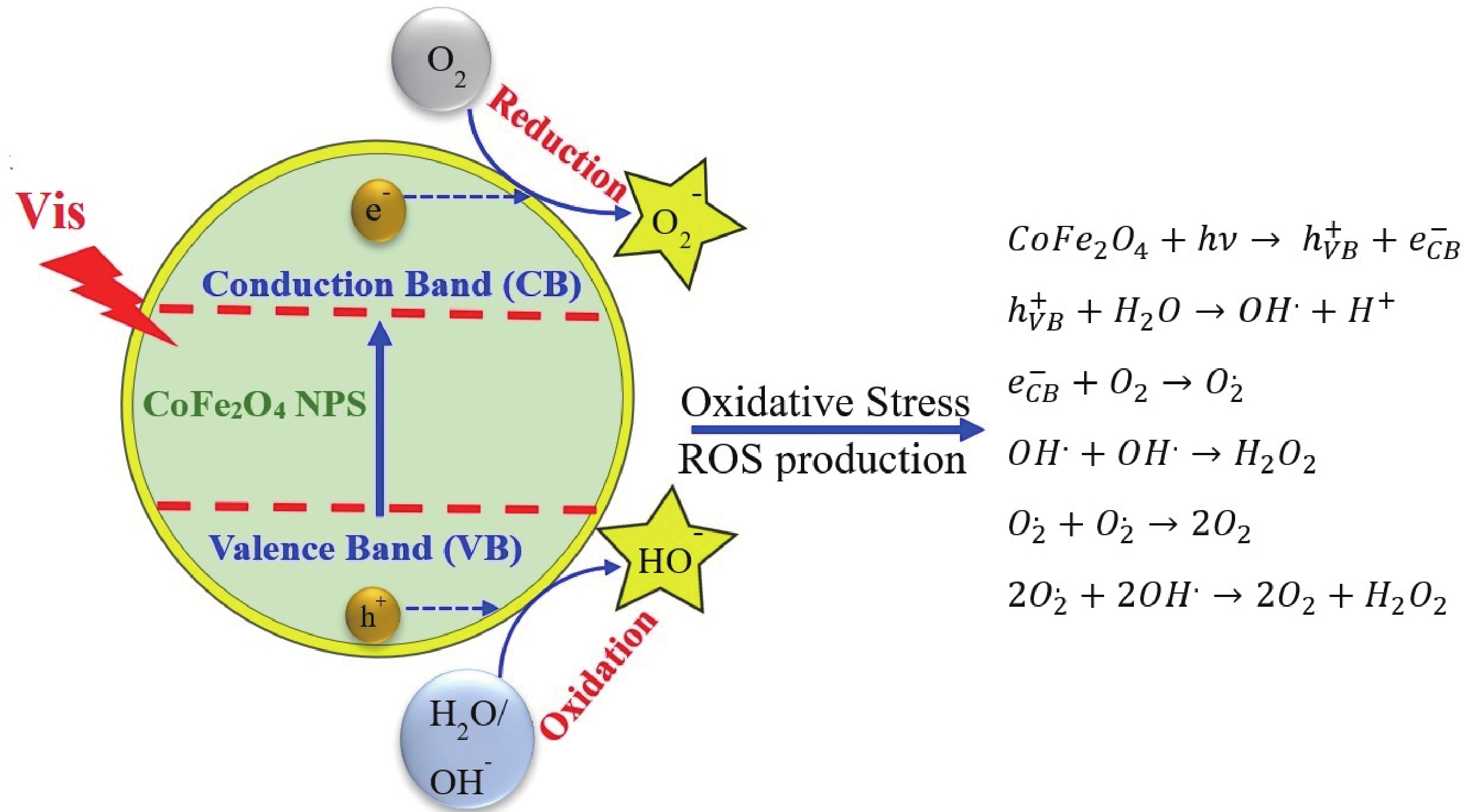
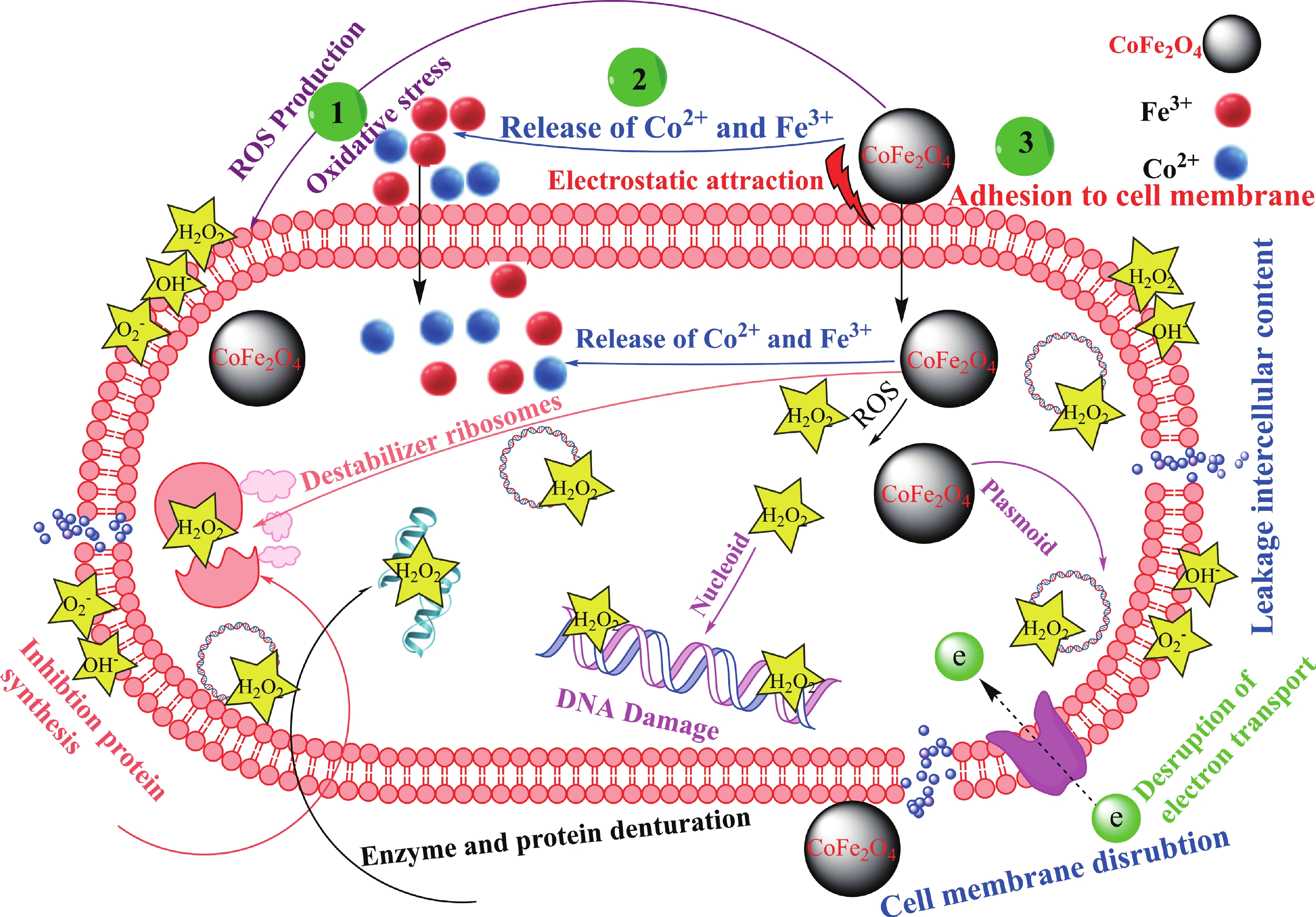
This study investigates the effect of apple extract on CoFe2O4 nanoparticles synthesized via a green self-ignition method. High resolution transmission electron microscope (HRTEM) showed nanometric particles with varied shapes, while X-ray diffraction (XRD) and Rietveld refinement confirmed a facecentered cubic (Fd3̅m) structure. Mössbauer spectroscopy revealed a Zeeman sextet pattern with only Fe3+ ions. Ultra violet vissible nearinfrared (UV–Vis–NIR) spectra indicated strong absorbance in the visible and NIR regions, suggesting optoelectronic potential. The nanoparticles demonstrated high photo-Fenton catalytic efficiency, degrading 96.88% of Methylene Blue under visible light. They also exhibited 100% adsorption capacities for Cr3+ and Pb2+, making them effective for water treatment. These properties were attributed to a large surface area (347.04 m2/g), mesoporous structure, and mixed spinel phase. ANOVA and Tukey’s honestly significant difference (HSD) tests confirmed that contact time and adsorbent dosage significantly affected pollutant removal. Additionally, strong antimicrobial activity highlighted their biotechnological relevance. The inclusion of apple extract enhanced structural and functional features, expanding application prospects in spin valves, magnetic recording, refrigeration, microwave technologies (C to Ku bands), optoelectronics, and biotechnology. Future work should explore the photo-Fenton degradation mechanism and optimize synthesis for scalable production, aiming to maximize their industrial utility. -
References
[1] Ahmad Wani T, Suresh G. Plant-mediated green synthesis of magnetic spinel ferrite nanoparticles: A sustainable trend in nanotechnology. Adv Sustain Syst, 2022, 6(6), 2200035 doi: 10.1002/adsu.202200035[2] El-Masry M M, Ramadan R, Ahmed M K. The effect of adding cobalt ferrite nanoparticles on the mechanical properties of epoxy resin. Results Mater, 2020, 8, 100160 doi: 10.1016/j.rinma.2020.100160[3] Mmelesi O K, Masunga N, Kuvarega A, et al. Cobalt ferrite nanoparticles and nanocomposites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater Sci Semicond Process, 2021, 123, 105523 doi: 10.1016/j.mssp.2020.105523[4] Tatarchuk T, Shyichuk A, Trawczyńska I, et al. Spinel cobalt(II) ferrite-chromites as catalysts for H2O2 decomposition: Synthesis, morphology, cation distribution and antistructure model of active centers formation. Ceram Int, 2020, 46(17), 27517 doi: 10.1016/j.ceramint.2020.07.243[5] Jeseentharani V, George M, Jeyaraj B, et al. Synthesis of metal ferrite (MFe2O4, M = Co, Cu, Mg, Ni, Zn) nanoparticles as humidity sensor materials. J Exp Nanosci, 2013, 8(3), 358 doi: 10.1080/17458080.2012.690893[6] Ghanbari M, Davar F, Shalan A E. Effect of rosemary extract on the microstructure, phase evolution, and magnetic behavior of cobalt ferrite nanoparticles and its application on anti-cancer drug delivery. Ceram Int, 2021, 47(7), 9409 doi: 10.1016/j.ceramint.2020.12.073[7] Karakas İ H. The effects of fuel type onto the structural, morphological, magnetic and photocatalytic properties of nanoparticles in the synthesis of cobalt ferrite nanoparticles with microwave assisted combustion method. Ceram Int, 2021, 47(4), 5597 doi: 10.1016/j.ceramint.2020.10.144[8] Ali Dheyab M, Oladzadabbasabadi N, Aziz A A, et al. Recent advances of plant-mediated metal nanoparticles: Synthesis, properties, and emerging applications for wastewater treatment. J Environ Chem Eng, 2024, 12(2), 112345 doi: 10.1016/j.jece.2024.112345[9] Osman A I, Zhang Y B, Farghali M, et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ Chem Lett, 2024, 22(2), 841 doi: 10.1007/s10311-023-01682-3[10] Zulfiqar Z, Khan R R M, Summer M, et al. Plant-mediated green synthesis of silver nanoparticles: Synthesis, characterization, biological applications, and toxicological considerations: A review. Biocatal Agric Biotechnol, 2024, 57, 103121 doi: 10.1016/j.bcab.2024.103121[11] Villagrán Z, Anaya-Esparza L M, Velázquez-Carriles C A, et al. Plant-based extracts as reducing, capping, and stabilizing agents for the green synthesis of inorganic nanoparticles. Resources, 2024, 13(6), 70 doi: 10.3390/resources13060070[12] Pirsaheb M, Gholami T, Seifi H, et al. Green synthesis of nanomaterials by using plant extracts as reducing and capping agents. Environ Sci Pollut Res, 2024, 31(17), 24768 doi: 10.1007/s11356-024-32983-x[13] Nadaf S, Jena G K, Rarokar N, et al. Biogenic and biomimetic functionalized magnetic nanosystem: Synthesis, properties, and biomedical applications. Hybrid Adv, 2023, 3, 100038 doi: 10.1016/j.hybadv.2023.100038[14] Kurian M. Green synthesis routes for spinel ferrite nanoparticles: A short review on the recent trends. J Aust Ceram Soc, 2023, 59(5), 1161 doi: 10.1007/s41779-023-00917-4[15] Saod W M, Al-Janaby M S, Gayadh E W, et al. Biogenic synthesis of iron oxide nanoparticles using Hibiscus sabdariffa extract: Potential for antibiotic development and antibacterial activity against multidrug-resistant bacteria. Curr Res Green Sustain Chem, 2024, 8, 100397 doi: 10.1016/j.crgsc.2024.100397[16] Sanni S E, Oni B A, Okoro E E, et al. Recent advances in the use of biogenic nanomaterials and photocatalysts for wastewater treatment: Challenges and future prospects. Front Nanotechnol, 2024, 6, 1469309 doi: 10.3389/fnano.2024.1469309[17] Saud A, Gupta S, Allal A, et al. Progress in the sustainable development of biobased (nano)materials for application in water treatment technologies. ACS Omega, 2024, 9(27), 29088 doi: 10.1021/acsomega.3c08883[18] Nagime P V, Singh S, Shaikh N M, et al. Biogenic fabrication of silver nanoparticles using calotropis procera flower extract with enhanced biomimetics attributes. Materials, 2023, 16(11), 4058 doi: 10.3390/ma16114058[19] Mohammed S A J, Al-Rawi B K, Al-Haddad R M S. Fe3O4@SiO2 core–shell nanoparticles: Synthesis, characterization prepared by green method for Iraqi aloe vera extract. Int J Nanosci, 2023, 22(2), 2350009 doi: 10.1142/S0219581X23500096[20] Nithya K, Sathish A, Sanganathan A, et al. Rapid sorption of chromium ions using neem extract capped green synthesized magnetic nanoparticles. Mater Today Proc, 2021, 46, 5085 doi: 10.1016/j.matpr.2020.10.427[21] El-Beltagi H S, Ragab M, Osman A, et al. Biosynthesis of zinc oxide nanoparticles via neem extract and their anticancer and antibacterial activities. PeerJ, 2024, 12, e17588 doi: 10.7717/peerj.17588[22] Aida M S, Alonizan N, Zarrad B, et al. Green synthesis of iron oxide nanoparticles using Hibiscus plant extract. J Taibah Univ Sci, 2023, 17(1), 2221827 doi: 10.1080/16583655.2023.2221827[23] Kushwaha P, Chauhan P. Facile green synthesis of CoFe2O4nanoparticles using hibiscus extract and their application in humidity sensing properties. Inorg Nano Met Chem, 2023, 53(7), 664 doi: 10.1080/24701556.2021.1992432[24] Sharma D, Kumar S, Sharma D K, et al. The role of Vitex negundo leaf extract in enhancing antioxidant properties of Ag-doped ZnFe2O4 nanoparticles for industrial water purification. J Water Process Eng, 2024, 68, 106478 doi: 10.1016/j.jwpe.2024.106478[25] Kundu D, Mukherjee N, Ranu B C. A general and green procedure for the synthesis of organochalcogenides by CuFe2O4 nanoparticle catalysed coupling of organoboronic acids and dichalcogenides in PEG-400. RSC Adv, 2013, 3(1), 117 doi: 10.1039/C2RA22415A[26] Jamal Salih S. Green synthesis and characterization of polyphenol-coated magnesium-substituted manganese ferrite nanoparticles: Antibacterial and antioxidant properties. Heliyon, 2024, 10(10), e31428 doi: 10.1016/j.heliyon.2024.e31428[27] Patle S, Meshram R S, Chaudhari D L, et al. A Comprehensive Review on synthesis, properties and applications of Cobalt Spinel Ferrites. FOUNDRY J, 2024, 27(10), 117[28] Al Mahmood A, Bin Syed S, Hasan Z, et al. Results Surf. Interfaces, 2024, 17, 100303 doi: 10.1016/j.rsurfi.2024.100303[29] Singaravelan K, Krishnamoorthi G, Jeevanantham V, et al. Antibacterial and methylene blue dye degradation activity of hibiscus rosa-sinensis synthesized CoFe2O4 nanocomposites. ChemistrySelect, 2024, 9(41), e202402697 doi: 10.1002/slct.202402697[30] Jawed A, Saxena V, Pandey L M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review. J Water Process Eng, 2020, 33, 101009 doi: 10.1016/j.jwpe.2019.101009[31] Azeez N A, Dash S S, Gummadi S N, et al. Nano-remediation of toxic heavy metal contamination: Hexavalent chromium [Cr(VI)]. Chemosphere, 2021, 266, 129204 doi: 10.1016/j.chemosphere.2020.129204[32] Balali-Mood M, Naseri K, Tahergorabi Z, et al. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol, 2021, 12, 643972 doi: 10.3389/fphar.2021.643972[33] Raj D, Maiti S K. Sources, bioaccumulation, health risks and remediation of potentially toxic metal(loid)s (As, Cd, Cr, Pb and Hg): An epitomised review. Environ Monit Assess, 2020, 192(2), 108 doi: 10.1007/s10661-019-8060-5[34] Verma A, Roy A, Bharadvaja N. Remediation of heavy metals using nanophytoremediation. Advanced Oxidation Processes for Effluent Treatment Plants. Amsterdam: Elsevier, 2021, 273 doi: 10.1016/B978-0-12-821011-6.00013-X[35] Kalra A, Gupta A. Recent advances in decolourization of dyes using iron nanoparticles: A mini review. Mater Today Proc, 2021, 36, 689 doi: 10.1016/j.matpr.2020.04.677[36] Tizaoui C, Grima N. Kinetics of the ozone oxidation of reactive orange 16 azo-dye in aqueous solution. Chem Eng J, 2011, 173(2), 463 doi: 10.1016/j.cej.2011.08.014[37] Kishor R, Purchase D, Saratale G D, et al. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J Environ Chem Eng, 2021, 9(2), 105012 doi: 10.1016/j.jece.2020.105012[38] K J P. A prospective clinical study of myocarditis in cases of paraphenylenediamine (hair dye) poisoning in Northern India. J Toxicol Environ Health Sci, 2012, 4(7), 633 doi: 10.5897/JTEHS11.031[39] Fjellsbø L M, Van Rompay A R, Hooyberghs J, et al. Screening for potential hazard effects from four nitramines on human eye and skin. Toxicol Vitro, 2013, 27(4), 1205 doi: 10.1016/j.tiv.2013.02.004[40] Peydayesh M, Suter M K, Bolisetty S, et al. Amyloid fibrils aerogel for sustainable removal of organic contaminants from water. Adv Mater, 2020, 32(12), e1907932 doi: 10.1002/adma.201907932[41] Liu Q. Pollution and treatment of dye waste-water. IOP Conf Ser: Earth Environ Sci, 2020, 514(5), 052001 doi: 10.1088/1755-1315/514/5/052001[42] Wang X N, Zhang X C, Zhang Y, et al. Nanostructured semiconductor supported iron catalysts for heterogeneous photo-Fenton oxidation: A review. J Mater Chem A, 2020, 8(31), 15513 doi: 10.1039/D0TA04541A[43] Ngo T P H, Le T K. Polyethylene glycol-assisted Sol-gel synthesis of magnetic CoFe2O4 powder as photo-Fenton catalysts in the presence of oxalic acid. J Sol Gel Sci Technol, 2018, 88(1), 211 doi: 10.1007/s10971-018-4783-y[44] Chauhan R, Dinesh G K, Alawa B, et al. A critical analysis of sono-hybrid advanced oxidation process of ferrioxalate system for degradation of recalcitrant pollutants. Chemosphere, 2021, 277, 130324 doi: 10.1016/j.chemosphere.2021.130324[45] Guo X J, Wang D G. Photo-Fenton degradation of methylene blue by synergistic action of oxalic acid and hydrogen peroxide with NiFe2O4 hollow nanospheres catalyst. J Environ Chem Eng, 2019, 7(1), 102814 doi: 10.1016/j.jece.2018.102814[46] Stefan M I. Advanced oxidation processes for water treatment-fundamentals and applications. Water Intell Online, 2017, 16, 9781780407197 doi: 10.2166/9781780407197[47] Barros W R P, Steter J R, Lanza M R V, et al. Catalytic activity of Fe3–CuO4 (0 ≤x≤ 0.25) nanoparticles for the degradation of Amaranth food dye by heterogeneous electro-Fenton process. Appl Catal B Environ, 2016, 180, 434 doi: 10.1016/j.apcatb.2015.06.048[48] Xie J, Meng W N, Wu D Y, et al. Removal of organic pollutants by surfactant modified zeolite: Comparison between ionizable phenolic compounds and non-ionizable organic compounds. J Hazard Mater, 2012, 231, 57 doi: 10.1016/j.jhazmat.2012.06.035[49] Ai L H, Jiang J. Catalytic reduction of 4-nitrophenol by silver nanoparticles stabilized on environmentally benign macroscopic biopolymer hydrogel. Bioresour Technol, 2013, 132, 374 doi: 10.1016/j.biortech.2012.10.161[50] Chang Y C, Chen D H. Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J Hazard Mater, 2009, 165(1/2/3), 664 doi: 10.1016/j.jhazmat.2008.10.034[51] Koga H, Kitaoka T. One-step synthesis of gold nanocatalysts on a microstructured paper matrix for the reduction of 4-nitrophenol. Chem Eng J, 2011, 168(1), 420 doi: 10.1016/j.cej.2010.08.073[52] Pradhan N, Pal A, Pal T. Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf A Physicochem Eng Aspects, 2002, 196(2/3), 247 doi: 10.1016/S0927-7757(01)01040-8[53] Zhang W, Tan F T, Wang W, et al. Facile, template-free synthesis of silver nanodendrites with high catalytic activity for the reduction of p-nitrophenol. J Hazard Mater, 2012, 217, 36 doi: 10.1016/j.jhazmat.2012.01.056[54] Feng J, Su L, Ma Y H, et al. CuFe2O4 magnetic nanoparticles: A simple and efficient catalyst for the reduction of nitrophenol. Chem Eng J, 2013, 221, 16 doi: 10.1016/j.cej.2013.02.009[55] Gupta V K, Atar N, Yola M L, et al. A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res, 2014, 48, 210 doi: 10.1016/j.watres.2013.09.027[56] Yang P, Xu A D, Xia J, et al. Facile synthesis of highly catalytic activity Ni–Co–Pd–P composite for reduction of the p-Nitrophenol. Appl Catal A Gen, 2014, 470, 89 doi: 10.1016/j.apcata.2013.10.043[57] Arade S, Agale P, Balgude S, et al. Zn0.5Ni0.5MnxFe2-xO4 magnetically separable nano ferrite: A highly efficient photocatalyst for environmental remediation. Inorg Chem Commun, 2024, 170, 113170 doi: 10.1016/j.inoche.2024.113170[58] Jangam K, Balgude S, Pawar H, et al. Effect of cobalt substitution in Zn1-xCoxFeCrO4 ferri-chromate: Emerging light absorber for degradation of model textile dye. Surf Interfaces, 2022, 33, 102189 doi: 10.1016/j.surfin.2022.102189[59] Borhan A I, Samoila P, Hulea V, et al. Effect of Al3+ substituted zinc ferrite on photocatalytic degradation of Orange I azo dye. J Photochem Photobiol A Chem, 2014, 279, 17 doi: 10.1016/j.jphotochem.2014.01.010[60] Rahimi-Nasrabadi M, Behpour M, Sobhani-Nasab A, et al. Nanocrystalline Ce-doped copper ferrite: Synthesis, characterization, and its photocatalyst application. J Mater Sci Mater Electron, 2016, 27(11), 11691 doi: 10.1007/s10854-016-5305-8[61] Sharma R, Singhal S. Photodegradation of textile dye using magnetically recyclable heterogeneous spinel ferrites. J Chemical Tech & Biotech, 2015, 90(5), 955 doi: 10.1002/jctb.4409[62] Jesudoss S K, Vijaya J J, Kennedy L J, et al. Studies on the efficient dual performance of Mn1–x Nix Fe2O4 spinel nanoparticles in photodegradation and antibacterial activity. J Photochem Photobiol B Biol, 2016, 165, 121 doi: 10.1016/j.jphotobiol.2016.10.004[63] Li Z L, Ai J Z, Ge M. A facile approach assembled magnetic CoFe2O4/AgBr composite for dye degradation under visible light. J Environ Chem Eng, 2017, 5(2), 1394 doi: 10.1016/j.jece.2017.02.024[64] Revathi J, Abel M J, Archana V, et al. Synthesis and characterization of CoFe2O4 and Ni-doped CoFe2O4 nanoparticles by chemical co-precipitation technique for photo-degradation of organic dyestuffs under direct sunlight. Phys B Condens Matter, 2020, 587, 412136 doi: 10.1016/j.physb.2020.412136[65] Sundararajan M, Sailaja V, John Kennedy L, et al. Photocatalytic degradation of rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: Kinetics and mechanism. Ceram Int, 2017, 43(1), 540 doi: 10.1016/j.ceramint.2016.09.191[66] Kalam A, Al-Sehemi A G, Assiri M, et al. Modified solvothermal synthesis of cobalt ferrite (CoFe2O4) magnetic nanoparticles photocatalysts for degradation of methylene blue with H2O2/visible light. Results Phys, 2018, 8, 1046 doi: 10.1016/j.rinp.2018.01.045[67] Gan L, Shang S M, Yuen C W M, et al. Hydrothermal synthesis of magnetic CoFe2O4/graphene nanocomposites with improved photocatalytic activity. Appl Surf Sci, 2015, 351, 140 doi: 10.1016/j.apsusc.2015.05.130[68] Hussein H, Ibrahim S S, Khairy S A. Biosynthesis of CoFe2O4 ferrite nanoparticles using Greek yogurt solution: Deep structural insights and appraisal for ecological mitigation via quartz crystal microbalance. J Water Process Eng, 2024, 65, 105856 doi: 10.1016/j.jwpe.2024.105856[69] Maslov Bandić L, Žulj M M, Fruk G, et al. The profile of organic acids and polyphenols in apple wines fermented with different yeast strains. J Food Sci Technol, 2019, 56(2), 599 doi: 10.1007/s13197-018-3514-2[70] Espíndola K M M, Ferreira R G, Narvaez L E M, et al. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol, 2019, 9, 541 doi: 10.3389/fonc.2019.00541[71] Hussein H, Ibrahim S S, Khairy S A. Impact of carrot extract and pomegranate juice on the structure, magnetic properties and Cr3+ ion removal efficiency of eco-friendly CoFe2O4 nanoparticles prepared via self-ignition technique. Desalination, 2025, 597, 118324 doi: 10.1016/j.desal.2024.118324[72] Lai W F, Tang R, Wong W T. Ionically crosslinked complex gels loaded with oleic acid-containing vesicles for transdermal drug delivery. Pharmaceutics, 2020, 12(8), 725 doi: 10.3390/pharmaceutics12080725[73] Lai W F, Gui D Y, Wong M, et al. A self-indicating cellulose-based gel with tunable performance for bioactive agent delivery. J Drug Deliv Sci Technol, 2021, 63, 102428 doi: 10.1016/j.jddst.2021.102428[74] Stein C R, Bezerra M T S, Holanda G H A, et al. Structural and magnetic properties of cobalt ferrite nanoparticles synthesized by co-precipitation at increasing temperatures. AIP Adv, 2018, 8(5), 056303 doi: 10.1063/1.5006321[75] Ballirano P. Effects of the choice of different ionization level for scattering curves and correction for small preferred orientation in Rietveld refinement: The MgAl2O4 test case. J Appl Crystallogr, 2003, 36(4), 1056 doi: 10.1107/S0021889803010410[76] Khedri H, Gholizadeh A. Experimental comparison of structural, magnetic and elastic properties of M0.3Cu0.2Zn0.5Fe2O4 (M = Cu, Mn, Fe, Co, Ni, Mg) nanoparticles. Appl Phys A, 2019, 125(10), 709 doi: 10.1007/s00339-019-3010-1[77] Dhanda N, Thakur P, Thakur A. Green synthesis of cobalt ferrite: A study of structural and optical properties. Mater Today Proc, 2023, 73, 237 doi: 10.1016/j.matpr.2022.07.202[78] Cullity B D. Elements of X-ray Diffraction. Addison-Wesley Publishing, 1956[79] Vara Prasad B B V S, Ramesh K V, Srinivas A. Structural and magnetic studies of nano-crystalline ferrites MFe2O4 (M = Zn, Ni, Cu, and co) synthesized via citrate gel autocombustion method. J Supercond Nov Magn, 2017, 30(12), 3523 doi: 10.1007/s10948-017-4153-y[80] Prasad B B V S V, Ramesh K V, Srinivas A. Structural and magnetic studies on Co-Zn nanoferrite synthesized via Sol-gel and combustion methods. Mater Sci Pol, 2019, 37(1), 39 doi: 10.2478/msp-2019-0013[81] Lakhani V K, Pathak T K, Vasoya N H, et al. Structural parameters and X-ray Debye temperature determination study on copper-ferrite-aluminates. Solid State Sci, 2011, 13(3), 539 doi: 10.1016/j.solidstatesciences.2010.12.023[82] Arean C O, Diaz E G, Rubio Gonzalez J M, et al. Crystal chemistry of cadmium-zinc ferrites. J Solid State Chem, 1988, 77(2), 275 doi: 10.1016/0022-4596(88)90249-6[83] Dhiman R L, Taneja S P, Reddy V R. Preparation and characterization of manganese ferrite aluminates. Adv Condens Matter Phys, 2008, 2008, 703479 doi: 10.1155/2008/703479[84] Kumar R, Rawat D, Barman P B, et al. Experimental and theoretical verification of cation distribution and spin canting effect via structural and magnetic studies of NiZnCo ferrite nanoparticles. J Aust Ceram Soc, 2022, 58(1), 101 doi: 10.1007/s41779-021-00671-5[85] Kuntscher C A, Frank S, Loa I, et al. Infrared properties of the quasi-one-dimensional superconductor β–Na0.33V2O5 under pressure. Phys Rev B, 2005, 71(22), 220502 doi: 10.1103/PhysRevB.71.220502[86] Mazen S A, Abdallah M H, Sabrah B A, et al. The effect of titanium on some physical properties of CuFe2O4. Phys Stat Sol (a), 1992, 134(1), 263 doi: 10.1002/pssa.2211340123[87] Ghosh M P, Datta S, Sharma R, et al. Copper doped nickel ferrite nanoparticles: Jahn-Teller distortion and its effect on microstructural, magnetic and electronic properties. Mater Sci Eng B, 2021, 263, 114864 doi: 10.1016/j.mseb.2020.114864[88] Modi K B, Gajera J D, Chhantbar M C, et al. Structural properties of magnesium and aluminium co-substituted lithium ferrite. Mater Lett, 2003, 57(24/25), 4049 doi: 10.1016/S0167-577X(03)00263-5[89] Jain R. A review on the development of XRD in ferrite nanoparticles. J Supercond Nov Magn, 2022, 35(5), 1033 doi: 10.1007/s10948-022-06213-9[90] Abu El-Fadl A, Hassan A M, Mahmoud M H, et al. Synthesis and magnetic properties of spinel Zn1–xNixFe2O4 (0.0 ≤ x ≤ 1.0) nanoparticles synthesized by microwave combustion method. J Magn Magn Mater, 2019, 471, 192 doi: 10.1016/j.jmmm.2018.09.074[91] Elius I B, Hossain S, Aktar M S, et al. Structural and magnetic characterizations of NixCu0.8-x Zn0.2Fe2O4 spinel ferrites. Ferroelectrics, 2021, 572(1), 36 doi: 10.1080/00150193.2020.1868871[92] Islam M A, Hossain A K M A, Ahsan M Z, et al. Structural characteristics, cation distribution, and elastic properties of Cr3+ substituted stoichiometric and non-stoichiometric cobalt ferrites. RSC Adv, 2022, 12(14), 8502 doi: 10.1039/D1RA09090A[93] Gorter E, Gorter W. Saturation magnetization and crystal chemistry of ferrimagnetic oxides. I. II. Theory of ferrimagnetism. Philips Res Rep, 1954, 9, 295[94] Barrett C, Massalski. TB structure of metals. Pergamon Press: Oxford, 1980, 1[95] Caglar M , Caglar Y, Ilican S. The determination of the thickness and optical constants of the ZnO crystalline thin film by using envelope method. J Optoelectron AdvMater, 2006, 8(4), 1410. doi: https://old.joam.inoe.ro/arhiva/pdf8_4/4Caglar.pdf[96] Varchola M, Saksl K, Durišin J, et al. Structural analysis of dispersion strengthened Al-Al4C3 material by XRD method. High Temp Mater Process, 2011, 30, 127 doi: 10.1515/HTMP.2011.018[97] Mahmoud M H, Elshahawy A M, Makhlouf S A, et al. Synthesis of highly ordered 30nm NiFe2O4 particles by the microwave-combustion method. J Magn Magn Mater, 2014, 369, 55 doi: 10.1016/j.jmmm.2014.06.011[98] Paswan S K, Kumari S, Kar M, et al. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J Phys Chem Solids, 2021, 151, 109928 doi: 10.1016/j.jpcs.2020.109928[99] Milam-Guerrero J, Neer A J, Melot B C. Crystal chemistry and competing magnetic exchange interactions in oxide garnets and spinels. J Solid State Chem, 2019, 274, 1 doi: 10.1016/j.jssc.2019.02.007[100] Ibrahim A H, Abbas Y M, Mohammed S E, et al. Investigation of structural and magnetic properties of multiferroic La1-XYxFeO3 perovskites, prepared by citrate auto-combustion technique. J Adv Phys, 2019, 15, 6056 doi: 10.24297/jap.v15i0.8056[101] Singh O, Agarwal A, Sanghi S, et al. Variation of crystal structure, magnetization, and dielectric properties of Nd and Ba Co-doped BiFeO3multiferroics. Int J Appl Ceram Technol, 2019, 16(1), 119 doi: 10.1111/ijac.13052[102] Waldron R D. Infrared spectra of ferrites. Phys Rev, 1955, 99(6), 1727 doi: 10.1103/PhysRev.99.1727[103] Dixit G, Singh J P, Srivastava R C, et al. Structural, optical and magnetic studies of Ce doped NiFe2O4 nanoparticles. J Magn Magn Mater, 2013, 345, 65 doi: 10.1016/j.jmmm.2013.05.060[104] Bouokkeze D, Massoudi J, Hzez W, et al. Investigation of the structural, optical, elastic and electrical properties of spinel LiZn2Fe3O8 nanoparticles annealed at two distinct temperatures. RSC Adv, 2019, 9(70), 40940 doi: 10.1039/C9RA07569K[105] Chandekar K V, Kant K M. Size-strain analysis and elastic properties of CoFe2O4 nanoplatelets by hydrothermal method. J Mol Struct, 2018, 1154, 418 doi: 10.1016/j.molstruc.2017.09.104[106] Modi K B, Shah S J, Pujara N B, et al. Infrared spectral evolution, elastic, optical and thermodynamic properties study on mechanically milled Ni0.5Zn0.5Fe2O4 spinel ferrite. J Mol Struct, 2013, 1049, 250 doi: 10.1016/j.molstruc.2013.06.051[107] Bhatu S S, Lakhani V, Tanna A, et al. Effect of nickel substitution on structural, infrared and elastic properties of lithium ferrite. Indian J Pure Appl Phys, 2007, 45, 596[108] Bouhadouza N, Rais A, Kaoua S, et al. Structural and vibrational studies of NiAlxFe2–xO4 ferrites (0≤x≤1). Ceram Int, 2015, 41(9), 11687 doi: 10.1016/j.ceramint.2015.05.132[109] Modi K B, Raval P Y, Shah S J, et al. Raman and Mossbauer spectroscopy and X-ray diffractometry studies on quenched copper–ferri–aluminates. Inorg Chem, 2015, 54(4), 1543 doi: 10.1021/ic502497a[110] Mohammed K A, Al-Rawas A D, Gismelseed A M, et al. Infrared and structural studies of Mg1–xZnxFe2O4 ferrites. Phys B Condens Matter, 2012, 407(4), 795 doi: 10.1016/j.physb.2011.12.097[111] Singh Yadav R, Havlica J, Masilko J, et al. Effects of annealing temperature variation on the evolution of structural and magnetic properties of NiFe2O4 nanoparticles synthesized by starch-assisted Sol–gel auto-combustion method. J Magn Magn Mater, 2015, 394, 439 doi: 10.1016/j.jmmm.2015.07.012[112] Patange S M, Shirsath S E, Jadhav S P, et al. Elastic properties of nanocrystalline aluminum substituted nickel ferrites prepared by co-precipitation method. J Mol Struct, 2013, 1038, 40 doi: 10.1016/j.molstruc.2012.12.053[113] Modi K B, Chhantbar M C, Joshi H H. Study of elastic behaviour of magnesium ferri aluminates. Ceram Int, 2006, 32(2), 111 doi: 10.1016/j.ceramint.2005.01.005[114] Modi K B, Rangolia M K, Chhantbar M C, et al. Study of infrared spectroscopy and elastic properties of fine and coarse grained nickel–cadmium ferrites. J Mater Sci, 2006, 41(22), 7308 doi: 10.1007/s10853-006-0929-3[115] Hasselman D, Fulrath R. Effect of small fraction of spherical porosity on elastic moduli of glass. J Am Ceram Soc, 1964, 47(1), 52[116] Sharma P U, Modi K B. Effect of Fe3+ substitution on elastic properties of yttrium iron garnet. Phys Scr, 2010, 81(1), 015601 doi: 10.1088/0031-8949/81/01/015601[117] Rice R W. Microstructure dependence of mechanical behavior of ceramics. Properties and Microstructure. Amsterdam: Elsevier, 1977, 199[118] Algude S G, Patange S M, Shirsath S E, et al. Elastic behaviour of Cr3+ substituted Co–Zn ferrites. J Magn Magn Mater, 2014, 350, 39 doi: 10.1016/j.jmmm.2013.09.021[119] Kudriavtsev B B. Ultrasonic studies in aqueous solution of sodium and potassium halides, alkali nitrates and sulphates of cadmium and magnesium. Soviet Phys Acoust, 1956, 2, 172[120] De Podesta M. Understanding the properties of matter. Boca Raton: CRC Press, 2020[121] Pradhan D K, Misra P, Puli V S, et al. Studies on structural, dielectric, and transport properties of Ni0.65Zn0.35Fe2O4. J Appl Phys, 2014, 115(24), 243904 doi: 10.1063/1.4885420[122] Singh J P, Srivastava R C, Agrawal H M, et al. Micro-Raman investigation of nanosized zinc ferrite: Effect of crystallite size and fluence of irradiation. J Raman Spectrosc, 2011, 42(7), 1510 doi: 10.1002/jrs.2902[123] Jacintho G V M, Brolo A G, Corio P, et al. Structural investigation of MFe2O4 (M = Fe, Co) magnetic fluids. J Phys Chem C, 2009, 113(18), 7684 doi: 10.1021/jp9013477[124] Ansari S M, Ghosh K C, Devan R S, et al. Eco-friendly synthesis, crystal chemistry, and magnetic properties of manganese-substituted CoFe2O4 nanoparticles. ACS Omega, 2020, 5(31), 19315 doi: 10.1021/acsomega.9b02492[125] Mai Oanh L, Do D B, Phu N D, et al. Influence of Mn doping on the structure, optical, and magnetic properties of PbTiO3 material. IEEE Trans Magn, 2014, 50(6), 2502004 doi: 10.1109/TMAG.2013.2297516[126] Chandramohan P, Srinivasan M P, Velmurugan S, et al. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J Solid State Chem, 2011, 184(1), 89 doi: 10.1016/j.jssc.2010.10.019[127] Lazarević Z Ž, Jovalekić Č, Recnik A, et al. Study of manganese ferrite powders prepared by a soft mechanochemical route. J Alloys Compd, 2011, 509(41), 9977 doi: 10.1016/j.jallcom.2011.08.004[128] Ansari S M, Sinha B B, Phase D, et al. Particle size, morphology, and chemical composition controlled CoFe2O4 nanoparticles with tunable magnetic properties via oleic acid based solvothermal synthesis for application in electronic devices. ACS Appl Nano Mater, 2019, 2(4), 1828 doi: 10.1021/acsanm.8b02009[129] Kumar Y, Sharma A, Shirage P M. Impact of different morphologies of CoFe2O4 nanoparticles for tuning of structural, optical and magnetic properties. J Alloys Compd, 2019, 778, 398 doi: 10.1016/j.jallcom.2018.11.128[130] Majumdar M G. Analysis of stress-coupled magneto-electric effect in BaTiO3-CoFe2O4 composites using Raman spectroscopy. Int J Sci Eng Res, 2012, 3(11), 1[131] Ramasamy T, Satheesh L G, Selvaraj V, et al. Spinel CoFe2O4 nanoflakes: A path to enhance energy generation and environmental remediation potential of waste-derived rGO. Nanomaterials, 2022, 12(21), 3822 doi: 10.3390/nano12213822[132] Sagu J S, Wijayantha K G U, Tahir A A. The pseudocapacitive nature of CoFe2O4 thin films. Electrochim Acta, 2017, 246, 870 doi: 10.1016/j.electacta.2017.06.110[133] Yadav R S, Kuřitka I, Vilcakova J, et al. Impact of grain size and structural changes on magnetic, dielectric, electrical, impedance and modulus spectroscopic characteristics of CoFe2O4 nanoparticles synthesized by honey mediated Sol-gel combustion method. Adv Nat Sci: Nanosci Nanotechnol, 2017, 8(4), 045002 doi: 10.1088/2043-6254/aa853a[134] Abbas M, Parvatheeswara Rao B, Nazrul Islam M, et al. Size-controlled high magnetization CoFe2O4 nanospheres and nanocubes using rapid one-pot sonochemical technique. Ceram Int, 2014, 40(2), 3269 doi: 10.1016/j.ceramint.2013.09.109[135] de Medeiros F, Madigou V, Lopes-Moriyama A L, et al. Synthesis of CoFe2O4 nanocubes. Nano Struct Nano Objects, 2020, 21, 100422 doi: 10.1016/j.nanoso.2019.100422[136] Cianciara A J, Coleman Z, Gates S J Jr, et al. N= 2 SUSY and the hexipentisteriruncicantitruncated 7-simplex. arXiv, 2023, 2304.09830[137] Lopes-Moriyama A L, Madigou V, de Souza C P, et al. Controlled synthesis of CoFe2O4 nano-octahedra. Powder Technol, 2014, 256, 482 doi: 10.1016/j.powtec.2014.01.080[138] Gabal M A, El-Shishtawy R M, Al Angari Y M. Structural and magnetic properties of nano-crystalline Ni–Zn ferrites synthesized using egg-white precursor. J Magn Magn Mater, 2012, 324(14), 2258 doi: 10.1016/j.jmmm.2012.02.112[139] Jaffari G H, Ceylan A, Bui H P, et al. Non-equilibrium cation distribution and enhanced spin disorder in hollow CoFe2O4 nanoparticles. J Phys: Condens Matter, 2012, 24(33), 336004 doi: 10.1088/0953-8984/24/33/336004[140] Allen G C, Hallam K R. Characterisation of the spinels MxCo1−x Fe2O4 (M = Mn, Fe or Ni) using X-ray photoelectron spectroscopy. Appl Surf Sci, 1996, 93(1), 25 doi: 10.1016/0169-4332(95)00186-7[141] Kim J G, Pugmire D L, Battaglia D, et al. Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl Surf Sci, 2000, 165(1), 70 doi: 10.1016/S0169-4332(00)00378-0[142] Yin L, Adler I, Tsang T, et al. Paramagnetism and shake-up satellites in X-ray photoelectron spectra. Chem Phys Lett, 1974, 24(1), 81 doi: 10.1016/0009-2614(74)80219-8[143] Anantharamaiah P N, Joy P A. Enhancing the strain sensitivity of CoFe2O4 at low magnetic fields without affecting the magnetostriction coefficient by substitution of small amounts of Mg for Fe. Phys Chem Chem Phys, 2016, 18(15), 10516 doi: 10.1039/C6CP00369A[144] Bonnelle J P, Grimblot J, D’Huysser A. Influence de la polarisation des liaisons sur les spectres esca des oxydes de cobalt. J Electron Spectrosc Relat Phenom, 1975, 7(2), 151 doi: 10.1016/0368-2048(75)80047-8[145] Barik A, Sahoo M R, Tiwary S, et al. Investigation of cation distributions and temperature-dependent magnetic properties of polycrystalline CoFe2O4. Dae Solid State Physics Symposium 2019, 2020 doi: 10.1063/5.0017171[146] Ahamad T, Naushad M, Ubaidullah M, et al. Fabrication of highly porous polymeric nanocomposite for the removal of radioactive U(VI) and Eu(III) ions from aqueous solution. Polymers, 2020, 12(12), 2940 doi: 10.3390/polym12122940[147] Mickevičius S, Grebinskij S, Bondarenka V, et al. Investigation of epitaxial LaNiO3−x thin films by high-energy XPS. J Alloys Compd, 2006, 423(1/2), 107 doi: 10.1016/j.jallcom.2005.12.038[148] Zhou Z P, Zhang Y, Wang Z Y, et al. Electronic structure studies of the spinel CoFe2O4 by X-ray photoelectron spectroscopy. Appl Surf Sci, 2008, 254(21), 6972 doi: 10.1016/j.apsusc.2008.05.067[149] More P, Kadam S, Lokhande P, et al. Effect of sintering temperature on the structural, morphological, and the magnetic properties of Ni0.25Cu0.55 Zn0.20 Fe2O4 nano ferrite. J Magn Magn Mater, 2023, 586, 171192 doi: 10.1016/j.jmmm.2023.171192[150] Cullity B D, Graham C D. Introduction to Magnetic Materials. John Wiley & Sons, 2011[151] Hochepied J F, Pileni M P. Magnetic properties of mixed cobalt–zinc ferrite nanoparticles. J Appl Phys, 2000, 87(5), 2472 doi: 10.1063/1.372205[152] Rani R, Sharma S K, Pirota K R, et al. Effect of zinc concentration on the magnetic properties of cobalt–zinc nanoferrite. Ceram Int, 2012, 38(3), 2389 doi: 10.1016/j.ceramint.2011.11.004[153] Sharifi I, Shokrollahi H. Nanostructural, magnetic and Mössbauer studies of nanosized Co1−xZnxFe2O4 synthesized by co-precipitation. J Magn Magn Mater, 2012, 324(15), 2397 doi: 10.1016/j.jmmm.2012.03.008[154] Humbe A V, Kounsalye J S, Somvanshi S B, et al. Cation distribution, magnetic and hyperfine interaction studies of Ni–Zn spinel ferrites: Role of Jahn Teller ion (Cu2+) substitution. Mater Adv, 2020, 1(4), 880 doi: 10.1039/D0MA00251H[155] Ajmal M, Maqsood A. Structural, electrical and magnetic properties of Cu1–xZnxFe2O4 ferrites (0≤x≤1). J Alloys Compd, 2008, 460(1/2), 54 doi: 10.1016/j.jallcom.2007.06.019[156] Patil K, Jangam K, Patange S, et al. Influence of Cu–Mg substituted ZnFe2O4 ferrite as a highly efficient nanocatalyst for dye degradation and 4-nitrophenol reduction. J Phys Chem Solids, 2022, 167, 110783 doi: 10.1016/j.jpcs.2022.110783[157] Ahmed M A, Mansour S F, Ismael H. A comparative study on the magnetic and electrical properties of MFe12O19 (M=Ba and Sr)/BiFeO3 nanocomposites. J Magn Magn Mater, 2015, 378, 376 doi: 10.1016/j.jmmm.2014.10.173[158] Ahmed M A, Okasha N, Hussein B. Enhancement of the magnetic properties of Al/La multiferroic. J Magn Magn Mater, 2012, 324(15), 2349 doi: 10.1016/j.jmmm.2012.02.036[159] Wang Z L, Liu Y, Zhang Z. Handbook of nanophase and nanostructured materials. Kluwer Academic/Plenum, 2003[160] Maher Wahba A, Bakr Mohamed M. Structural, magnetic, and dielectric properties of nanocrystalline Cr-substituted Co0.8Ni0.2Fe2O4 ferrite. Ceram Int, 2014, 40(4), 6127 doi: 10.1016/j.ceramint.2013.11.064[161] Ramadan R, El-Masry M M. Effect of (Co and Zn) doping on structural, characterization and the heavy metal removal efficiency of CuFe2O4 nanoparticles. J Aust Ceram Soc, 2024, 60(2), 509 doi: 10.1007/s41779-023-00932-5[162] Singh R P, Tomy C V, Grover A K. Observation of tunable exchange bias in Sr2YbRuO6. Appl Phys Lett, 2010, 97(18), 182505 doi: 10.1063/1.3505525[163] Kamel R, Reffat A. On the effect of lattice disorders on the reversible magnetization process in pure nickel. Solid State Commun, 1970, 8(11), 821 doi: 10.1016/0038-1098(70)90266-8[164] Nogués J, Schuller I K. Exchange bias. J Magn Magn Mater, 1999, 192(2), 203 doi: 10.1016/S0304-8853(98)00266-2[165] Park B G, Wunderlich J, Martí X, et al. A spin-valve-like magnetoresistance of an antiferromagnet-based tunnel junction. Nature Mater, 2011, 10(5), 347 doi: 10.1038/nmat2983[166] Sahoo R C, Giri S K, Dasgupta P, et al. Exchange bias effect in ferromagnetic LaSrCoMnO6 double perovskite: Consequence of spin glass-like ordering at low temperature. J Alloys Compd, 2016, 658, 1003 doi: 10.1016/j.jallcom.2015.11.025[167] Kuo M F, Hung Y H, Hung C C, et al. Structure and magnetic properties of Mn and Al doped magnesium ferrite. China Steel Technical Report, 2016, 29, 44[168] Shafaay A S, Ramadan R. The influence of Zn doping on the cation distribution and antibacterial activity of CoFe2O4. J Supercond Nov Magn, 2023, 36(5), 1465 doi: 10.1007/s10948-023-06589-2[169] Jones N, Ray B, Ranjit K T, et al. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett, 2008, 279(1), 71 doi: 10.1111/j.1574-6968.2007.01012.x[170] Kumar L, Kumar P, Srivastava S K, et al. Low temperature and high magnetic field dependence and magnetic properties of nanocrystalline cobalt ferrite. J Supercond Nov Magn, 2014, 27(7), 1677 doi: 10.1007/s10948-014-2519-y[171] Maaz K, Mumtaz A, Hasanain S K, et al. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J Magn Magn Mater, 2007, 308(2), 289 doi: 10.1016/j.jmmm.2006.06.003[172] Ferenc J, Kowalczyk M, Wróblewski R, et al. Entropy change calculations for pure Gd and a Ni-Mn-Cu-Ga heusler alloy: Constant field vs. constant temperature experiment. Acta Phys Pol A, 2015, 128(1), 111 doi: 10.12693/APhysPolA.128.111[173] Pubby K, Meena S S, Yusuf S M, et al. Cobalt substituted nickel ferrites via Pechini’s Sol–gel citrate route: X-band electromagnetic characterization. J Magn Magn Mater, 2018, 466, 430 doi: 10.1016/j.jmmm.2018.07.038[174] Tatarchuk T, Danyliuk N, Shyichuk A, et al. Green synthesis of cobalt ferrite using grape extract: The impact of cation distribution and inversion degree on the catalytic activity in the decomposition of hydrogen peroxide. Emergent Mater, 2022, 5(1), 89 doi: 10.1007/s42247-021-00323-1[175] Banerjee A M, Pai M R, Meena S S, et al. Catalytic activities of cobalt, nickel and copper ferrospinels for sulfuric acid decomposition: The high temperature step in the sulfur based thermochemical water splitting cycles. Int J Hydrog Energy, 2011, 36(8), 4768 doi: 10.1016/j.ijhydene.2011.01.073[176] Ok H N, Evans B J. Collective electron description of the conduction mechanism in MxFe3–xO4, M=Cd, Zn. Phys Rev B, 1976, 14(7), 2956 doi: 10.1103/PhysRevB.14.2956[177] Pujari V C, Mhase P D, Patange S M, et al. Influence of Dy3+ doping on Mössbauer, magnetic and microwave absorption properties of M-type Ba0.5Ca0.5DyxFe12-xO19 hexaferrites. J Magn Magn Mater, 2024, 610, 172555 doi: 10.1016/j.jmmm.2024.172555[178] Halasa N A, DePasquali G, Drickamer H G. High-pressure studies on ferrites. Phys Rev B, 1974, 10(1), 154 doi: 10.1103/PhysRevB.10.154[179] Nikolaev V I, Rusakov V S, Chistyakova N I. Covalency effects and the pressure dependence of the mössbauer line shifts for spinel-type ferrites. Phys Stat Sol (a), 1985, 91(2), K139 doi: 10.1002/pssa.2210910257[180] Cross W B, Affleck L, Kuznetsov M V, et al. Self-propagating high-temperature synthesis of ferrites MFe2O4 (M = Mg, Ba, Co, Ni, Cu, Zn); reactions in an external magnetic field. J Mater Chem, 1999, 9(10), 2545 doi: 10.1039/A904431K[181] Nguyen T K C, Nguyen A T. Structural, optical and magnetic properties of Y-doped CoFe2O4 nanoparticles prepared by a simple co-precipitation method. J Mater Sci Mater Electron, 2023, 34(5), 448 doi: 10.1007/s10854-023-09914-6[182] Rai R C, Wilser S, Guminiak M, et al. Optical and electronic properties of NiFe2O4 and CoFe2O4 thin films. Appl Phys A, 2012, 106(1), 207 doi: 10.1007/s00339-011-6549-z[183] Zhou B, Zhang Y W, Yu Y J, et al . Correlation between structure and intervalence charge-transfer transitions in nanocrystalline CoFe2−xMxO4 (M= M n, A l, S c) thin films. Physical Review B, 2003, 68(2), 024426 doi: 10.1103/PhysRevB.68.024426[184] Rejaiba O, Khirouni K, Dhaou M H, et al. Investigation study of optical and dielectric parameters using absorption and diffuse reflectance spectroscopy method on La0.57Nd0.1Sr0.13Ag0.2MnO3 perovskite for optoelectronic application. Opt Quantum Electron, 2022, 54(5), 315 doi: 10.1007/s11082-022-03633-8[185] Raddaoui G, Rejaiba O, Nasri M, et al. Investigation studies of structural, electrical, dielectric, and optical of DyTi0.5 Mn0.5O3 multiferroic for optoelectronics applications. J Mater Sci Mater Electron, 2022, 33(27), 21890 doi: 10.1007/s10854-022-08976-2[186] Lal G, Punia K, Bhoi H, et al. Exploring the structural, elastic, optical, dielectric and magnetic characteristics of Ca2+ incorporated superparamagnetic Zn0.5−xCa0.1Co0.4+xFe2O4 (x = 0.0, 0.05 & 0.1) nanoferrites. J Alloys Compd, 2021, 886, 161190 doi: 10.1016/j.jallcom.2021.161190[187] Lal G, Punia K, Dolia S N, et al. Rietveld refinement, Raman, optical, dielectric, Mössbauer and magnetic characterization of superparamagnetic fcc-CaFe2O4 nanoparticles. Ceram Int, 2019, 45(5), 5837 doi: 10.1016/j.ceramint.2018.12.050[188] Chandekar K V, Kant K M. Relaxation phenomenon and relaxivity of cetrimonium bromide (CTAB) coated CoFe2O4 nanoplatelets. Phys B Condens Matter, 2018, 545, 536 doi: 10.1016/j.physb.2018.07.010[189] Adamson A W, Gast A P. Physical chemistry of surfaces. Interscience publishers, 1967, 150[190] Harkins W D, Jura G. Surfaces of solids. XIII. A vapor adsorption method for the determination of the area of a solid without the assumption of a molecular area, and the areas occupied by nitrogen and other molecules on the surface of a solid. J Am Chem Soc, 1944, 66(8), 1366 doi: 10.1021/ja01236a048[191] Chella S, Kollu P, Komarala E V P R, et al. Solvothermal synthesis of MnFe2O4-graphene composite: Investigation of its adsorption and antimicrobial properties. Appl Surf Sci, 2015, 327, 27 doi: 10.1016/j.apsusc.2014.11.096[192] Al-Zaqri N, Umamakeshvari K, Mohana V, et al. Green synthesis of nickel oxide nanoparticles and its photocatalytic degradation and antibacterial activity. J Mater Sci Mater Electron, 2022, 33(15), 11864 doi: 10.1007/s10854-022-08149-1[193] Haron W, Wisitsoraat A, Sirimahachai U, et al. Removal of toxic heavy metal ions from water with LaAlO3 perovskite. Songklanakarin J Sci Technol, 2018, 40(5), 993 doi: 10.14456/sjst-psu.2018.121[194] Ahmed M A, Bishay S T, Abd-Elwahab S M, et al. Removing lead ions from water by using nanocomposite (rare earth oxide/alumina). Journal of Molecular Liquids, 2017, 240, 604 doi: 10.1016/j.molliq.2017.05.122[195] Liu F, Zhou K, Chen Q, et al. Application of magnetic ferrite nanoparticles for removal of Cu (II) from copper-ammonia wastewater. Journal of Alloys and Compounds, 2019, 773, 140 doi: 10.1016/j.jallcom.2018.09.240[196] Wang G, Chang Q, Zhang M, et al. Effect of pH on the removal of Cr (III) and Cr (VI) from aqueous solution by modified polyethyleneimine. Reactive and Functional Polymers, 2013, 73(11), 1439 doi: 10.1016/j.reactfunctpolym.2013.07.009[197] Ateia E E, Ramadan R, Shafaay A S. Efficient treatment of lead-containing wastewater by CoFe2O4/graphene nanocomposites. Applied Physics A, 2020, 126(3), 222 doi: 10.1007/s00339-020-3401-3[198] Arade S, Balgude S, Kounsalye J, et al. Magnetically separable Cu0.5Ni0.5MnFeO4@GO nanocomposites for efficient methylene blue removal. J Mater Sci Mater Electron, 2024, 35(24), 1636 doi: 10.1007/s10854-024-13405-7[199] Israelachvili J N, Tabor D. Van der waals forces: Theory and experiment. Progress in Surface and Membrane Science. Amsterdam: Elsevier, 1973, 1[200] Lim K R G, Kaiser S K, Wu H C, et al. Nanoparticle proximity controls selectivity in benzaldehyde hydrogenation. Nat Catal, 2024, 7, 172 doi: 10.1038/s41929-023-01104-1[201] Obayomi K S, Lau S Y, Zahir A, et al. Removing methylene blue from water: A study of sorption effectiveness onto nanoparticles-doped activated carbon. Chemosphere, 2023, 313, 137533 doi: 10.1016/j.chemosphere.2022.137533[202] Vareda J P. On validity, physical meaning, mechanism insights and regression of adsorption kinetic models. J Mol Liq, 2023, 376, 121416 doi: 10.1016/j.molliq.2023.121416[203] Ren B Q, Zhang Q, Zhang X C, et al. Biosorption of Cr(vi) from aqueous solution using dormant spores of Aspergillus niger. RSC Adv, 2018, 8(67), 38157 doi: 10.1039/C8RA07084A[204] Jasrotia R, Suman, Verma A, et al. Photocatalytic dye degradation efficiency and reusability of Cu-substituted Zn-Mg spinel nanoferrites for wastewater remediation. J Water Process Eng, 2022, 48, 102865 doi: 10.1016/j.jwpe.2022.102865[205] Madhukara Naik M, Bhojya Naik H S, Nagaraju G, et al. Green synthesis of zinc doped cobalt ferrite nanoparticles: Structural, optical, photocatalytic and antibacterial studies. Nano Struct Nano Objects, 2019, 19, 100322 doi: 10.1016/j.nanoso.2019.100322[206] Hatchard C, Parker C A. A new sensitive chemical actinometer - II. Potassium ferrioxalate as a standard chemical actinometer. Proc R Soc Lond A, 1956, 235(1203), 518 doi: 10.1098/rspa.1956.0102[207] Mulazzani Q G, D’Angelantonio M, Venturi M, et al. Interaction of formate and oxalate ions with radiation-generated radicals in aqueous solution. Methylviologen as a mechanistic probe. J Phys Chem, 1986, 90(21), 5347 doi: 10.1021/j100412a090[208] Walling C. Fenton’s reagent revisited. Acc Chem Res, 1975, 8(4), 125 doi: 10.1021/ar50088a003[209] Sedlak D L, Hoigné J. The role of copper and oxalate in the redox cycling of iron in atmospheric waters. Atmos Environ Part A Gen Top, 1993, 27(14), 2173 doi: 10.1016/0960-1686(93)90047-3[210] Hussein H, Ibrahim S, Khairy S A. Sustainable synthesis of CoFe2O4 nanoparticles with tailored physical properties using Hibiscus extract for photo-Fenton catalytic degradation of methylene blue dye: Response surface methodology. J Water Process Eng, 2025, 76, 1078 doi: 10.1016/j.jwpe.2025.107893[211] Dean J A. Lange's handbook of chemistry. Materials and Manufacturing Processes, 1999, 5(4), 687[212] Kumar S, Kaushik R D, Purohit L P. Novel ZnO tetrapod-reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of phenolic compounds and MB dye. J Mol Liq, 2021, 327, 114814 doi: 10.1016/j.molliq.2020.114814[213] Rauf M A, Meetani M A, Khaleel A, et al. Photocatalytic degradation of Methylene Blue using a mixed catalyst and product analysis by LC/MS. Chem Eng J, 2010, 157(2/3), 373 doi: 10.1016/j.cej.2009.11.017[214] Nguyen C H, Tran M L, Van Tran T T, et al. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep Purif Technol, 2020, 232, 115962 doi: 10.1016/j.seppur.2019.115962[215] Hussein H, Ibrahim S S, Khairy S A. Green synthesis of ZnO nanoparticles using Hibiscus sabdariffa L: Rapid Pb2+ ion removal, photocatalytic degradation of methylene blue, and biomedical applications. J Water Process Eng, 2025, 69, 106649 doi: 10.1016/j.jwpe.2024.106649[216] Ansari M A, Murali M, Prasad D, et al. Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules, 2020, 10(2), 336 doi: 10.3390/biom10020336[217] Satheeshkumar M K, Kumar E R, Srinivas C, et al. Study of structural, morphological and magnetic properties of Ag substituted cobalt ferrite nanoparticles prepared by honey assisted combustion method and evaluation of their antibacterial activity. J Magn Magn Mater, 2019, 469, 691 doi: 10.1016/j.jmmm.2018.09.039[218] Mejías Carpio I E, Santos C M, Wei X, et al. Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale, 2012, 4(15), 4746 doi: 10.1039/c2nr30774j[219] Cobos M, De-La-Pinta I, Quindós G, et al. One-step eco-friendly synthesized silver-graphene oxide/poly(vinyl alcohol) antibacterial nanocomposites. Carbon, 2019, 150, 101 doi: 10.1016/j.carbon.2019.05.011[220] Ma Y, Bai D C, Hu X J, et al. Robust and antibacterial polymer/mechanically exfoliated graphene nanocomposite fibers for biomedical applications. ACS Appl Mater Interfaces, 2018, 10(3), 3002 doi: 10.1021/acsami.7b17835[221] Cobos M, De-La-Pinta I, Quindós G, et al. Synthesis, physical, mechanical and antibacterial properties of nanocomposites based on poly(vinyl alcohol)/graphene oxide–silver nanoparticles. Polymers, 2020, 12(3), 723 doi: 10.3390/polym12030723[222] Ameen F, Majrashi N. Recent trends in the use of cobalt ferrite nanoparticles as an antimicrobial agent for disability infections: A review. Inorg Chem Commun, 2023, 156, 111187 doi: 10.1016/j.inoche.2023.111187[223] Bouarab-Chibane L, Forquet V, Lantéri P, et al. Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure-activity relationship) models. Front Microbiol, 2019, 10, 829 doi: 10.3389/fmicb.2019.00829[224] Lopes M, Sanches-Silva A, Castilho M, et al. Halophytes as source of bioactive phenolic compounds and their potential applications. Critical Reviews in Food Science and Nutrition, 2023, 63(8), 1078 doi: 10.1080/10408398.2021.1959295[225] Parra-Ortiz E, Malmsten M. Photocatalytic nanoparticles–From membrane interactions to antimicrobial and antiviral effects. Adv Colloid Interface Sci, 2022, 299, 102526 doi: 10.1016/j.cis.2021.102526[226] Zhang H J, Chen G H. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot Sol–gel method. Environ Sci Technol, 2009, 43(8), 2905 doi: 10.1021/es803450f[227] Mahdy S A, Raheed Q J, Kalaichelvan P T. Antimicrobial activity of zero-valent iron nanoparticles. International Journal of Modern Engineering Research, 2012, 2(1), 578 doi: 10.5812/jjm.10054[228] Rehman S, Jermy B R, Akhtar S, et al. Isolation and characterization of a novel thermophile; Bacillus haynesii, applied for the green synthesis of ZnO nanoparticles. Artif Cells Nanomed Biotechnol, 2019, 47(1), 2072 doi: 10.1080/21691401.2019.1620254[229] Prabhu Y T, Rao K V, Kumari B S, et al. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int Nano Lett, 2015, 5(2), 85 doi: 10.1007/s40089-015-0141-z[230] Kalpana V N, Kataru B A S, Sravani N, et al. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano, 2018, 3, 48 doi: 10.1016/j.onano.2018.06.001[231] Sumanth B, Lakshmeesha T R, Ansari M A, et al. Mycogenic synthesis of extracellular zinc oxide nanoparticles from Xylaria acuta and its nanoantibiotic potential. Int J Nanomed, 2020, 15, 8519 doi: 10.2147/IJN.S271743[232] Sharmila G, Muthukumaran C, Sandiya K, et al. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J Nanostruct Chem, 2018, 8(3), 293 doi: 10.1007/s40097-018-0271-8[233] Saemi R, Taghavi E, Jafarizadeh-Malmiri H, et al. Fabrication of green ZnO nanoparticles using walnut leaf extract to develop an antibacterial film based on polyethylene–starch–ZnO NPs. Green Process Synth, 2021, 10(1), 112 doi: 10.1515/gps-2021-0011 -
Supplements
 25040013-Supplementary Information.pdf
25040013-Supplementary Information.pdf

-
Proportional views





 Heba Hussein is a doctoral researcher in the Department of Physics, Faculty of Science-Cairo University. She received her B.Sc. in Physics in 2006 and her M.Sc. in 2020, both from Cairo University. Currently pursuing her Ph.D. under the supervision of Prof. Dr. Sobhy Sayed Ibrahim and Prof. Dr. Sherif Ahmed Khairy, her research centers on the eco-sustainable synthesis of nanoparticles using plant-based and natural extracts. Her work bridges green chemistry and nanotechnology, with a focus on designing materials for advanced environmental remediation and biomedical applications. She integrates statistical optimization techniques such as response surface methodology to precisely tailor the physical, optical, and magnetic properties of nanomaterials. Her research contributes to the growing field of sustainable nanoscience, aiming to replace conventional synthesis methods with environmentally benign alternatives.
Heba Hussein is a doctoral researcher in the Department of Physics, Faculty of Science-Cairo University. She received her B.Sc. in Physics in 2006 and her M.Sc. in 2020, both from Cairo University. Currently pursuing her Ph.D. under the supervision of Prof. Dr. Sobhy Sayed Ibrahim and Prof. Dr. Sherif Ahmed Khairy, her research centers on the eco-sustainable synthesis of nanoparticles using plant-based and natural extracts. Her work bridges green chemistry and nanotechnology, with a focus on designing materials for advanced environmental remediation and biomedical applications. She integrates statistical optimization techniques such as response surface methodology to precisely tailor the physical, optical, and magnetic properties of nanomaterials. Her research contributes to the growing field of sustainable nanoscience, aiming to replace conventional synthesis methods with environmentally benign alternatives. Sobhy Sayed Ibrahim is a Professor Emeritus of Materials Science in the Department of Physics, Faculty of Science-Cairo University. He began his academic career as a teaching assistant and rose through the ranks to become a full professor in material science, focusing on the electrical, thermal, and optical properties of materials. He has published extensively in polymers, ceramics, and polymer nanocomposites. He completed a six-month postdoctoral fellowship at Tokyo University of Science. Until 2001, he served as a professor at Cairo University and held advisory roles to the Minister of Youth and Sports and the Vice President of Cairo University. He later joined King Faisal University in Saudi Arabia, where he worked as a physics professor for 12 years and served as a scientific advisor to the university’s Vice President.
Sobhy Sayed Ibrahim is a Professor Emeritus of Materials Science in the Department of Physics, Faculty of Science-Cairo University. He began his academic career as a teaching assistant and rose through the ranks to become a full professor in material science, focusing on the electrical, thermal, and optical properties of materials. He has published extensively in polymers, ceramics, and polymer nanocomposites. He completed a six-month postdoctoral fellowship at Tokyo University of Science. Until 2001, he served as a professor at Cairo University and held advisory roles to the Minister of Youth and Sports and the Vice President of Cairo University. He later joined King Faisal University in Saudi Arabia, where he worked as a physics professor for 12 years and served as a scientific advisor to the university’s Vice President. Sherif Ahmed Khairy is a Professor Emeritus of Materials Science in the Department of Physics, Faculty of Science-Cairo University. He obtained his PhD from Moscow State university. He began his academic career as a lecturer at Cairo University and progressed to the rank of full professor in materials science. He served as Vice Dean for Student Affairs at the Faculty of Science, Cairo University for three years. He is a founding member of the Solid State Society in Egypt and has supervised numerous MSc and PhD theses. He also worked for several years in Qatar at Qatar university Department of Physics. His main research interests focus on dielectric and electrical properties of materials, along with their technological applications.
Sherif Ahmed Khairy is a Professor Emeritus of Materials Science in the Department of Physics, Faculty of Science-Cairo University. He obtained his PhD from Moscow State university. He began his academic career as a lecturer at Cairo University and progressed to the rank of full professor in materials science. He served as Vice Dean for Student Affairs at the Faculty of Science, Cairo University for three years. He is a founding member of the Solid State Society in Egypt and has supervised numerous MSc and PhD theses. He also worked for several years in Qatar at Qatar university Department of Physics. His main research interests focus on dielectric and electrical properties of materials, along with their technological applications.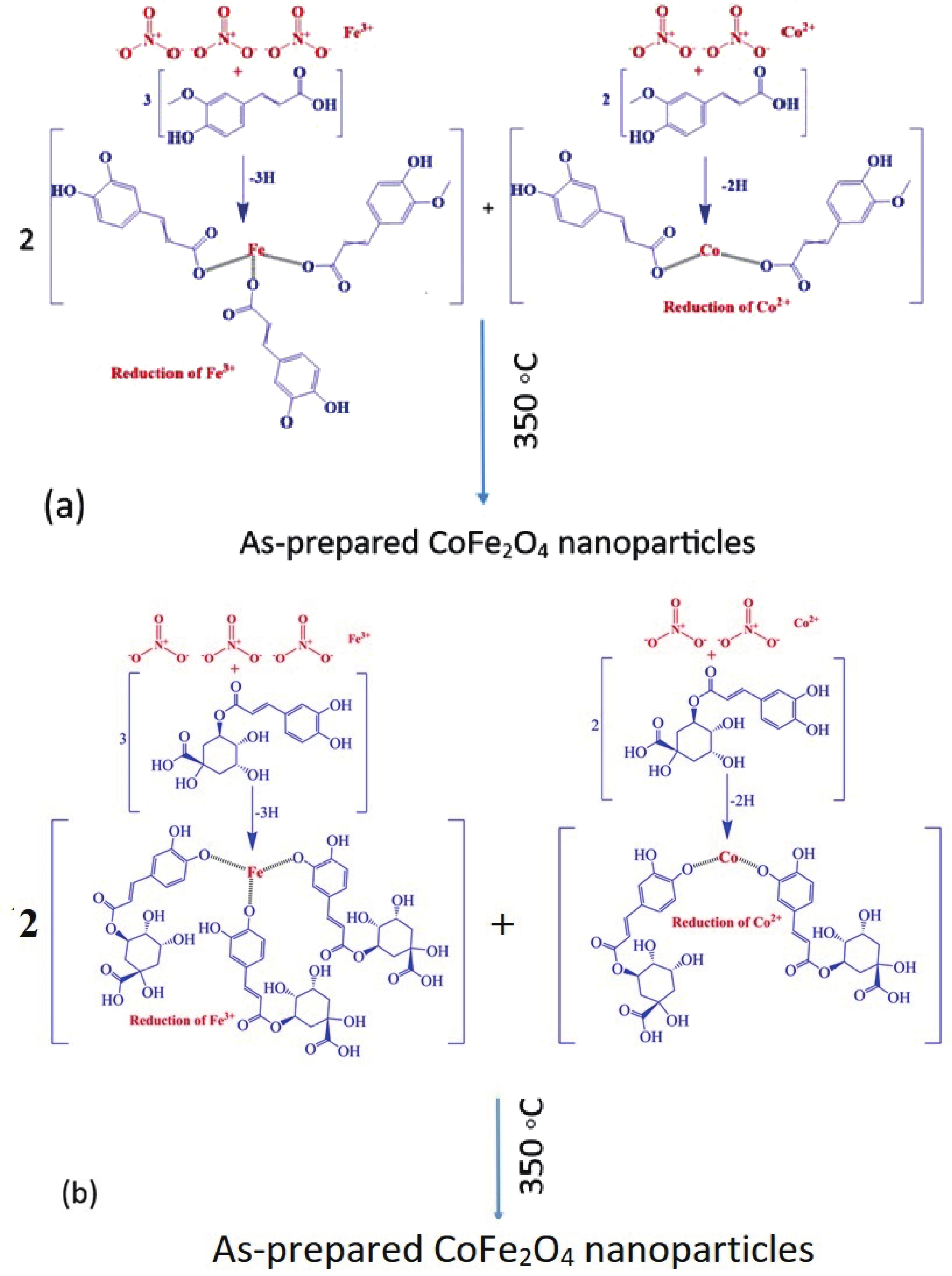
 DownLoad:
DownLoad: