| Citation: |
Lang Wen, Liang Shan, Yunhan Hu, Yiyong Zhang, Wen Lu, Wen-Hua Zhang, Junqiao Ding. Synergistic aluminum lattice doping and surface coating for high-performance Co-free Ni-rich cathodes[J]. Journal of Semiconductors, 2026, In Press. doi: 10.1088/1674-4926/25110009
****
L Wen, L Shan, Y H Hu, Y Y Zhang, W Lu, W - H Zhang, and J Q Ding, Synergistic aluminum lattice doping and surface coating for high-performance Co-free Ni-rich cathodes[J]. J. Semicond., 2026, accepted doi: 10.1088/1674-4926/25110009
|
Synergistic aluminum lattice doping and surface coating for high-performance Co-free Ni-rich cathodes
DOI: 10.1088/1674-4926/25110009
CSTR: 32376.14.1674-4926.25110009
More Information-
Abstract
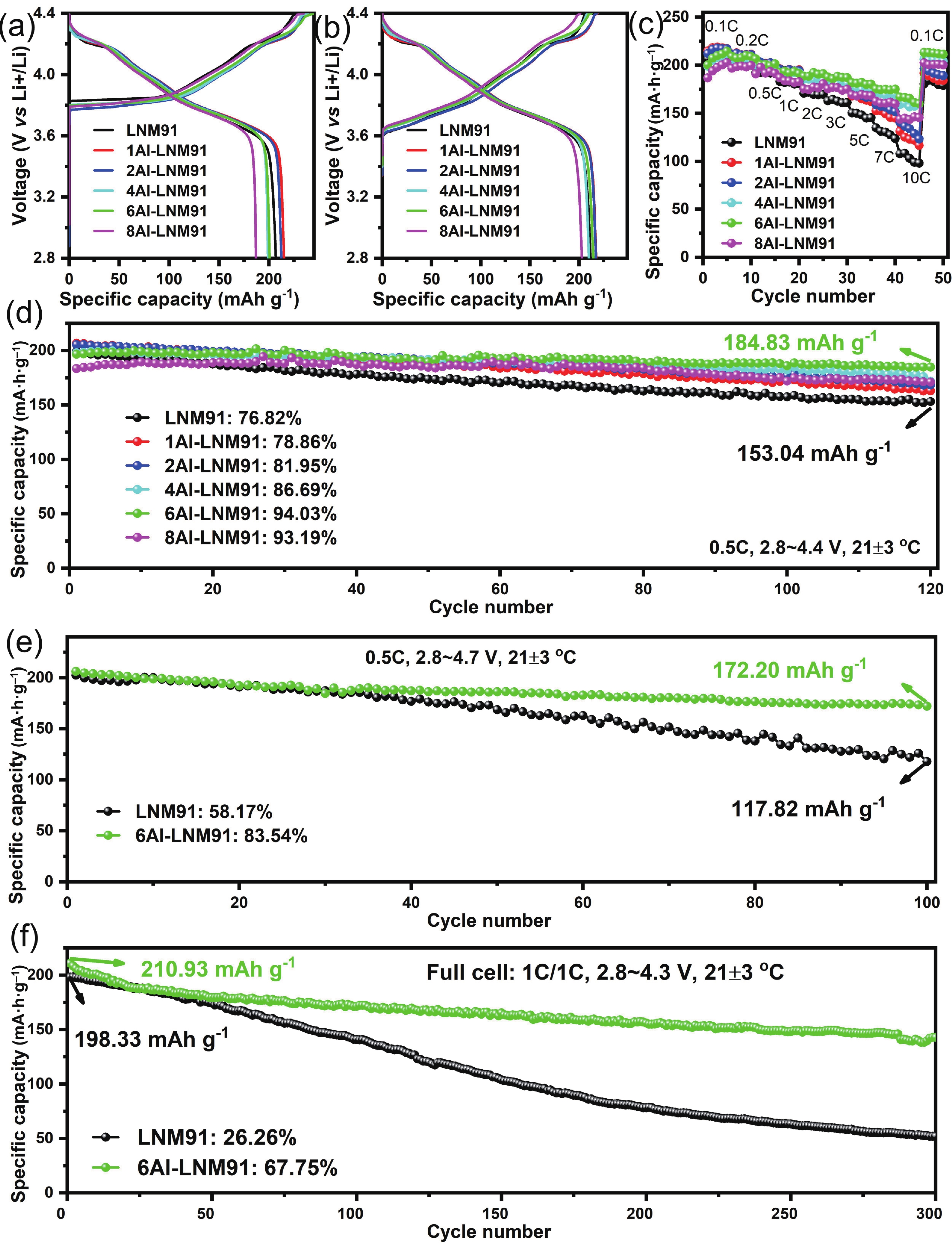
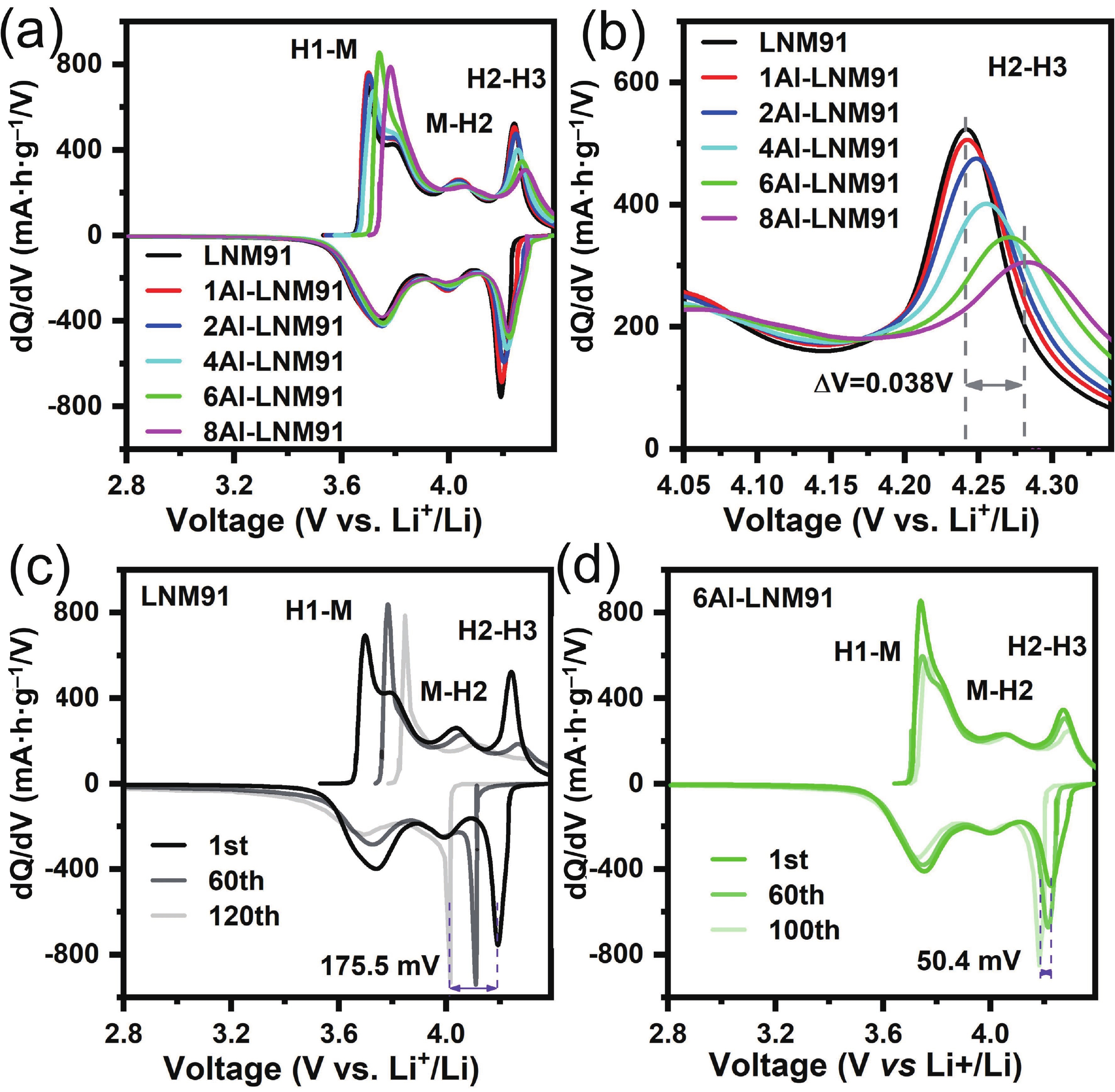
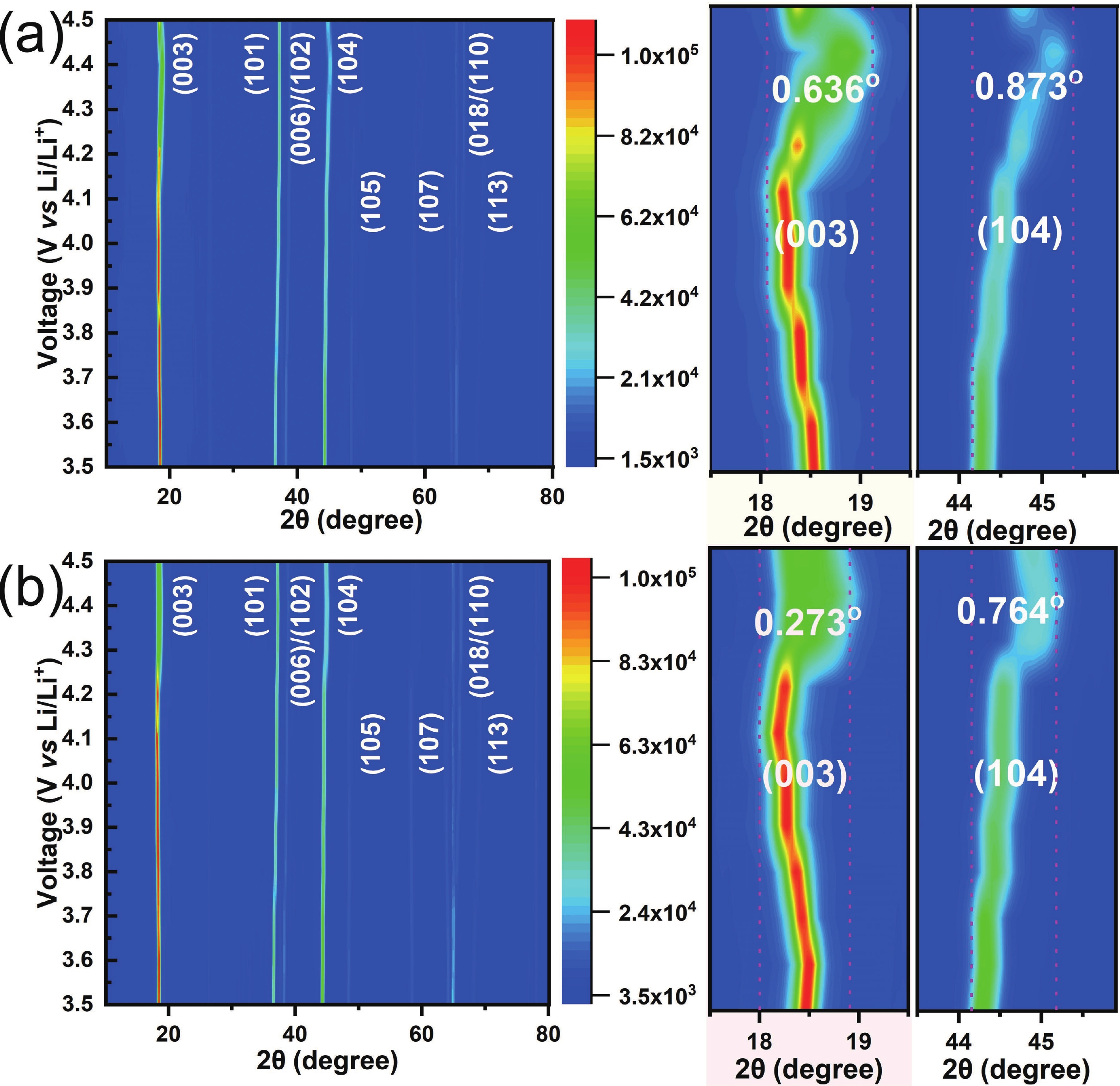
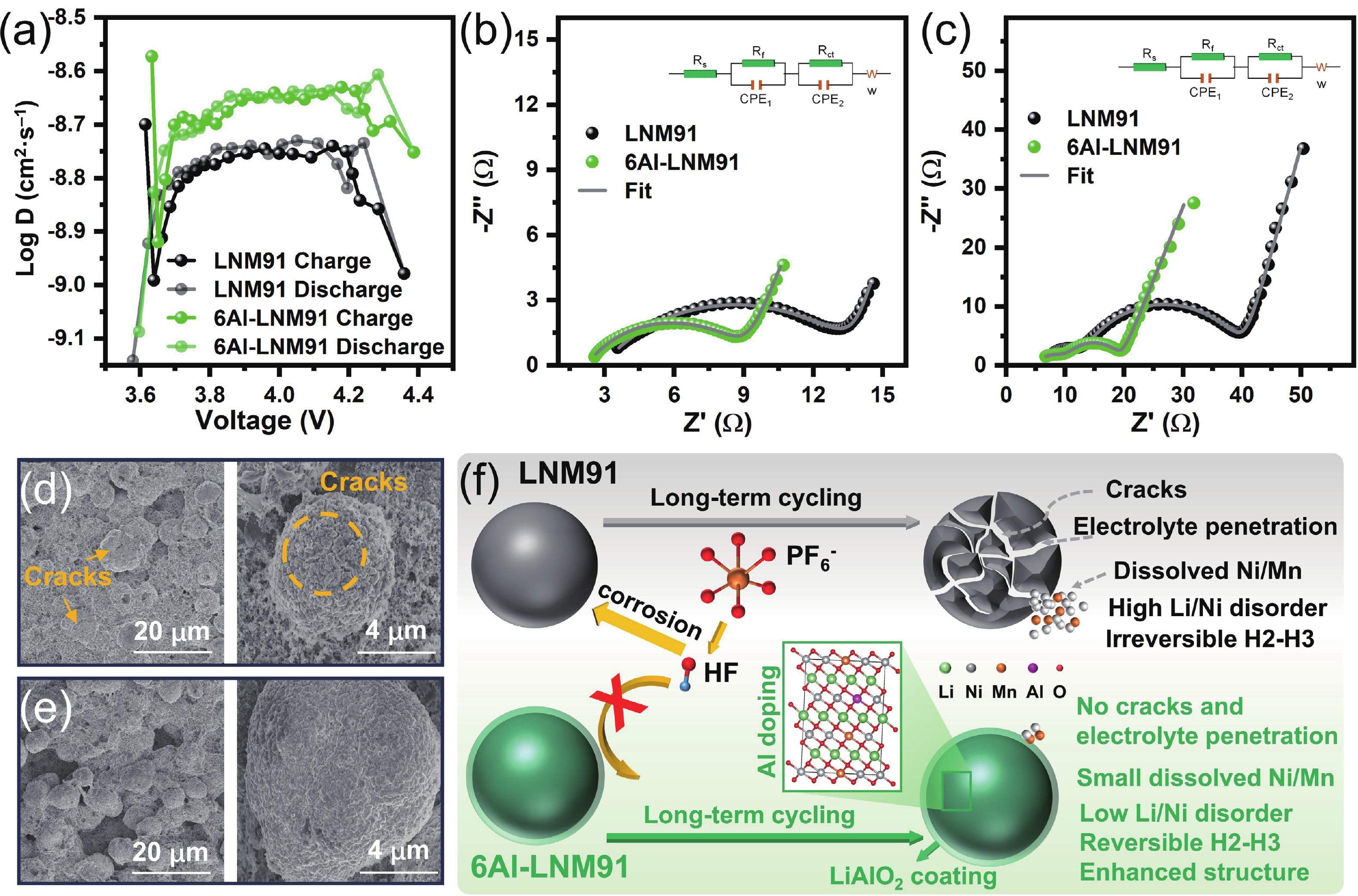
LiNi0.9Mn0.1O2 (LNM91) is a promising cobalt-free, high-energy cathode material for next-generation lithium-ion batteries, but its commercialization is challenged by rapid capacity fading resulting from bulk and interfacial structural degradation. Herein, an in-situ surface-to-bulk dual-modification strategy is developed to synthesize 6Al-LNM91 (6 mol% Al modified LNM91) via a one-step calcination process based on Al diffusion chemistry. This method concurrently constructs a protective LiAlO2 coating and incorporates Al3+ into the bulk lattice, effectively enhancing the structural integrity of the cathode during cycling. The optimized 6Al-LNM91 cathode delivers a remarkable rate capability of 165 mA∙h∙g−1 at 10 C and maintains 94.03% capacity retention after 120 cycles at 0.5 C (2.8 − 4.4 V), substantially outperforming the pristine material (76.82% of LNM91). This organic solvent-free, single-step modification approach offers a scalable and efficient route for improving high-nickel layered oxide cathodes. -
References
[1] Li W D, Erickson E M, Manthiram A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat Energy, 2020, 5(1): 26 doi: 10.1038/s41560-019-0513-0[2] Lee S, Li C, Manthiram A. Effects of calcination conditions on the structural and electrochemical behaviors of high-nickel, cobalt-free LiNi0.9Mn0. 1O2 cathode. Adv Energy Mater, 2024, 14(24): 2400662[3] Co-free Ni-rich layered cathode with long-term cycling stability. Nat Energy, 2022, 7(10): 914[4] Park G T, Namkoong B, Kim S B, et al. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat Energy, 2022, 7(10): 946 doi: 10.1038/s41560-022-01106-6[5] Aishova A, Park G T, Yoon C S, et al. Cobalt-free high-capacity Ni-rich layered Li [Ni0.9Mn0. 1] O2 cathode. Adv Energy Mater, 2020, 10(4): 1903179[6] Wei S X, Cui C J, Jin Y, et al. Enhancement of Li intercalation kinetics of LiFePO4 nanoparticles with mesoporous carbon. Energy Mater, 2024, 4(5): 400062 doi: 10.20517/energymater.2024.20[7] Huang M T, Wang M, Yang L M, et al. Direct regeneration of spent lithium-ion battery cathodes: From theoretical study to production practice. Nano Micro Lett, 2024, 16(1): 207 doi: 10.1007/s40820-024-01434-0[8] Liu Y, Dong J Y, Guan Y B, et al. In situ 3D conductive networks and interfacial bonding to stabilize oxygen vacancies for single-crystal Ni-rich cathodes. Adv Mater, 2026, 38(5): e15106 doi: 10.1002/adma.202515106[9] Wang H Y, Dong J Y, Zhang H Y, et al. Enhancing structural and thermal stability of ultrahigh-Ni cathodes via anion-cation codoping induced surface reconstruction strategy. J Energy Chem, 2025, 106: 9 doi: 10.1016/j.jechem.2025.01.077[10] Li C, Liu J Z, Su Y F, et al. Enhancing chemomechanical stability and high-rate performance of nickel-rich cathodes for lithium-ion batteries through three-in-one modification. Energy Storage Mater, 2025, 74: 103893 doi: 10.1016/j.ensm.2024.103893[11] Wang H Y, Shi Q, Dong J Y, et al. Consolidating surface lattice via facile self-anchored oxygen layer reconstruction toward superior performance and high safety nickel-rich oxide cathodes. Adv Funct Mater, 2025, 35(20): 2422806 doi: 10.1002/adfm.202422806[12] Zheng J X, Ye Y K, Liu T C, et al. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control. Acc Chem Res, 2019, 52(8): 2201 doi: 10.1021/acs.accounts.9b00033[13] Hu C Z, Ma J T, Li A F, et al. Structural reinforcement through high-valence Nb doping to boost the cycling stability of co-free and Ni-rich LiNi0.9Mn0. 1O2 cathode materials. Energy Fuels, 2023, 37(11): 8005[14] Li L J, Chen Q H, Jiang M Z, et al. Uncovering mechanism behind tungsten bulk/grain-boundary modification of Ni-rich cathode. Energy Storage Mater, 2025, 75: 104016 doi: 10.1016/j.ensm.2025.104016[15] Shi Q, Wu F, Wang H Y, et al. Smart-responsive sustained-release capsule design enables superior air storage stability and reinforced electrochemical performance of cobalt-free nickel-rich layered cathodes for lithium-ion batteries. Energy Storage Mater, 2024, 67: 103264 doi: 10.1016/j.ensm.2024.103264[16] Wang L, Wang J Q, Lu Y F, et al. A review of Ni-based layered oxide cathode materials for alkali-ion batteries. Chem Soc Rev, 2025, 54(9): 4419 doi: 10.1039/D3CS00911D[17] Yoon C S, Jun D W, Myung S T, et al. Structural stability of LiNiO2 cycled above 4. 2 V. ACS Energy Lett, 2017, 2(5): 1150[18] Li H Y, Cormier M, Zhang N, et al. Is cobalt needed in Ni-rich positive electrode materials for lithium ion batteries? J Electrochem Soc, 2019, 166(4): A429[19] Adamo J B, Manthiram A. Understanding the effects of Al and Mn doping on the H2–H3 phase transition in high-nickel layered oxide cathodes. Chem Mater, 2024, 36(12): 6226 doi: 10.1021/acs.chemmater.4c01033[20] Sim R, Cui Z H, Manthiram A. Impact of dopants on suppressing gas evolution from high-nickel layered oxide cathodes. ACS Energy Lett, 2023, 8(12): 5143 doi: 10.1021/acsenergylett.3c02024[21] Lee S, Kmiec S, Manthiram A. Effects of coprecipitation conditions on the electrochemical properties of cobalt-free LiNi0.9Mn0. 1-xAlxO2 cathodes. Small, 2024, 20(50): 2406947[22] Feng H L, Xu Y X, Zhou Y C, et al. The Y3+ and W6+ co-doping into Ni-rich Co-free single-crystal cathode LiNi0.9Mn0. 1O2 for achieving high electrochemical properties in lithium-ion batteries. J Alloys Compd, 2024, 976: 173043[23] Guo S N, Lei X C, Wang J Y, et al. Doping of group IVB elements for nickel-rich cobalt-free cathodes. J Energy Chem, 2023, 86: 559 doi: 10.1016/j.jechem.2023.07.041[24] Wang X Y, Zhang B, Xiao Z M, et al. Enhanced rate capability and mitigated capacity decay of ultrahigh-nickel cobalt-free LiNi0.9Mn0. 1O2 cathode at high-voltage by selective tungsten substitution. Chin Chem Lett, 2023, 34(7): 107772[25] Zhao B Y, Sun X, Bi H W, et al. Design high-entropy core-shell nickel-rich cobalt-free cathode material toward high performance lithium batteries. Adv Funct Mater, 2025, 35(26): 2423717[26] Zhou J H, Hu J H, Zhou X, et al. High-entropy doping for high-performance zero-cobalt high-nickel layered cathode materials. Energy Environ Sci, 2025, 18(1): 347 doi: 10.1039/D4EE05020G[27] Shen L N, Gu Y H, Xu T, et al. Dual modification of phosphate toward improving electrochemical performance of LiNiO2 cathode materials. J Colloid Interface Sci, 2024, 662: 505 doi: 10.1016/j.jcis.2024.01.181[28] Geng C X, Rathore D, Heino D, et al. Impact of Tantalum added to Ni-based positive electrode materials for Lithium-ion Batteries. J Power Sources, 2024, 590: 233803 doi: 10.1016/j.jpowsour.2023.233803[29] Wu J, Wen Y L, Zhou Q, et al. Simultaneous bulk doping and surface coating of Sn to boost the electrochemical performance of LiNiO2. ACS Appl Energy Mater, 2023, 6(5): 3010 doi: 10.1021/acsaem.2c04125[30] Chu Y H, Zhou J W, Liu W X, et al. Cobalt-free LiNiO2 with a selenium coating as a high-energy layered cathode material for lithium-ion batteries. Small Sci, 2023, 3(7): 2300023[31] Zhang L, Huang J F, Tang H Y, et al. B/Al codoped/coated ultra-high nickel cobalt-free material with excellent high voltage/rate cycle stability. ACS Sustainable Chem Eng, 2024, 12(24): 9168 doi: 10.1021/acssuschemeng.4c01847[32] Brow R, Donakowski A, Mesnier A, et al. Mechanical pulverization of co-free nickel-rich cathodes for improved high-voltage cycling of lithium-ion batteries. ACS Appl Energy Mater, 2022, 5(6): 6996 doi: 10.1021/acsaem.2c00606[33] Han G M, Kim Y S, Ryu H H, et al. Structural stability of single-crystalline Ni-rich layered cathode upon delithiation. ACS Energy Lett, 2022, 7(9): 2919 doi: 10.1021/acsenergylett.2c01521[34] Lee D, Mesnier A, Manthiram A. Crack-free single-crystalline LiNiO2 for high energy density all-solid-state batteries. Adv Energy Mater, 2024, 14(19): 2303490[35] Sun Y J, Wang Y S, Wang S, et al. Bulk-to-surface engineering allows for extremely stable co-free Ni-rich cathodes for rechargeable batteries. J Mater Sci, 2023, 58(25): 10428 doi: 10.1007/s10853-023-08676-0[36] Zhang F L, Li B Q, Li C Y, et al. In-situ conversion of residual alkali into fast-ion conductor coating and synchronously realizing gradient Mo4+ doping to stabilize LiNi0.9Mn0.1O2 cathode. J Alloys Compd, 2024, 991: 174457[37] Zheng C, Xiao Z M, Xian K Y, et al. Reinforcing ion diffusion and controlling microcrack of nickel-rich cobalt-free single-crystalline cathodes via interfacial protection and bulk optimization. J Colloid Interface Sci, 2025, 684: 138 doi: 10.1016/j.jcis.2025.01.079[38] Peng J Q, Wei Y Y, Hu B, et al. Ultra-high nickel cobalt-free layered cathode material NM90 for power batteries modified collaboratively by La2O3 coating and La3+ doping. Ionics, 2023, 29(7): 2549 doi: 10.1007/s11581-023-05010-1[39] Kaneda H, Furuichi Y, Ikezawa A, et al. Effects of aluminum substitution in nickel-rich layered LiNixAl1−xO2 (x = 0. 92, 0. 95) positive electrode materials for Li-ion batteries on high-rate cycle performance. J Mater Chem A, 2021, 9(38): 21981[40] Shen Y B, Yin D M, Wang L M, et al. A universal multifunctional dual cation doping strategy towards stabilized ultra-high nickel cobalt-free lithium layered oxide cathode. J Energy Chem, 2024, 95: 296 doi: 10.1016/j.jechem.2024.03.030[41] Yao J, Li Y Y, Xiong T T, et al. Scalable precise nanofilm coating and gradient Al doping enable stable battery cycling of LiCoO2 at 4. 7 V. Angew Chem Int Ed, 2024, 63(32): e202407898 doi: 10.1002/anie.202407898[42] Sun Y J, Wang C H, Huang W J, et al. One-step calcination synthesis of bulk-doped surface-modified Ni-rich cathodes with superlattice for long-cycling Li-ion batteries. Angew Chem Int Ed, 2023, 62(20): e202300962[43] Li J Z, Luo S H, Ding X Y, et al. Hydrothermal synthesis of LiAlO2 nanostructures with high specific surface area by using anodized aluminum oxide template. Mater Lett, 2017, 196: 183 doi: 10.1016/j.matlet.2017.03.051[44] Wu Y, Li Y F, Wang L Y, et al. Enhancing the Li-ion storage performance of graphite anode material modified by LiAlO2. Electrochim Acta, 2017, 235: 463 doi: 10.1016/j.electacta.2017.03.129[45] Zhou J W, Chu Y H, Liu W X, et al. Mg/Al double-pillared LiNiO2 as a co-free ternary cathode material ensuring stable cycling at 4. 6 V. ACS Appl Mater Interfaces, 2024, 16(11): 13948 doi: 10.1021/acsami.3c17457[46] Marezio M. The crystal structure and anomalous dispersion of γ-LiAlO2. Acta Cryst, 1965, 19(3): 396[47] Zhang B, Wen H, Xian K Y, et al. Stabilizing ultrahigh-nickel cobalt-free cathode materials by using tri-element doping engineering. Mater Today Energy, 2025, 48: 101787 doi: 10.1016/j.mtener.2024.101787[48] Li M, Lu J. Cobalt in lithium-ion batteries. Science, 2020, 367(6481): 979 doi: 10.1126/science.aba9168[49] Cao H S, Du F H, Adkins J, et al. Al-doping induced superior lithium ion storage capability of LiNiO2 spheres. Ceram Int, 2020, 46(12): 20050 doi: 10.1016/j.ceramint.2020.05.078[50] Huang F Y, Zhu Y, Yao W L, et al. Aluminum/titanium bimetallic doping for boosting the high-voltage Li-storage performance of co-free high nickel cathode. J Alloys Compd, 2025, 1018: 179198 doi: 10.1016/j.jallcom.2025.179198[51] Liu T C, Yu L, Liu J J, et al. Understanding Co roles towards developing co-free Ni-rich cathodes for rechargeable batteries. Nat Energy, 2021, 6(3): 277 doi: 10.1038/s41560-021-00776-y[52] Tian R Z, Yin S, Zhang H Z, et al. Influence of Al doping on the structure and electrochemical performance of the co-free LiNi0.8Mn0. 2O2 cathode material. Dalton Trans, 2023, 52(33): 11716[53] Feng H, Leng Y, Chen T D, et al. Stabilizing LiNi0.9Mn0. 1O2 structure by Al3+ doping for cobalt-free lithium-ion batteries. J Alloys Compd, 2023, 960: 170676[54] Sun Y J, Huang W J, Zhao G F, et al. LiNi0.9Co0. 09Mo0. 01O2 cathode with Li3PO4 coating and Ti doping for next-generation lithium-ion batteries. ACS Energy Lett, 2023, 8(3): 1629[55] Li B Q, Zhang F L, Li C Y, et al. Insights into the effects of coating and single crystallization on the rate performance and cycle life of LiNi0.9Mn0. 1O2 cathode. J Colloid Interface Sci, 2024, 672: 776[56] Sun Y J, Shao H Y, Zou X X, et al. In-situ modification from surface to bulk for enhancing the performance of NCM811 cathode. EnLab, 2025, 2: 240020 doi: 10.54227/elab.20240020[57] Zou K Y, Xie S C, Jiang M Z, et al. Insights into the precursor specific surface area for engineering co-free Ni-rich cathodes with tailorable properties. Chem Eng J, 2024, 483: 149189 doi: 10.1016/j.cej.2024.149189[58] Tong H, Yuan X, Qin N B, et al. Plane-controlled growth strategy improves electrochemical performance of cobalt-free LiNi0.9Mn0. 1O2 cathode. Prog Nat Sci Mater Int, 2024, 34(3): 569[59] Shi T F, Liu F, Liu W H, et al. Cation mixing regulation of cobalt-free high-nickel layered cathodes enables stable and high-rate lithium-ion batteries. Nano Energy, 2024, 123: 109410 doi: 10.1016/j.nanoen.2024.109410[60] Dai P P, Kong X B, Yang H Y, et al. Single-crystal Ni-rich layered LiNi0.9Mn0. 1O2 enables superior performance of co-free cathodes for lithium-ion batteries. ACS Sustainable Chem Eng, 2022, 10(14): 4381[61] Cui B C, Xiao Z X, Cui S L, et al. Safety issues and improvement measures of Ni-rich layered oxide cathode materials for Li-ion batteries. Electrochem Energy Rev, 2024, 7(1): 27 doi: 10.1007/s41918-024-00211-2 -
Proportional views





 Lang Wen got his master’s degree in 2020 from Sichuan University of Science & Engineering, Zigong, China. Now he is a doctoral student at Yunnan University. His research focuses on cobalt-free and nickel-rich cathode materials.
Lang Wen got his master’s degree in 2020 from Sichuan University of Science & Engineering, Zigong, China. Now he is a doctoral student at Yunnan University. His research focuses on cobalt-free and nickel-rich cathode materials. Yiyong Zhang received his doctoral degree from Xiamen University, Xiamen, China, in 2018. He is currently an Associate Professor with the Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming, China. His current research interests focused on the electrochemical energy storage and electrochemical in-situ characterization technology including lithium-sulfur batteries, metal sulfides, carbon-based, and silicon-based lithium/sodium negative electrodes.
Yiyong Zhang received his doctoral degree from Xiamen University, Xiamen, China, in 2018. He is currently an Associate Professor with the Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming, China. His current research interests focused on the electrochemical energy storage and electrochemical in-situ characterization technology including lithium-sulfur batteries, metal sulfides, carbon-based, and silicon-based lithium/sodium negative electrodes. Wen Lu is currently a professor and Director of the Institute of Energy Storage Technologies at Yunnan University. He obtained his PhD from the University of Wollongong in Australia and conducted his postdoctoral research at Carleton University in Canada. His research activities have been focused on the applications of electrochemistry and new materials to the development of a range of electrochemical devices including energy conversion and storage devices, electrochromic devices, electrochemical sensors and biosensors, electromechanical actuators, and environmental remediation devices.
Wen Lu is currently a professor and Director of the Institute of Energy Storage Technologies at Yunnan University. He obtained his PhD from the University of Wollongong in Australia and conducted his postdoctoral research at Carleton University in Canada. His research activities have been focused on the applications of electrochemistry and new materials to the development of a range of electrochemical devices including energy conversion and storage devices, electrochromic devices, electrochemical sensors and biosensors, electromechanical actuators, and environmental remediation devices. Junqiao Ding received his doctoral degree from Changchun Insititute of Applied Chemistry Chinese Academy of Sciences, Changchun, China. He is currently a Researcher at the School of Chemical Science and Technology, Yunnan University, Kunming, China. He is an outstanding mentor of the Chinese Academy of Sciences, a Yunnan Province "Yunling Scholar", and a member of the editorial board of the Journal of Semiconductors. His research mainly focuses on organic polymer optoelectronic materials and devices. He has successfully developed low-cost, high-efficiency dendritic iridium phosphorescent complexes, thermally activated delayed fluorescence polymers, and pure organic room-temperature electrophosphorescent polymers.
Junqiao Ding received his doctoral degree from Changchun Insititute of Applied Chemistry Chinese Academy of Sciences, Changchun, China. He is currently a Researcher at the School of Chemical Science and Technology, Yunnan University, Kunming, China. He is an outstanding mentor of the Chinese Academy of Sciences, a Yunnan Province "Yunling Scholar", and a member of the editorial board of the Journal of Semiconductors. His research mainly focuses on organic polymer optoelectronic materials and devices. He has successfully developed low-cost, high-efficiency dendritic iridium phosphorescent complexes, thermally activated delayed fluorescence polymers, and pure organic room-temperature electrophosphorescent polymers.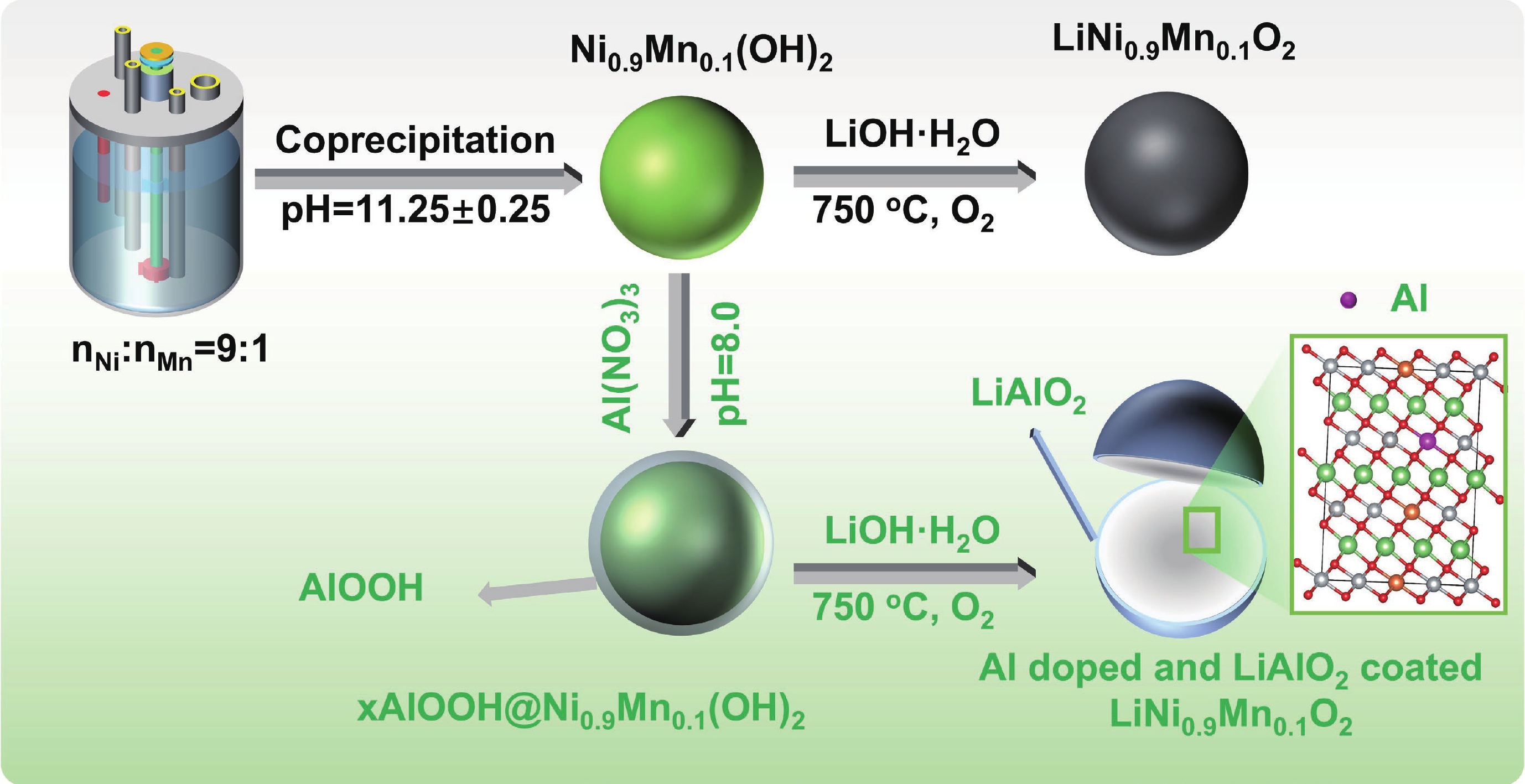
 DownLoad:
DownLoad: