| Citation: |
D. Djouadi, M. Meddouri, A. Chelouche, L. Hammiche, A. Aksas. Structural and morphological characterizations of ZnO nanopowder synthesized by hydrothermal route using inorganic reactants[J]. Journal of Semiconductors, 2014, 35(12): 123001. doi: 10.1088/1674-4926/35/12/123001
****
D. Djouadi, M. Meddouri, A. Chelouche, L. Hammiche, A. Aksas. Structural and morphological characterizations of ZnO nanopowder synthesized by hydrothermal route using inorganic reactants[J]. J. Semicond., 2014, 35(12): 123001. doi: 10.1088/1674-4926/35/12/123001.
|
Structural and morphological characterizations of ZnO nanopowder synthesized by hydrothermal route using inorganic reactants
DOI: 10.1088/1674-4926/35/12/123001
More Information
-
Abstract
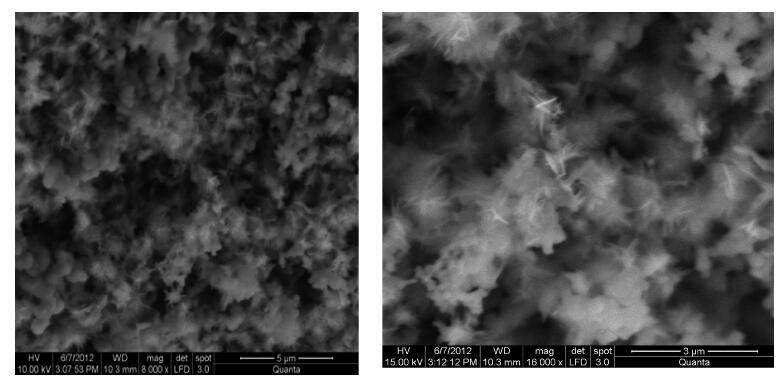
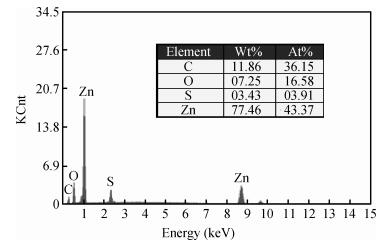
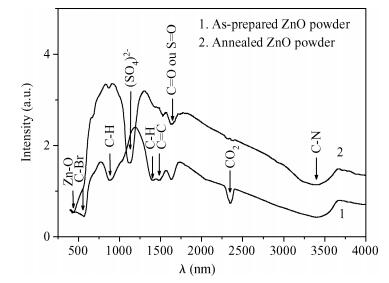
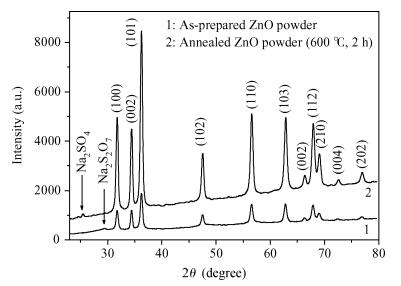
Zinc oxide nanoscale powder has been synthesized by a hydrothermal route using zinc sulfate and sodium hydroxide. The as-prepared powder was annealed at 600℃ for 2 h and then characterized by X-ray diffraction (XRD), scanning electron microscopy and infra-red Fourier transformed spectroscopy. XRD measurements have shown a ZnO hexagonal wurtzite polycrystalline structure with good crystallinity and the formation of a new sodium pyrosulfate phase in the as-prepared powder. The annealing improves the crystalline quality of the powder and transforms the sodium pyrosulfate phase to a sodium sulfate one. The thermal treatment does not affect the lattice parameters and the Zn-O bond length but improves the random orientation of the ZnO crystallites growth. ZnO crystallites have an interconnected-nano-needles morphology forming irregular shaped aggregates. The size of the crystallites is about 20 nm. EDX analysis has shown the presence of C and S in addition to Zn and O. FTIR spectra confirm the formation of ZnO and sodium sulfate. The synthesized ZnO powder has a very high crystalline quality and the used method is a very advantageous one for the fabrication of nanosized metal oxides from inorganic reactants for photo-catalysis applications.-
Keywords:
- ZnO,
- hydrothermal method,
- zinc sulfate,
- nanoneedles,
- DRX,
- SEM,
- FTIR
-
References
[1] Kim S J, Kim H H, Kwona J B, et al. Novel fabrication of various size ZnO nanorods using hydrothermal method. Microelectron Eng, 2010, 87:1534 doi: 10.1016/j.mee.2009.11.033[2] Djurisic A B, Ng A M C, Chen X Y. ZnO nanostructures for optoelectronics:material properties and device applications. Progress in Quantum Electron, 2010, 34:191 doi: 10.1016/j.pquantelec.2010.04.001[3] Akhtar M S, Khan M A, Jeon M S, et al. Controlled synthesis of various ZnO nanostructured materials by capping agents-assisted hydrothermal method for dye-sensitized solar cells. Electrochimica Acta, 2008, 53:7869 doi: 10.1016/j.electacta.2008.05.055[4] Das N C, Sokol P E. Hybrid photovoltaic devices from regioregular polythiophene and ZnO nanoparticles composites. Renewable Energy, 2010, 35:2683 doi: 10.1016/j.renene.2010.04.014[5] Ma J, Liu J, Bao Y, et al. Synthesis of large-scale uniform mulberry-like ZnO particles with microwave hydrothermal method and its antibacterial property. Ceramics International, 2013, 39:2803 doi: 10.1016/j.ceramint.2012.09.049[6] Liu Y L, Yang Y H, Yang H F, et al. Nanosized flower-like ZnO synthesized by a simple hydrothermal method and applied as matrix for horseradish peroxidase immobilization for electro-biosensing. Journal of Inorganic Biochemistry, 2005, 99:2046 doi: 10.1016/j.jinorgbio.2005.07.001[7] Sun L, Shao R, Chen Z, et al. Alkali-dependent synthesis of flower-like ZnO structures with enhanced photocatalytic activity via a facile hydrothermal method. Appl Surf Sci, 2012, 258:5455 doi: 10.1016/j.apsusc.2012.02.034[8] Donkova B, Dimitrov D, Kostadinov M, et al. Catalytic and photocatalytic activity of lightly doped catalysts M:ZnO (M=Cu, Mn). Mater Chem Phys, 2010, 123:563 doi: 10.1016/j.matchemphys.2010.05.015[9] Xie J, Wang H, Duan M. Synthesis and photocatalysis properties of ZnO structures with different morphologies via hydrothermal method. Appl Surf Sci, 2011, 257:6358 doi: 10.1016/j.apsusc.2011.01.105[10] Qiuxiang Z, Ke Y, Wei B, et al. Synthesis, optical and field emission properties of three different ZnO nanostructures. Mater Lett, 2007, 61:3890 doi: 10.1016/j.matlet.2006.12.064[11] Li Z, Huang X, Liu J, et al. Morphology control and transition of ZnO nanorod arrays by a simple hydrothermal method. Mater Lett, 2008, 62:1503 doi: 10.1016/j.matlet.2007.09.011[12] Kim J Y, Cho J W, Kim S H. The characteristic of the ZnO nanowire morphology grown by the hydrothermal method on various surface-treated seed layers. Mater Lett, 2011, 65:1161 doi: 10.1016/j.matlet.2010.10.092[13] Tao Y, Fu M, Zhao A, et al. The effect of seed layer on morphology of ZnO nanorod arrays grown by hydrothermal method. Journal of Alloys and Compounds, 2010, 489:99 doi: 10.1016/j.jallcom.2009.09.020[14] Wang Z, Huang B, Qin X, et al. Growth of high transmittance vertical aligned ZnO nanorod arrays with polyvinyl alcohol by hydrothermal method. Mater Lett, 2009, 63:130 doi: 10.1016/j.matlet.2008.09.042[15] Zhou Z, Zhao Y, Cai Z. Low-temperature growth of ZnOnanorods on PET fabrics with two-step hydrothermal method. Appl Surf Sci, 2010, 256:4724 doi: 10.1016/j.apsusc.2010.02.081[16] Lv W, Wei B, Xua L, et al. Photocatalytic properties of hierarchical ZnO flowers synthesized by a sucrose-assisted hydrothermal method. Appl Surf Sci, 2012, 259:557 doi: 10.1016/j.apsusc.2012.04.182[17] Wang Y, Chen X, Zhang J, et al. Fabrication of surface-patterned and free-standing ZnO nanobowls. Colloids and Surfaces, 2008, A329:184[18] Chiu W S, Khiew P S, Isa D, et al. Synthesis of two-dimensional ZnO nanopellets by pyrolysis of zinc oleate. Chem Eng J, 2008, 142:337 doi: 10.1016/j.cej.2008.04.034[19] Bacsa R, Kihn Y, Verelst M, et al. Large scale synthesis of zinc oxide nanorods by homogeneous chemical vapor deposition and their characterization. Surface Coating Technology, 2007, 201:9200 doi: 10.1016/j.surfcoat.2007.04.037[20] Ozcan S, Can M M, Ceylan A. Single step synthesis of nanocrystalline ZnO via wet-milling. Mater Lett, 2010, 64:2447 doi: 10.1016/j.matlet.2010.08.012[21] Djouadi D, Aksas A, Chelouche A. Elaboration et Caractérisations structurale et optique des nanocristallites toriques de ZnO. Annales de Chimie-Science des Matériaux, 2010, 35(5):255 doi: 10.3166/acsm.35.255-260[22] Zak A K, Abdol Majid W H, Darroudi M, et al. Synthesis and characterization of ZnO nanoparticles prepared in gelatin media. Mater Lett, 2011, 65:70 doi: 10.1016/j.matlet.2010.09.029[23] Li Z, Huang X, Liu J, et al. Morphology control and transition of ZnO nanorod arrays by a simple hydrothermal method. Mater Lett, 2008, 62:1503 doi: 10.1016/j.matlet.2007.09.011[24] He Y, Wang J. A novel simple method to prepare ZnS whiskers. Mater Lett, 2008, 62:1379 doi: 10.1016/j.matlet.2007.08.059[25] Da Costa J P, Girão A V, Lourenço J P, et al. Synthesis of nanocrystalline ZnS using biologically generated sulfide. Hydrometallurgy, 2012, 117/118:57 doi: 10.1016/j.hydromet.2012.02.005[26] Barret C, Massalski T B. Structures of metals:crystallographic methods, principles and data. Oxford:Pergamon Press, 1980[27] Sofiani Z, Derkowska B, Dalasiñski P, et al. Optical properties of ZnO and ZnO:Ce layers grown by spray pyrolysis. Opt Commun, 2006, 267:433 doi: 10.1016/j.optcom.2006.06.049[28] Singhal S, Kaur J, Namgyal T, et al. Cu -doped ZnO nanoparticles:synthesis, structural and electrical properties. Physica B, 2012, 407:1223 doi: 10.1016/j.physb.2012.01.103[29] Ahsanulhaq Q, Umar A, Hahn Y B. Growth of aligned ZnO nanorods and nanopencils on ZnO/Si in aqueous solution:growth mechanism and structural and optical properties. Nanotechnology, 2007, 18:115603 doi: 10.1088/0957-4484/18/11/115603[30] Lv W, Wei B, Xu L, et al. Photocatalytic properties of hierarchical ZnO flowers synthesized by a sucrose-assisted hydrothermal method. Appl Surf Sci, 2012, 259:557 doi: 10.1016/j.apsusc.2012.04.182[31] Kloprogge J T, Wharton D, Hickey L, et al. Infrared and Raman study of interlayer anions (CO3)2-, (NO3)-, (SO4)2- and (ClO4)- in Mg/Al-hydrotalcite. American Mineralogist, 2002, 87:623 doi: 10.2138/am-2002-5-604[32] Frost R L, Carmody O, Erickson K L, et al. Molecular structure of the uranyl mineral uranopilite-a Raman spectroscopic study. Journal of Molecular Structure, 2004, 733(1-3):203 -
Proportional views





 DownLoad:
DownLoad: