| Citation: |
Guodong Yan, Minqiang Wang, Zhi Yang. ZnO/Ag nanowires composite film ultraviolet photoconductive detector[J]. Journal of Semiconductors, 2015, 36(8): 084006. doi: 10.1088/1674-4926/36/8/084006
****
G D Yan, M Q Wang, Z Yang. ZnO/Ag nanowires composite film ultraviolet photoconductive detector[J]. J. Semicond., 2015, 36(8): 084006. doi: 10.1088/1674-4926/36/8/084006.
|
ZnO/Ag nanowires composite film ultraviolet photoconductive detector
DOI: 10.1088/1674-4926/36/8/084006
More Information
-
Abstract
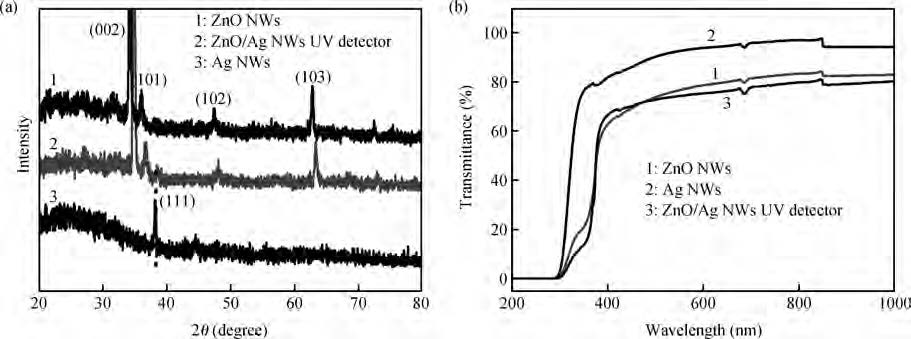
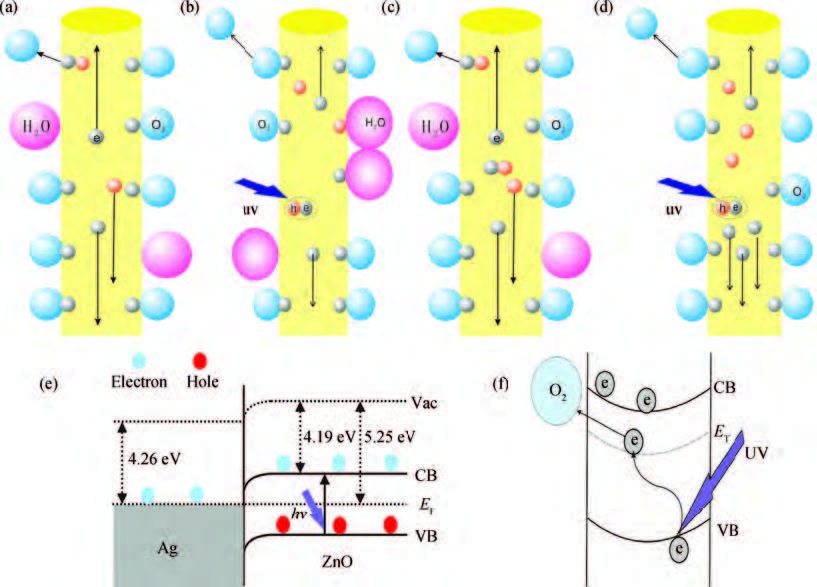
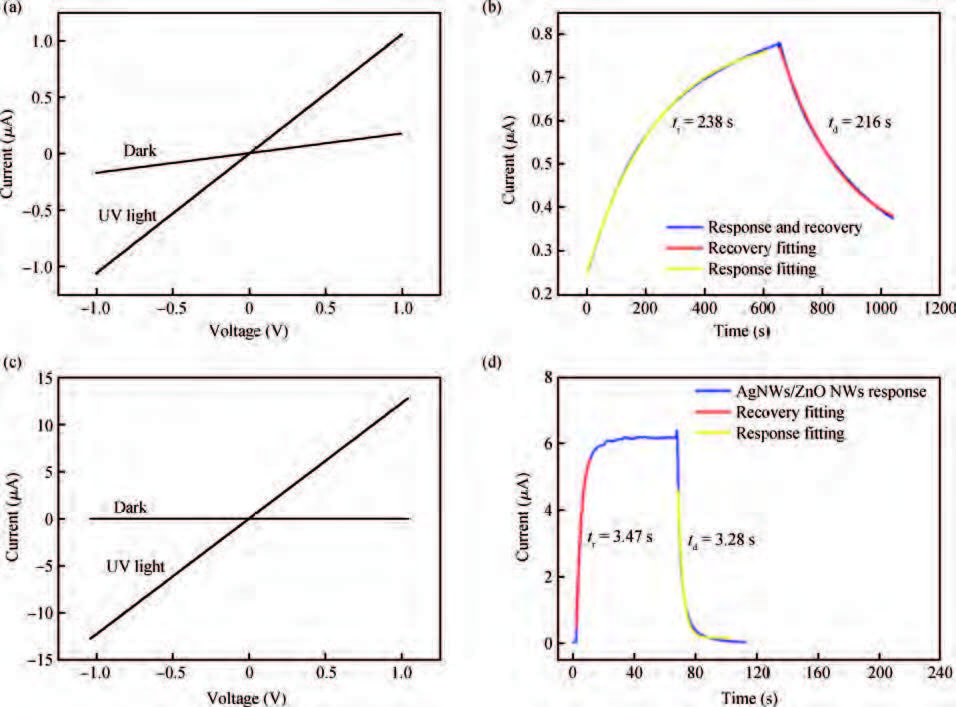
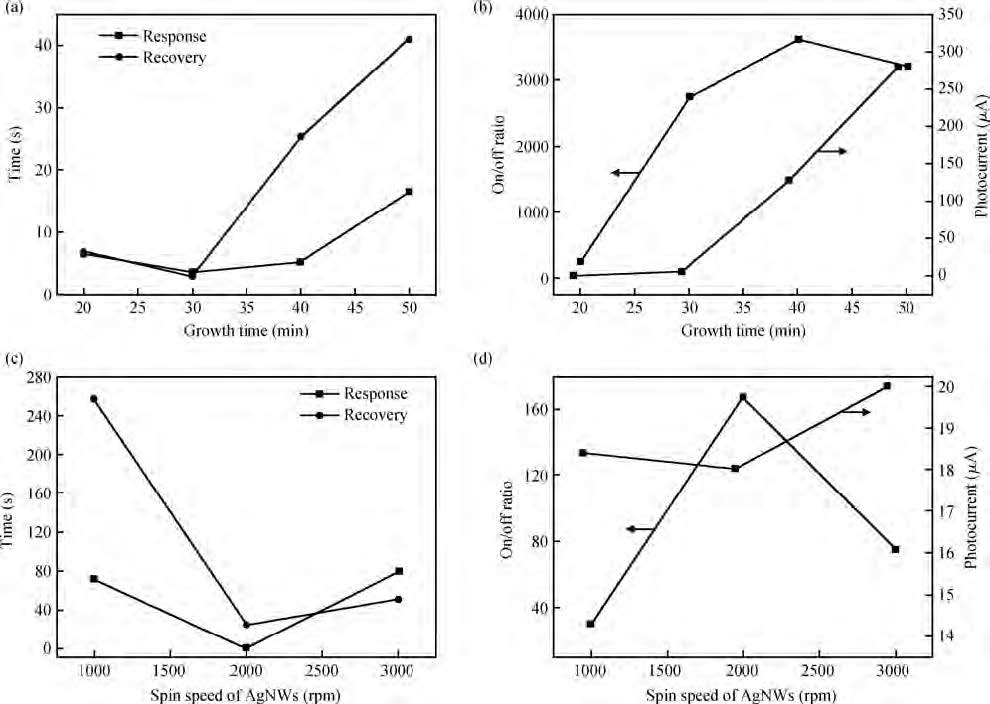
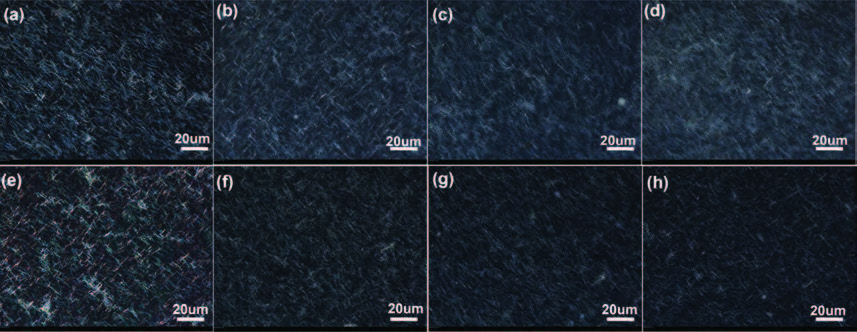
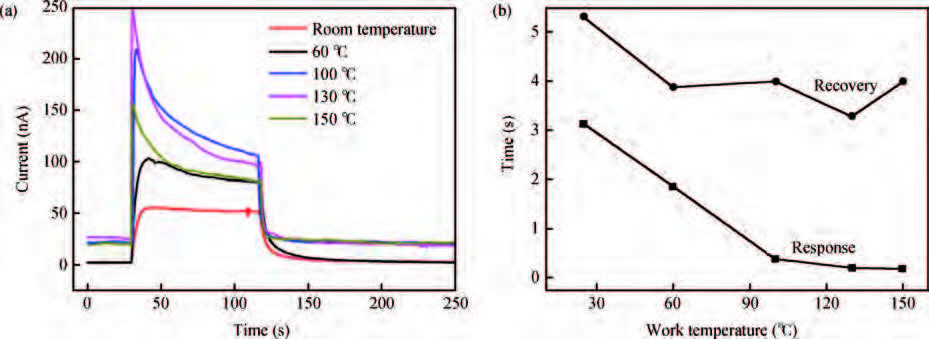
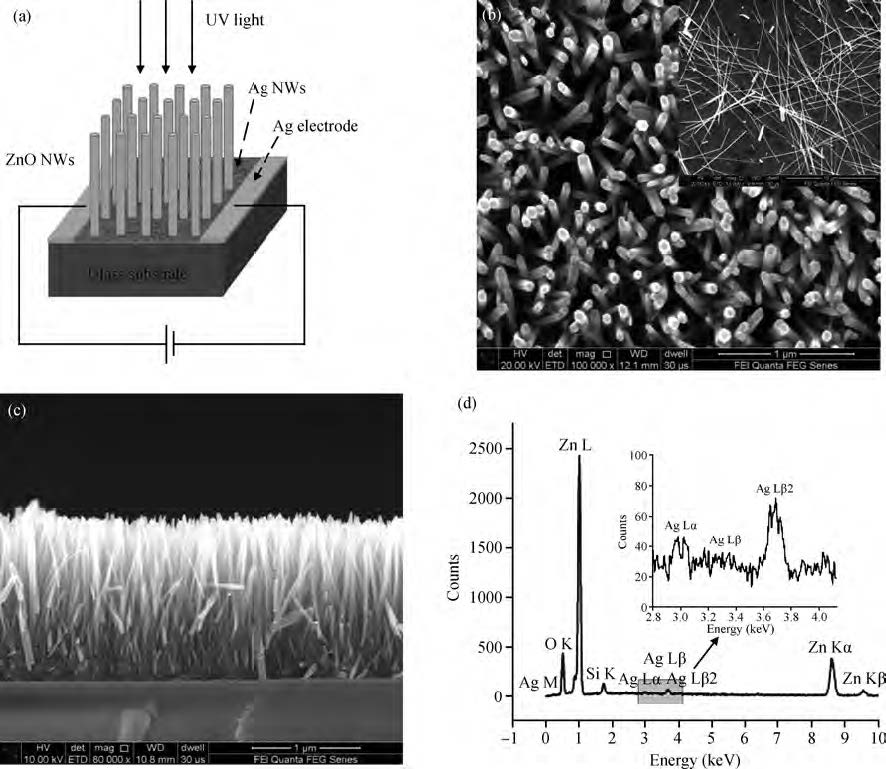
ZnO/Ag nanowires (NWs) film ultraviolet (UV) detector was fabricated by a simple and low-cost solution-processed method. In order to prepare this device, Ag NWs network was first spin-coated on glass substrate as a transparent conducting electrode, then ZnO NWs arrays were grown vertically on the Ag NWs network based on the hydrothermal method. This UV detector exhibited an excellent detection performance with large on/off ratio and short response time. Several process and working parameters were particularly investigated to analyze the relationship between structure and performance, which include growth time of ZnO NWs array, spin speed of Ag NWs network and working temperature. This UV photoconductive detector is based on two kinds of one-dimension nanomaterials, and it was regarded as a compromise between high performance with large area, low voltage and low cost.-
Keywords:
- UV detector,
- ZnO NWs array,
- Ag NWs
-
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] -
Proportional views





 DownLoad:
DownLoad: