| Citation: |
K S Divya, Athulya K Madhu, T U Umadevi, T Suprabha, P. Radhakrishnan Nair, Suresh Mathew. Improving the photocatalytic performance of TiO2 via hybridizing with graphene[J]. Journal of Semiconductors, 2017, 38(6): 063002. doi: 10.1088/1674-4926/38/6/063002
****
K S Divya, A K Madhu, T U Umadevi, T Suprabha, P R Nair, S Mathew. Improving the photocatalytic performance of TiO2 via hybridizing with graphene[J]. J. Semicond., 2017, 38(6): 063002. doi: 10.1088/1674-4926/38/6/063002.
|
Improving the photocatalytic performance of TiO2 via hybridizing with graphene
DOI: 10.1088/1674-4926/38/6/063002
More Information
-
Abstract
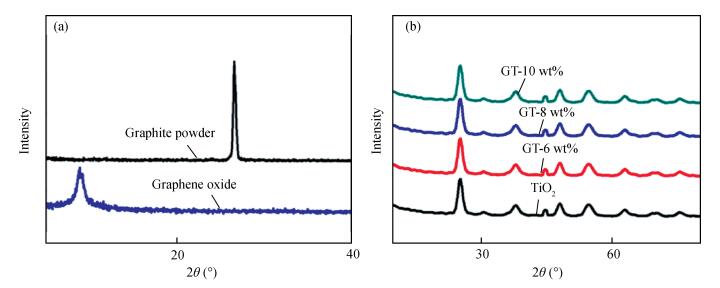
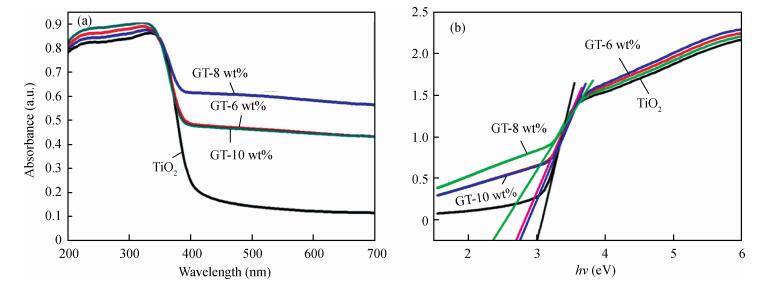
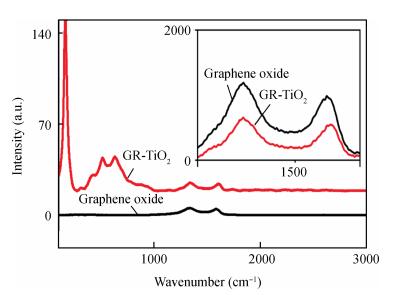
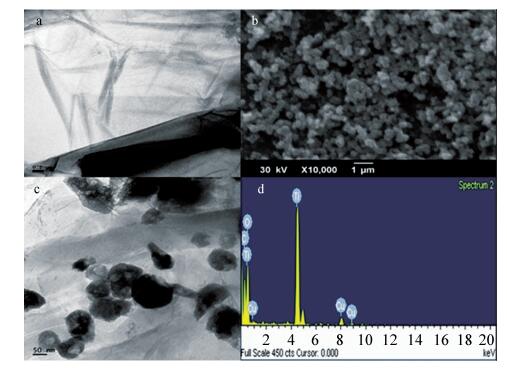
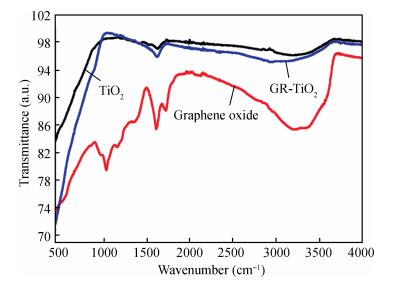
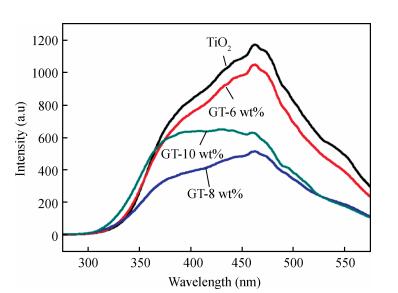
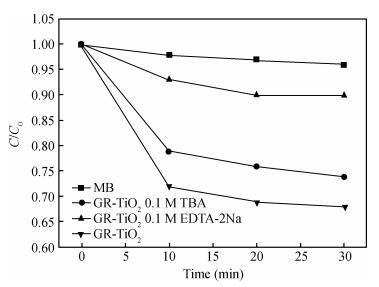
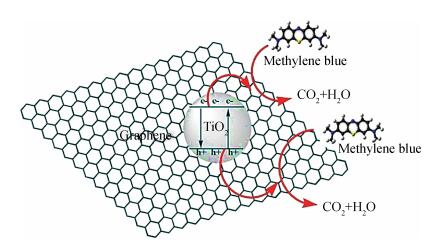
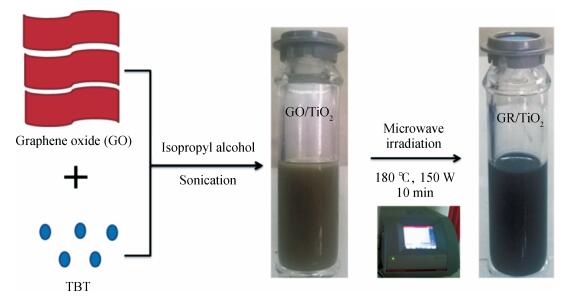
In this paper an improvement in the photocatalytic performance of TiO2 was carried out via hybridizing with graphene. Graphene-TiO2 (GR-TiO2)nanocomposites with different weight addition ratios of graphene oxide (GO) have been prepared via a facile microwave irradiation of GO and tetrabutyl titanate in isopropyl alcohol. Raman spectroscopy (RS), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-visible spectroscopy (UV-vis), Fourier transform infrared spectra (FTIR), energy dispersive X-ray spectroscopy (EDX) and photoluminescence spectra (PL) are employed to determine the properties of the samples. Microwave irradiation can heat the reactant to a higher temperature in a short time, simultaneously GO is reduced to graphene and TiO2 nanoparticles grown on the surface of GR. GR-TiO2 nanocomposites synthesized via this approach have efficient electron conductivity in GR, resulting in a reduced electron-hole recombination rate. Among the synthesized nanocomposites, GT-8wt% exhibited the best photocatalytic activity toward photocatalytic degradation of MB. Our current work provides a new insight for the fabrication of GR-TiO2 nanocomposites within a short reaction time and also explains the mechanism of photocatalysis employing radical and hole scavengers. -
References
[1] Zhang L, Diao S, Nie Y, et al. Photocatalytic patterning and modification of graphene. J Am Chem Soc, 2011, 133:2706 doi: 10.1021/ja109934b[2] Akhavan O, Abdolahad M, Esfandiar A, et al. Photodegradation of graphene oxide sheets by TiO2 nanoparticles after a photocatalytic reduction. J Phys Chem C, 2010, 114:1295 doi: 10.1021/jp103472c?src=recsys[3] Zhang J, Xiong Z, Zhao X. Graphene-meta-oxide composites for the degradation of dyes under visible light irradiation. J Mater Chem, 2011, 21:3634 doi: 10.1039/c0jm03827j[4] Meng X, Geng D, Liu J, et al. Non-aqueous approach to synthesize amorphous/crystalline metal oxide-graphene nanosheet hybrid composites. J Phys Chem C, 2010, 114:18330 doi: 10.1021/jp105852h[5] Deng S, Tjoa V, Fan H M, et al. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J Am Chem Soc, 2012, 134:490 https://www.researchgate.net/publication/221831108_Reduced_Graphene_Oxide_Conjugated_Cu2O_Nanowire_Mesocrystals_for_High-Performance_NO2_Gas_Sensor[6] Chu J, Li X, Qi J. Hydrothermal synthesis of CdS microparticlegraphene hybrid and its optical properties. Cryst Eng Comm, 2012, 14:1881 doi: 10.1039/c1ce06162c[7] Park J H, Kim S, Bard A J. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett, 2006, 6:24 doi: 10.1021/nl051807y[8] Divya K, Uma Devi T, Mahtew S. Graphene-based semiconductor nanocomposites for photocatalytic applications. J Nanosci Lett, 2014, 4:2 doi: 10.1201/b19460-26[9] Chen X, Mao S S. Titanium dioxide nanomaterials:synthesis, properties, modifications, and applications. Chem Rev, 2007, 107:2891 doi: 10.1021/cr0500535[10] Pan X, Zhao Y, Liu S, et al. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl Mater Interfaces, 2012, 4:3944 doi: 10.1021/am300772t[11] Thomas J, Kumar K P, Mathew S. Hydrothermal synthesis of samarium doped nanotitania as highly efficient solar photocatalyst. Sci Adv Mater, 2010, 2:48 https://www.researchgate.net/publication/272272530_Hydrothermal_Synthesis_of_Samarium_Doped_Nanotitania_as_Highly_Efficient_Solar_Photocatalyst[12] Liu S, Yang L, Xu S, et al. Photocatalytic activities of N-doped TiO2 nanotube array/carbon nanorod composite. Electrochem Commun, 2009, 11:174 doi: 10.1016/j.elecom.2008.10.056[13] Ji K H, Jang D M, Cho Y K, et al. Comparative photocatalytic ability of nanocrystal-carbon nanotube and TiO2 nanocrystal hybrid nanostructures. J Phys Chem C, 2009, 113:19966 doi: 10.1021/jp906476m[14] Suprabha T, Roy H G, Mathew S. Gold loaded titania nanostructures——synthesis, characterization and morphology dependence on photocatalysis. Sci Adv Mater, 2010, 2:10 https://www.researchgate.net/publication/233658942_Gold_Loaded_Titania_Nanostructures-Synthesis_Characterization_and_Morphology_Dependence_on_Photocatalysis[15] Yu H, Quan X, Chen S, et al. TiO2-multiwalled carbon nanotube heterojunction arrays and their charge separation capability. J Phys Chem C, 2007, 111:12987 doi: 10.1021/jp0728454[16] Zhang Y, Zhang N, Tang Z R, et al. Improving the photocatalytic performance of graphene-TiO2 nanocomposites via a combined strategy of decreasing defects of graphene and increasing interfacial contact. PCCP, 2012, 14:916 http://pubs.rsc.org/en/content/articlelanding/2012/cp/c2cp41318c/unauth#![17] Dong P, Wang Y, Guo L, et al. A facile one-step solvothermal synthesis of graphene/rod-shaped TiO2 nanocomposite and its improved photocatalytic activity. Nanoscale, 2012, 4:464 http://www.rsc.org/suppdata/nr/c2/c2nr31231j/c2nr31231j.pdf[18] Shah M S A S, Park A R, Zhang K, et al. Green synthesis of biphasic TiO-reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl Mater Interfaces, 2012, 4:3893 doi: 10.1021/am301287m[19] Balandin A A, Ghosh S, Bao W, et al. Superior thermal conductivity of single-layer graphene. Nano Lett, 2008, 8:90 doi: 10.1021/nl0731872[20] Stoller M D, Park S, Zhu Y, et al. Graphene-based ultracapacitors. Nano Lett, 2008, 8:3498 doi: 10.1021/nl802558y[21] Nair R R, Blake P, Grigorenko A N, et al. Fine structure constant defines visual transparency of graphene. Science, 2008, 320:1308 doi: 10.1126/science.1156965[22] Lee C, Wei X, Kysar J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 2008, 321:385 doi: 10.1126/science.1157996[23] Guo S, Dong D. Graphene nanosheet:synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem Soc Rev, 2011, 40:2644 doi: 10.1039/c0cs00079e[24] Zhang L, Hao W, Wang H, et al. Porous graphene frame supported silicon@graphitic carbon via in situ solid-state synthesis for high-performance lithium-ion anodes. J Mater Chem A, 2013, 1:760 https://www.researchgate.net/publication/255773845_Porous_graphene_frame_supported_silicongraphitic_carbon_via_in_situ_solid-state_synthesis_for_high-performance_lithium-ion_anodes[25] Anandan S, Rao T N, Sathish M, et al. Superhydrophilic graphene-loaded TiO2 thin film for self-cleaning applications. ACS Appl Mater Interfaces, 2012, 5:207 https://www.researchgate.net/publication/233929278_Superhydrophilic_Graphene-Loaded_TiO2_Thin_Film_for_Self-Cleaning_Applications[26] Xin X, Zhou X, Wu J, et al. Scalable synthesis of TiO2/graphene nanostructured composite with high-rate performance for lithium ion batteries. ACS Nano, 2012, 6:11035 doi: 10.1021/nn304725m[27] Sun J, Zhang H, Guo L H, et al. Two-dimensional interface engineering of a titani-graphene nanosheet composite for improved photocatalytic activity. ACS Appl Mater Interfaces, 2013, 5:13035 doi: 10.1021/am403937y[28] Moon G H, Kim D H, Kim H I, et al. Platinum-like behavior of reduced graphene oxide as a cocatalyst on TiO2 for the efficient photocatalytic oxidation of arsenite. Environ Sci Technol Lett, 2014, 1:185 doi: 10.1021/ez5000012[29] Gu L, Wang J, Cheng H, et al. One-step preparation of graphenesupported anatase TiO2 with exposed {00}facets and mechanism of enhanced photocatalytic properties. ACS Appl Mater Interfaces, 2013, 5:308 doi: 10.1021/am303274t?src=recsys[30] Liu B, Huang Y, Wen Y, et al. Highly dispersive {00} facetsexposed nanocrystalline TiO2 on high quality graphene as a high performance photocatalyst. J Mater Chem, 2012, 22:7484 doi: 10.1039/c2jm16114a[31] Zhang Y, Tang Z R, Fu X, et al. TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant:is TiO2-graphene truly different from other TiO2-carbon composite materials. ACS Nano, 2010, 4:7303 doi: 10.1021/nn1024219[32] Wang Z, Huang B, Dai Y, et al. Crystal facets controlled synthesis of graphene@TiO2 nanocomposites by a one-pot hydrothermal process. Cryst Eng Comm, 2012, 14:168 http://pubs.rsc.org/en/content/articlelanding/2012/ce/c1ce06193c/unauth#!divAbstract[33] Yan W, He F, Gai S, et al. A novel 3D structured reduced graphene oxide/TiO2 composite:synthesis and photocatalytic performance. J Mater Chem A, 2014, 2:360 doi: 10.1039/C3TA13584E[34] Liu X, Pan L, Lv T, et al. Microwave-assisted synthesis of TiO 2-reduced graphene oxide composites for the photocatalytic reduction of Cr (Ⅵ). RSC Adv, 2011, 1:124 http://pubs.rsc.org/en/content/articlelanding/2012/ce/c1ce06193c/unauth#!divAbstract[35] Bilecka I, Niederberger M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale, 2010, 2:135 https://www.researchgate.net/publication/46282847_Microwave_Chemistry_for_Inorganic_Nanomaterials_Synthesis[36] Baghbanzadeh M, Carbone L, Cozzoli P D, et al. Microwaveassisted synthesis of colloidal inorganic nanocrystals. Angew Chem Int Ed, 2011, 50:11312 doi: 10.1002/anie.v50.48[37] Hummers W S Jr, Offeman R E. Preparation of graphitic oxide. J Am Chem Soc, 1958, 80:1339 doi: 10.1021/ja01539a017[38] Che J, Shen L, Xiao Y. A new approach to fabricate graphene nanosheets in organic medium:combination of reduction and dispersion. J Mater Chem, 2010, 20:1722 doi: 10.1039/b922667b[39] Xu Y J, Zhuang Y, Fu X. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes:a case study on degradation of benzene and methyl orange. J Phys Chem C, 2010, 114:2669 doi: 10.1021/jp909855p[40] Yu J, Ma T, Liu S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. PCCP, 2011, 13:349 doi: 10.1039/c0cp90149k[41] Serpone N, Lawless D, Khairutdinov R. Size effects on the photophysical properties of colloidal anatase TiO2 particles:size quantization versus direct transitions in this indirect semiconductor. J Phys Chem, 1995, 99:1664 https://www.researchgate.net/publication/231396119_Size_Effects_on_the_Photophysical_Properties_of_Colloidal_Anatase_TiO2_Particles_Size_Quantization_Versus_Direct_Transitions_in_This_Indirect_Semiconductor[42] Kudin K N, Ozbas B, Schniepp H C, et al. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett, 2008, 8:36 doi: 10.1021/nl071822y[43] Yu J, Ma T, Liu G, et al. Enhanced photocatalytic activity of bimodal mesoporous titania powders by C60 modification. Dalton Trans, 2011, 40:663 https://www.researchgate.net/publication/51107858_Enhanced_photocatalytic_activity_of_bimodal_mesoporous_titania_powders_by_C-60_modification[44] Zhang X Y, Li H P, Cui X L, et al. Graphene/TiO2 nanocomposites:synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J Mater Chem, 2010, 20:2801 doi: 10.1039/b917240h[45] Jiang B, Tian C, Pan Q, et al. Enhanced photocatalytic activity and electron transfer mechanisms of graphene/TiO2 with exposed {00} facets. J Phys Chem C, 2011, 115:23718 doi: 10.1021/jp207624x[46] Yeh T F, Syu J M, Cheng C T, et al. Graphite oxide as a photocatalyst for hydrogen production from water. Adv Funct Mater, 2010, 20:2255 doi: 10.1002/adfm.v20:14[47] Tang F Q, Hou L P, Guo G S. Preparation of TiO2 nanometer powders. J Inorgan Mater, 2001, 16:615[48] Yu J C, Yu J, Ho W, et al. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem Mater, 2002, 14:3808 doi: 10.1021/cm020027c[49] Tu W, Zhou Y, Liu Q, et al. An in situ simultaneous reductionhydrolysis technique for fabrication of TiO2-graphene 2D sandwich-like hybrid nanosheets:graphene-promoted selectivity of photocatalytic-driven hydrogenation and coupling of CO2 into methane and ethane. Adv Funct Mater, 2013, 23:1743 doi: 10.1002/adfm.v23.14[50] Zhou K, Zhu Y, Yang X, et al. Preparation of graphene-TiO2 composites with enhanced photocatalytic activity. New J Chem, 2011, 35:35 https://www.researchgate.net/publication/255750422_Preparation_of_Graphene-TiO2_Composites_With_Enhanced_Photocatalytic_Activity[51] Zhang W, Zhang M, Yin Z, et al. Photoluminescence in anatase titanium dioxide nanocrystals. Appl Phys B, 2000, 70:26 https://www.researchgate.net/publication/226120273_Photoluminescence_in_Anatase_Titanium_Dioxide_Nanocrystals[52] Williams G, Seger B, Kamat P V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano, 2008, 2:1487 doi: 10.1021/nn800251f?src=recsys[53] Houas A, Lachheb H, Ksibi M, et al. Photocatalytic degradation pathway of methylene blue in water. Appl Catal B, 2001, 31:14 https://www.researchgate.net/publication/236880306_Photocatalytic_Degradation_Pathway_of_Methylene_Blue_in_Water -
Proportional views





 DownLoad:
DownLoad: