| Citation: |
R. Jayakrishnan, Varun G Nair, Akhil M Anand, Meera Venugopal. Gas selectivity of SILAR grown CdS nano-bulk junction[J]. Journal of Semiconductors, 2018, 39(3): 033002. doi: 10.1088/1674-4926/39/3/033002
****
R Jayakrishnan, V G Nair, A M Anand, M Venugopal, Gas selectivity of SILAR grown CdS nano-bulk junction[J]. J. Semicond., 2018, 39(3): 033002. doi: 10.1088/1674-4926/39/3/033002.
|
Gas selectivity of SILAR grown CdS nano-bulk junction
DOI: 10.1088/1674-4926/39/3/033002
More Information
-
Abstract
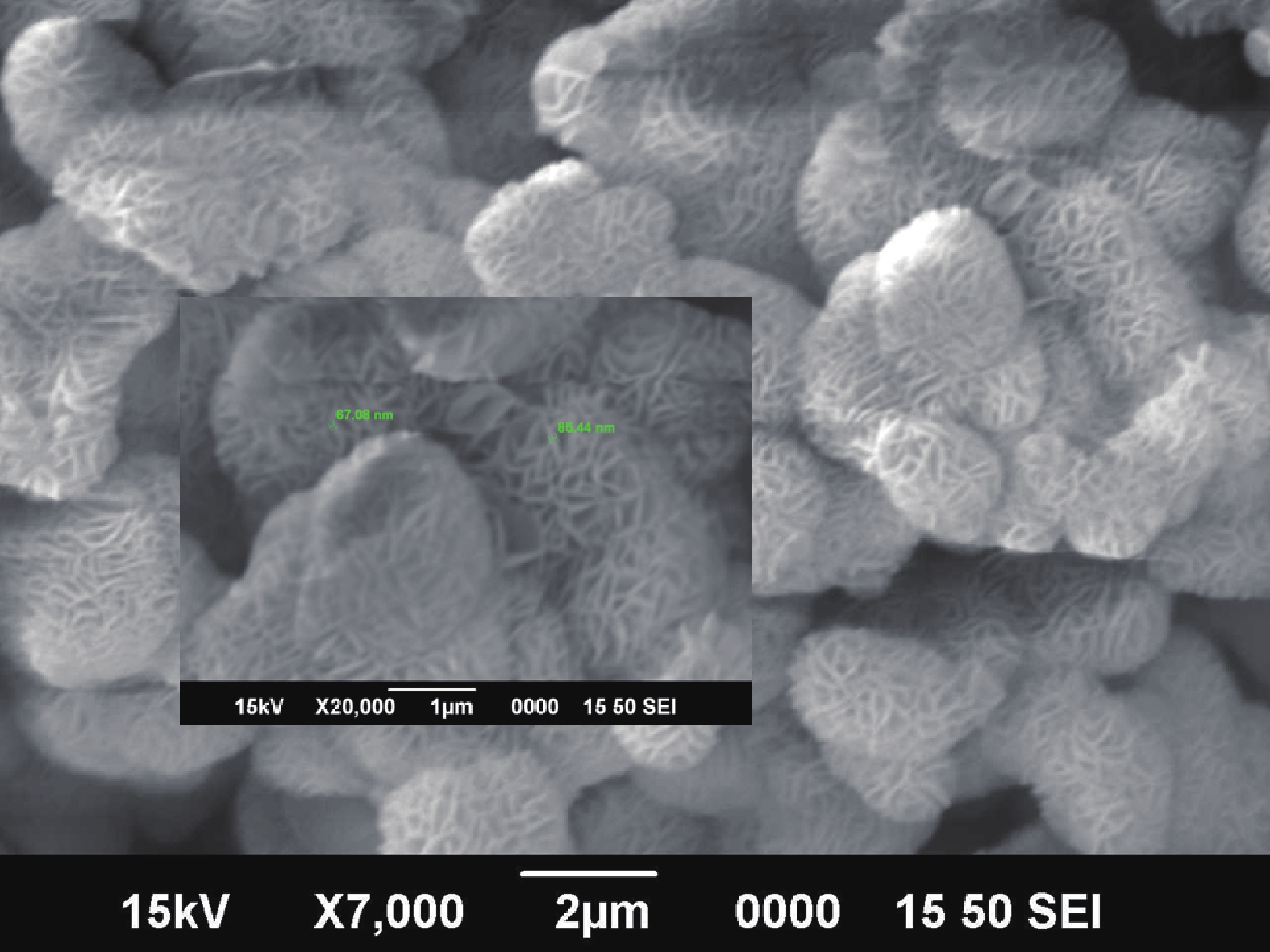
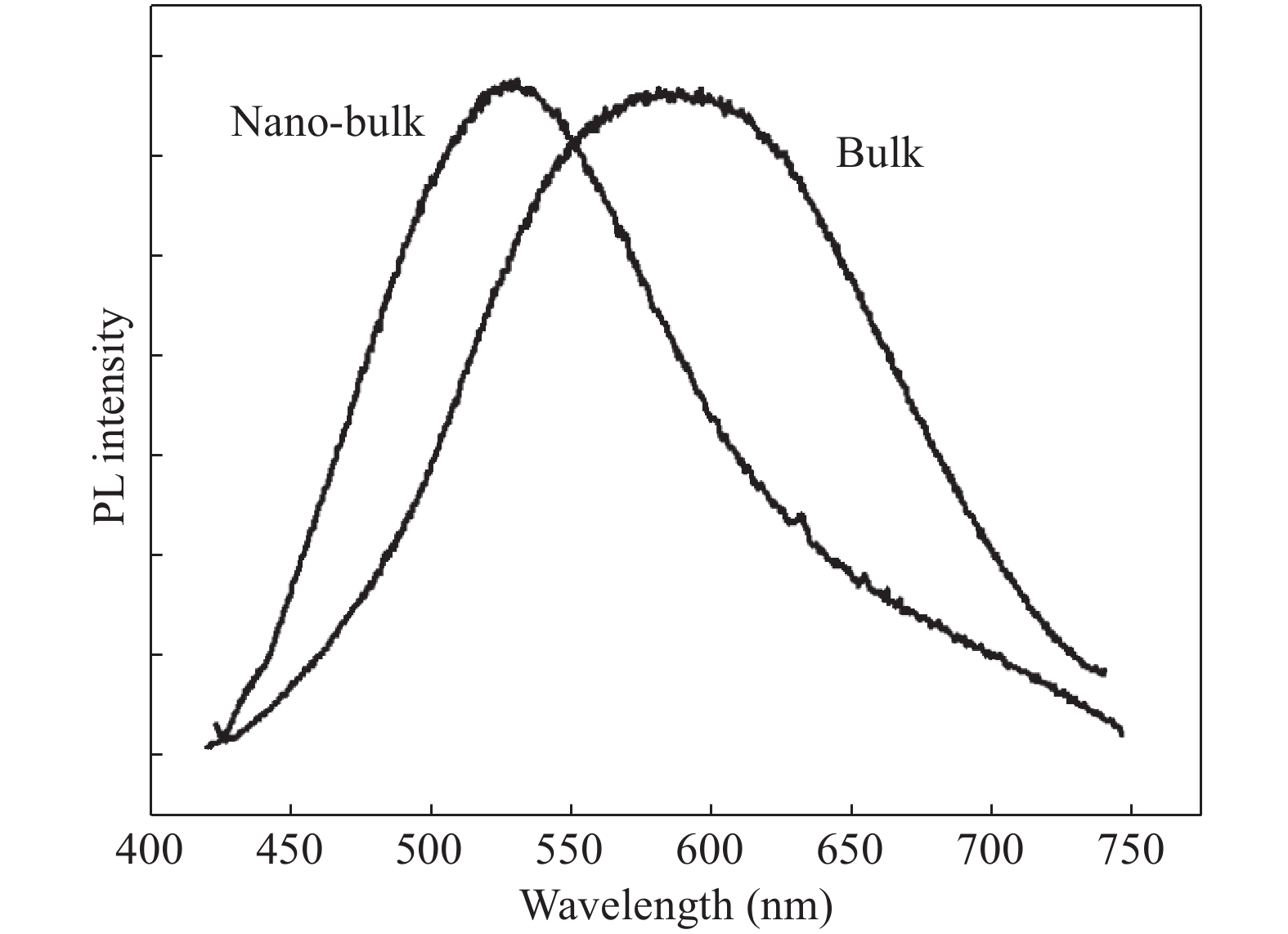
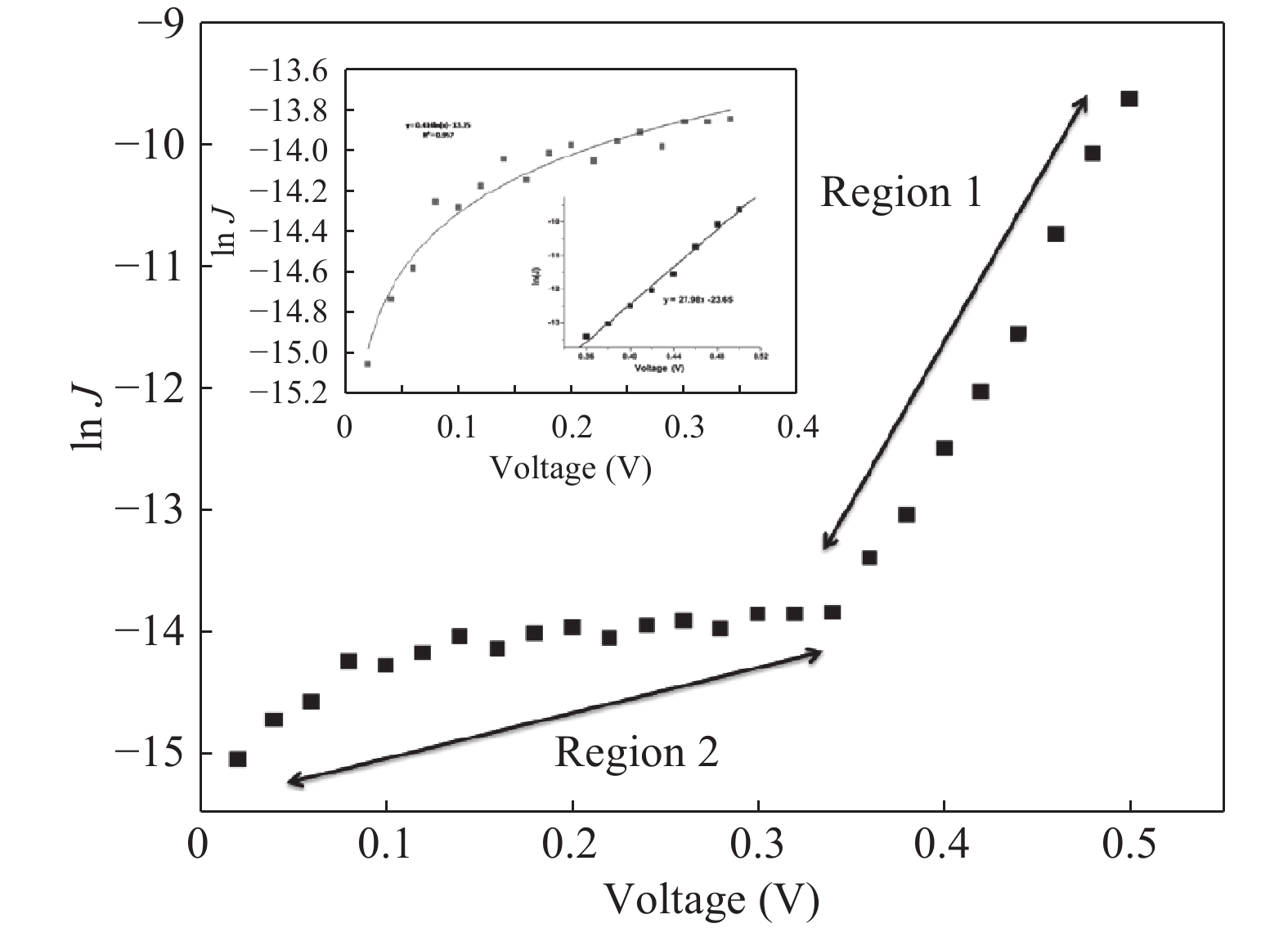
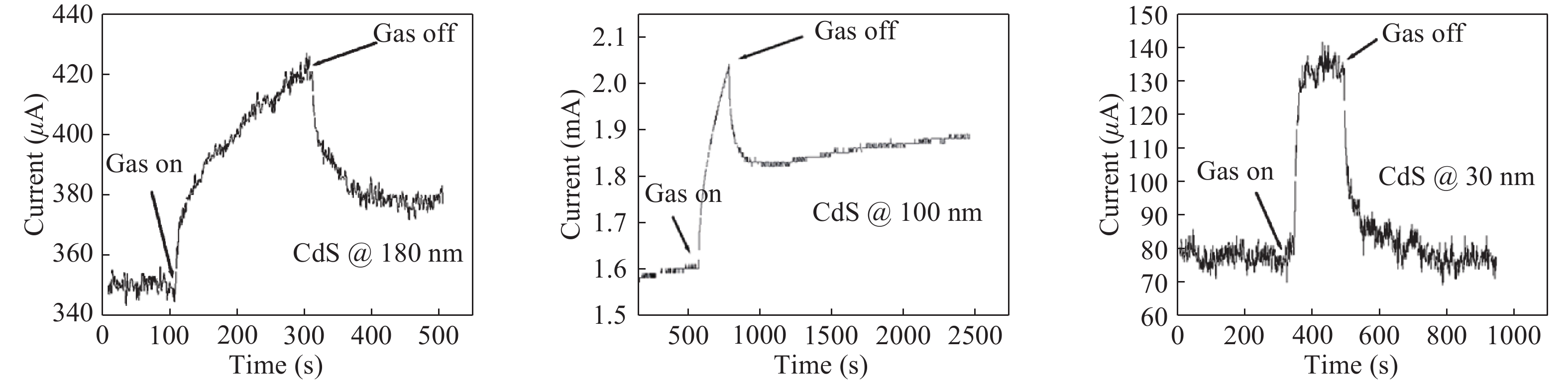
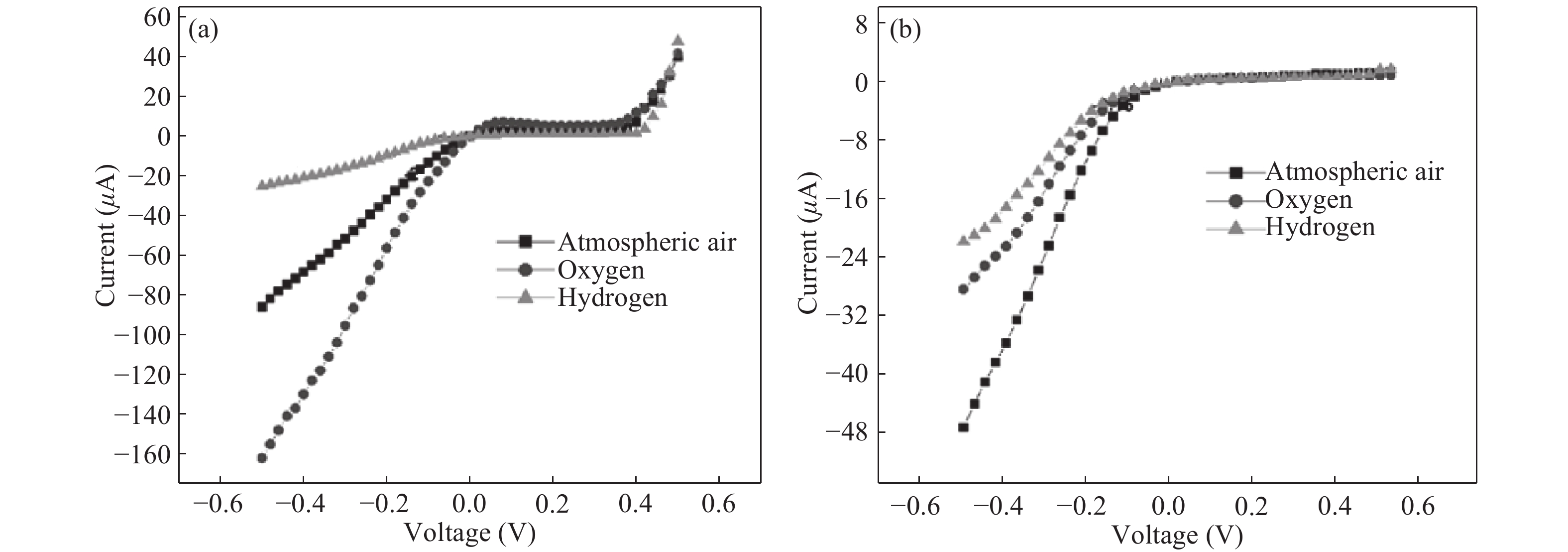
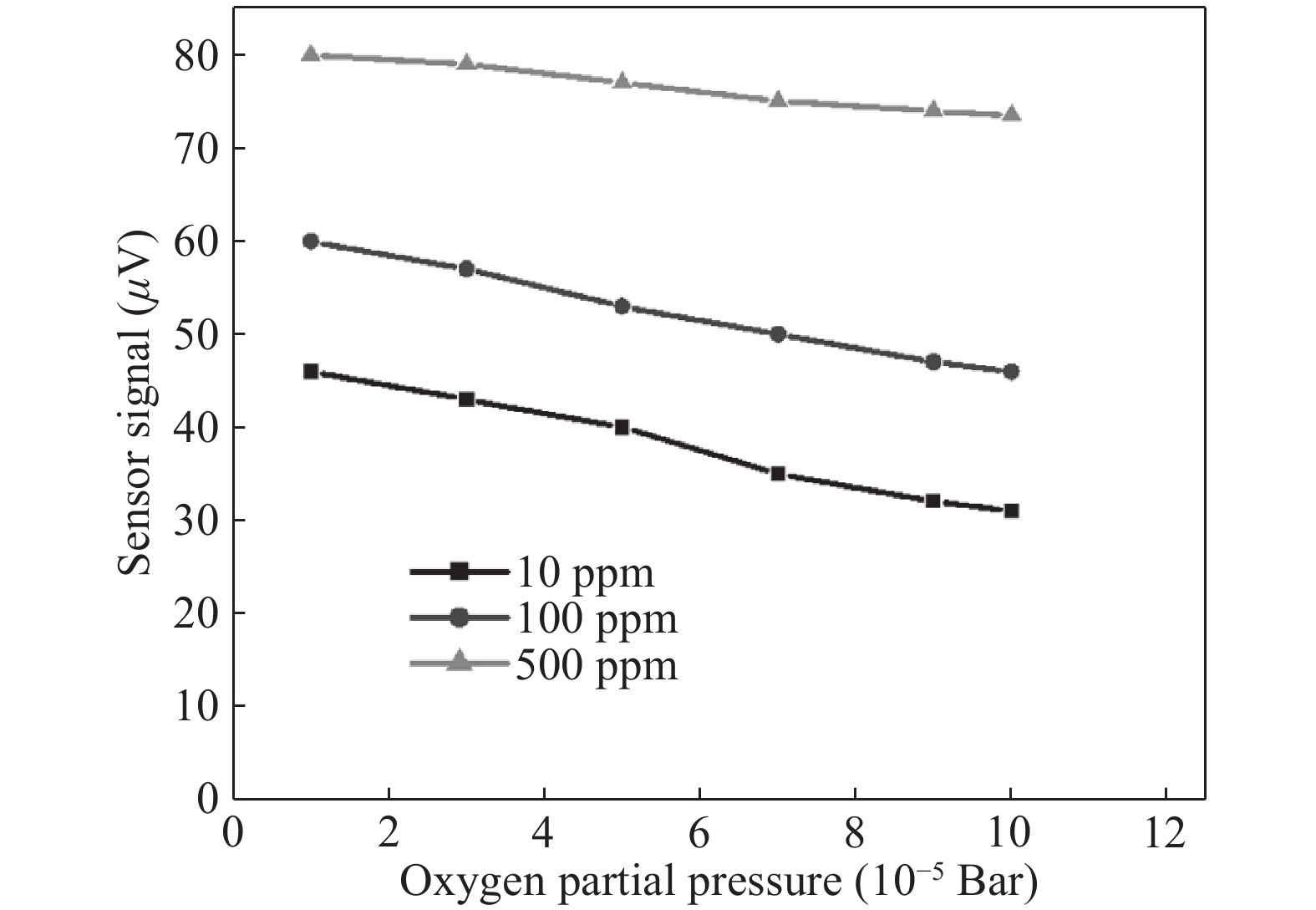
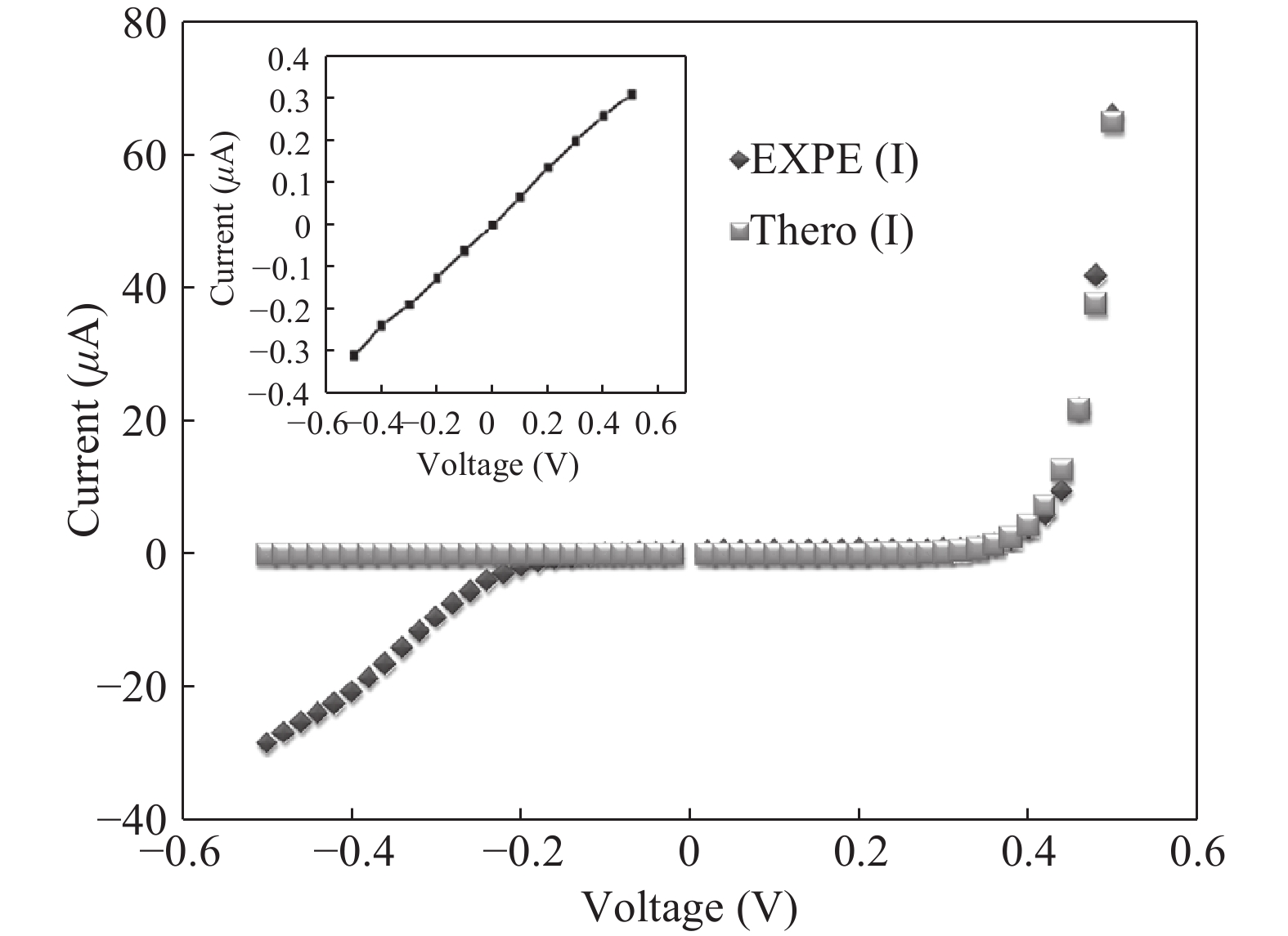
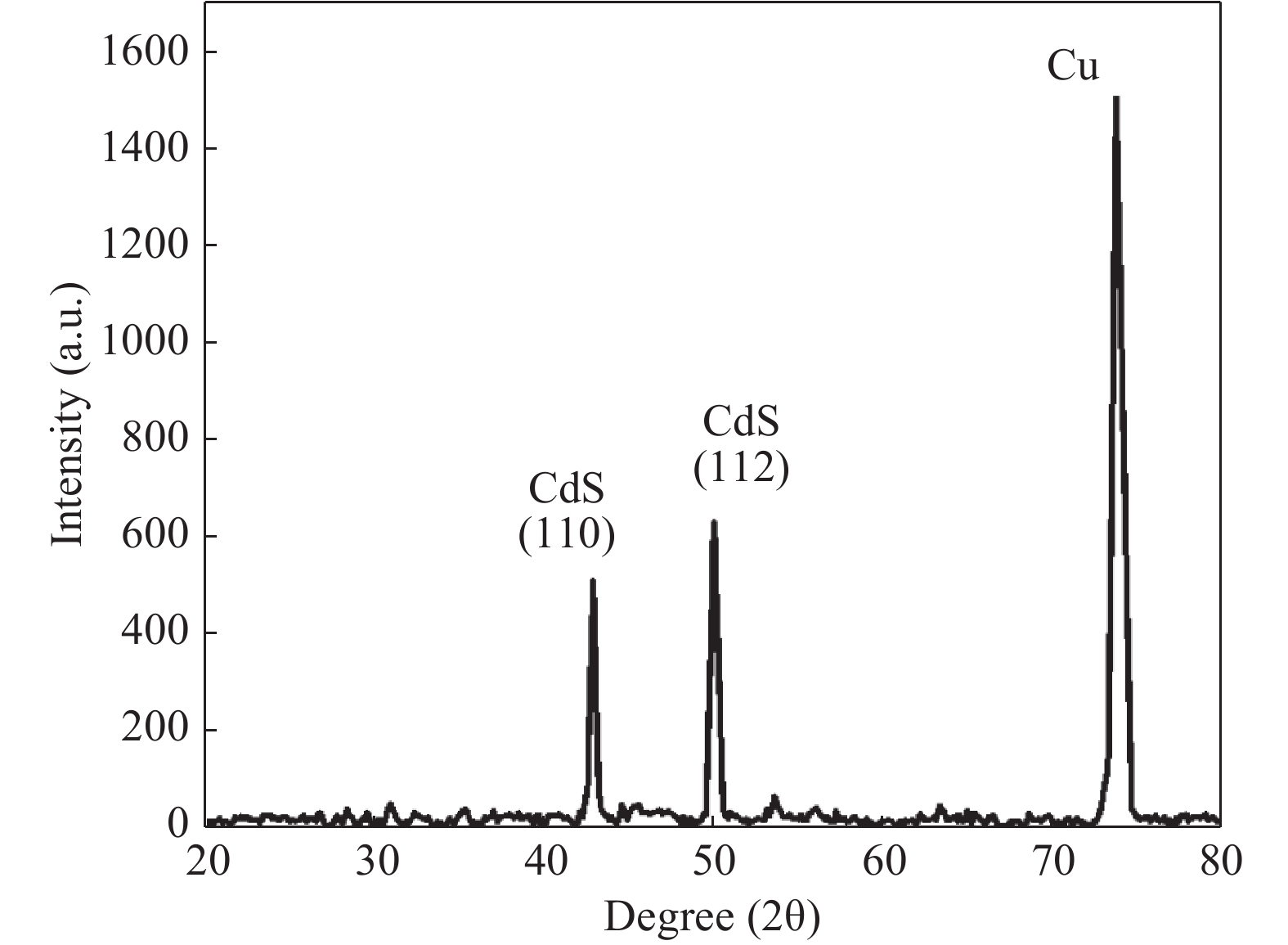
Nano-particles of cadmium sulphide were deposited on cleaned copper substrate by an automated sequential ionic layer adsorption reaction (SILAR) system. The grown nano-bulk junction exhibits Schottky diode behavior. The response of the nano-bulk junction was investigated under oxygen and hydrogen atmospheric conditions. The gas response ratio was found to be 198% for Oxygen and 34% for Hydrogen at room temperature. An increase in the operating temperature of the nano-bulk junction resulted in a decrease in their gas response ratio. A logarithmic dependence on the oxygen partial pressure to the junction response was observed, indicating a Temkin isothermal behavior. Work function measurements using a Kelvin probe demonstrate that the exposure to an oxygen atmosphere fails to effectively separate the charges due to the built-in electric field at the interface. Based on the benefits like simple structure, ease of fabrication and response ratio the studied device is a promising candidate for gas detection applications. -
References
[1] Azulay D, Millo O, Silbert S, et al. Where does photocurrent flow in polycrystalline CdS. Appl Phys Lett, 2005, 86: 212102 doi: 10.1063/1.1923157[2] Grus M, Sikorska A. Characterization of the absorption edge in crystalline CdS:Cu powder by use of photoacoustic and reflection spectroscopy. Physica B, 1999, 266: 139 doi: 10.1016/S0921-4526(98)01290-3[3] Rakhshani A E. Study of Urbach tail, bandgap energy and grain-boundary characteristics in CdS by modulated photocurrent spectroscopy. J Phys: Condens Matter, 2000, 12: 4391 doi: 10.1088/0953-8984/12/19/309[4] Kokaj J, Rakhshani A E. CdS thin film transistor for inverter and operational amplifier circuit. J Phys D, 2004, 37: 1970 doi: 10.1088/0022-3727/37/14/012[5] Kadam A N, Dhabbe R S, Kokate M R, et al. Room temperature synthesis of CdS nanoflakes for photocatalytic properties. J Mater Sci: Mater Electron, 2014, 25: 1887 doi: 10.1007/s10854-014-1816-3[6] Jayakrishnan R. Negative resistance in Cu2O/In2S3 heterostructure. Mater Chem Phys, 2015, 162: 542 doi: 10.1016/j.matchemphys.2015.06.025[7] Giberti A, Gaiardo A, Fabbri B, et al. Metal sulfides as sensing materials for chemoresistive gas sensors. Sens Actuators B, 2016, 223: 827 doi: 10.1016/j.snb.2015.10.007[8] Kim S, Park S, Park S, et al. Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sens Actuators B, 2015, 209: 180 doi: 10.1016/j.snb.2014.11.106[9] Dumbrava A, Badea C, Prodan G, et al. Zinc sulphide fine particles obtained at low temperature. Chalcogenide Lett, 2009, 6: 437[10] Wang Y, Herron N. Nanometer-sized semiconductor clusters: materials synthesis, quantum size effects, and photophysical properties. J Phys Chem, 1991, 95: 525 doi: 10.1021/j100155a009[11] Eranna G, Joshi B C, Runthala D P, et al. Oxide materials for development of integrated gas sensors—a comprehensive review. Crit Rev Solid State Mater Sci, 2004, 29: 111 doi: 10.1080/10408430490888977[12] Jayakrishnan R, Kurian A S, Nair V G, et al. Effect of vacuum annealing on the photoconductivity of CuO thin films grown using sequential ionic layer adsorption reaction. Mater Chem Phys, 2016, 180: 149 doi: 10.1016/j.matchemphys.2016.05.055[13] Shafiei M, Sadek A, Yu J, et al. Pt/WO3 nanoplatelet/SiC Schottky diode based hydrogen gas sensor. Sens Lett, 2011, 9: 11 doi: 10.1166/sl.2011.1409[14] Hu Y, Zhou X, Han Q, et al. Sensing properties of CuO–ZnO heterojunction gas sensors. Mater Sci Eng B, 2003, 99: 41 doi: 10.1016/S0921-5107(02)00446-4[15] Yoon D H, Yu J H, Choi G M. CO gas sensing properties of ZnO–CuO composite. Sens Actuator B, 1998, 46: 15 doi: 10.1016/S0925-4005(97)00317-1[16] Zhu C L, Chen Y J, Wang R X, et al. SnO2 surfactant composite films for superior gas sensitivity. Sens Actuator B, 2009, 140: 185 doi: 10.1016/j.snb.2009.04.011[17] Fergus J W. Perovskite oxides for semiconductor-based gas sensors. Sens Actuator B, 2007, 123: 1169 doi: 10.1016/j.snb.2006.10.051[18] Korotcenkov G. Metal oxides for solid-state gas sensors: what determines our choice. Mater Sci Eng B, 2007, 139: 1 doi: 10.1016/j.mseb.2007.01.044[19] Barsan N, Schweizer-Berberich M, Fresenius G W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: a status report. J Anal Chem, 1999, 365: 287 doi: 10.1007/s002160051490[20] Chopra K L. Thin film phenomena. New York: MC Graw Hill Co., 1969[21] Weaver J M R, Abraham D W. High resolution atomic force microscopy potentiometry. J Vac Sci Technol B, 1991, 9: 1559 doi: 10.1116/1.585423[22] Nonnenmacher M, O’Boyle M P, Wickeramasinghe H K. Kelvin probe force microscopy. Appl Phys Lett, 1991, 58: 2921 doi: 10.1063/1.105227[23] Scherrer P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Mathematisch-Physikalische Klasse, 1918, 2: 98[24] Rau U, Schock H W. Electronic properties of Cu(In,Ga)Se2 heterojunction solar cells—recent achievements, current understanding, and future challenges. Appl Phys A, 1999, 69: 131 doi: 10.1007/s003390050984[25] Clark V A. The theory of adsorption and catalysis. New York: Academic, 1970[26] Rhoderick E. Metal–semiconductor contacts. IEE Proc I, 1982, 129: 1 -
Proportional views





 DownLoad:
DownLoad: