| Citation: |
Lei Fu, Yuling Liu, Chenwei Wang, Linan Han. Effect of 1,2,4-triazole on galvanic corrosion between cobalt and copper in CMP based alkaline slurry[J]. Journal of Semiconductors, 2018, 39(4): 046001. doi: 10.1088/1674-4926/39/4/046001
****
L Fu, Y L Liu, C W Wang, L N Han. Effect of 1,2,4-triazole on galvanic corrosion between cobalt and copper in CMP based alkaline slurry[J]. J. Semicond., 2018, 39(4): 046001. doi: 10.1088/1674-4926/39/4/046001.
|
Effect of 1,2,4-triazole on galvanic corrosion between cobalt and copper in CMP based alkaline slurry
DOI: 10.1088/1674-4926/39/4/046001
More Information
-
Abstract
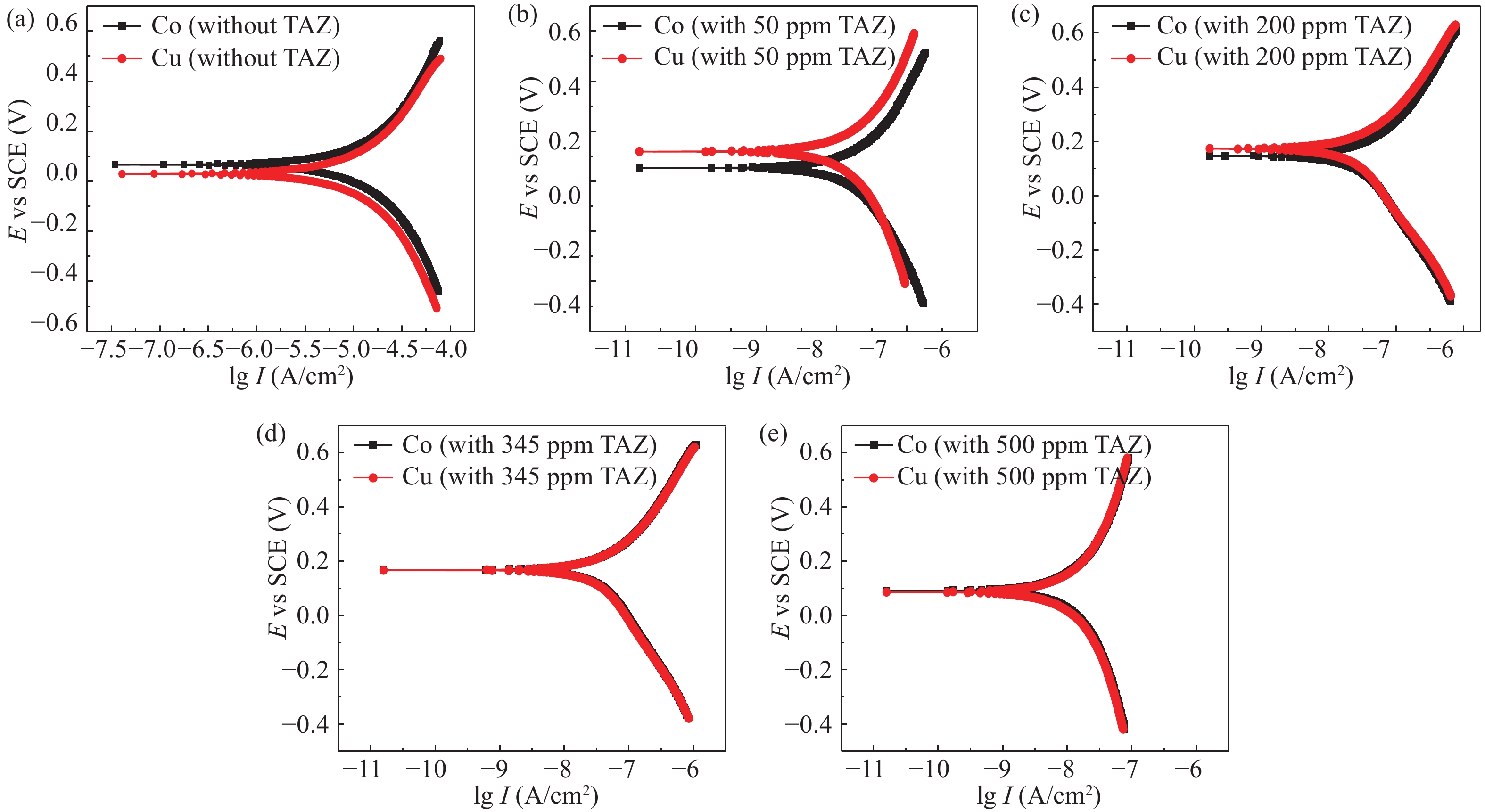
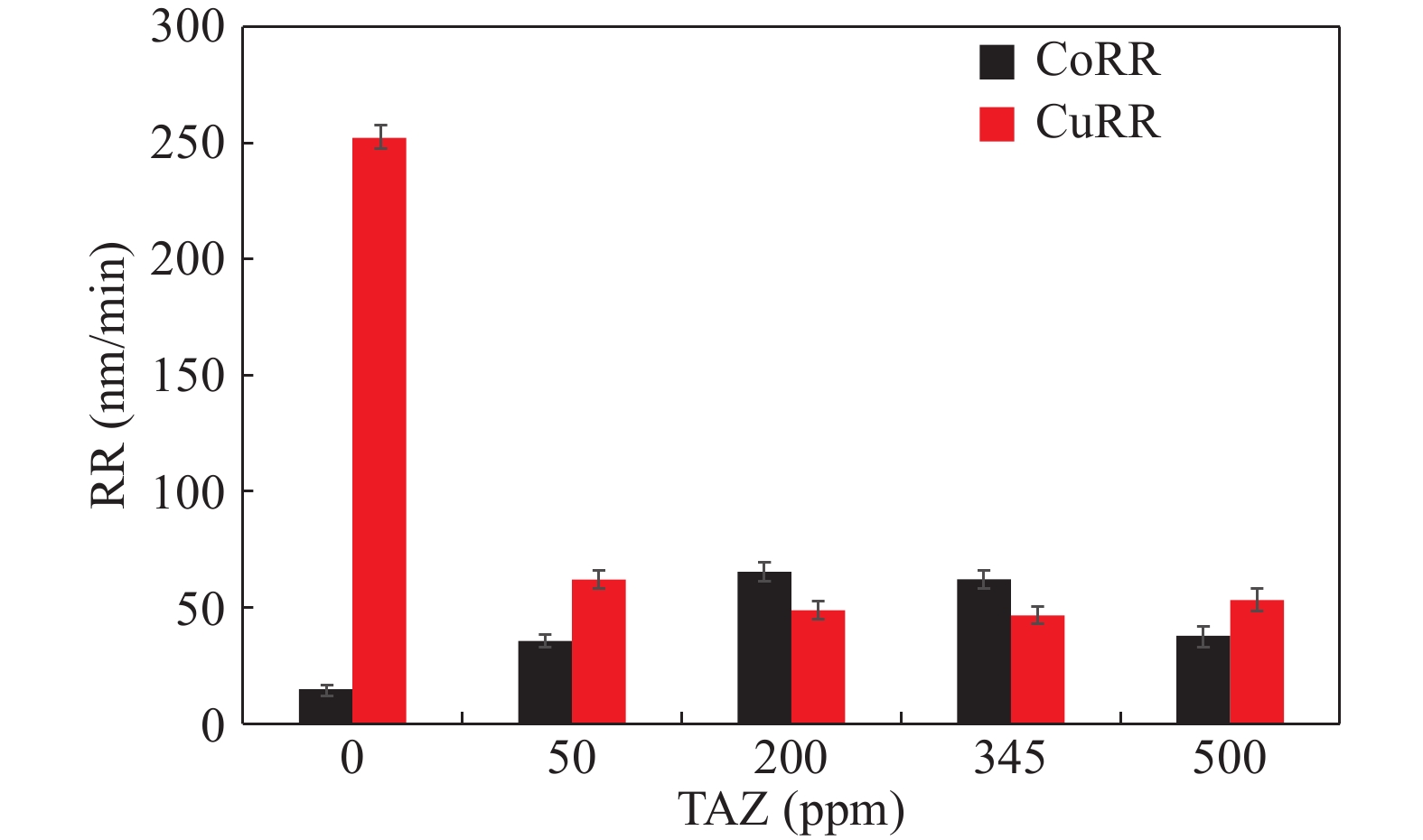
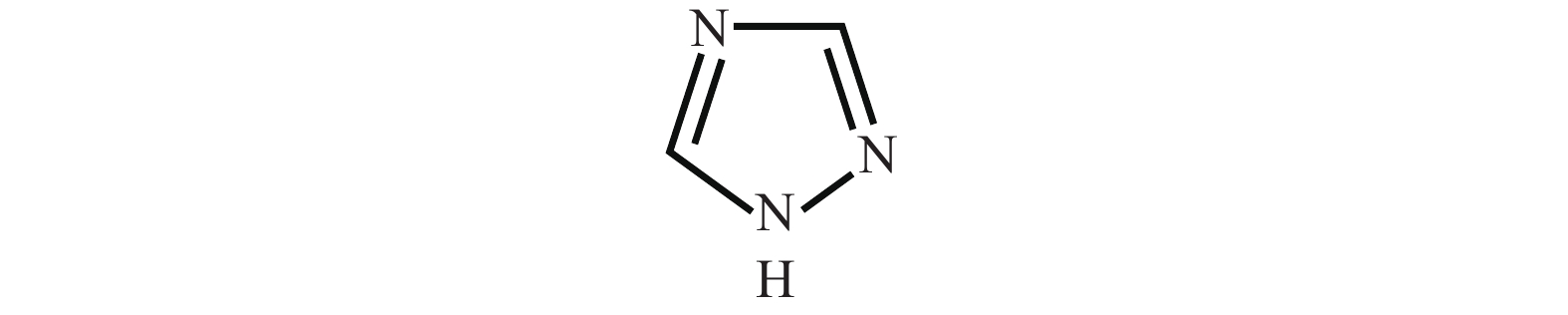
Cobalt has become a new type of barrier material with its unique advantages since the copper-interconnects in the great-large scale integrated circuits (GLSI) into 10 nm and below technical nodes, but cobalt and copper have severe galvanic corrosion during chemical–mechanical flattening. The effect of 1,2,4-triazole on Co/Cu galvanic corrosion in alkaline slurry and the control of rate selectivity of copper and cobalt were investigated in this work. The results of electrochemical experiments and polishing experiments had indicated that a certain concentration of 1,2,4-triazole could form a layer of insoluble and dense passive film on the surface of cobalt and copper, which reduced the corrosion potential difference between cobalt and copper. Meantime, the removal rate of cobalt and copper could be effectively controlled according to demand during the CMP process. When the study optimized slurry was composed of 0.5 wt% colloidal silica, 0.1 %vol. hydrogen peroxide, 0.05 wt% FA/O, 345 ppm 1,2,4-triazole, cobalt had higher corrosion potential than copper and the galvanic corrosion could be reduced effectively when the corrosion potential difference between them decreased to 1 mV and the galvanic corrosion current density reached 0.02 nA/cm2. Meanwhile, the removal rate of Co was 62.396 nm/min, the removal rate of Cu was 47.328 nm/min, so that the removal rate ratio of cobalt and copper was 1.32 : 1, which was a good amendment to the dishing pits. The contact potential corrosion of Co/Cu was very weak, which could be better for meeting the requirements of the barrier CMP.-
Keywords:
- cobalt,
- 1,2,4-triazole,
- galvanic corrosion,
- alkaline polishing slurry,
- CMP
-
References
[1] Roule A, Amuntencei M, Deronzier E, et al. Seed layer enhancement by electrochemical deposition: The copper seed solution for beyond 45 nm. Microelectron Eng, 2007, 84(11): 2610 doi: 10.1016/j.mee.2007.06.014[2] Tanwar K, Canaperi D, Lofaro M, et al. BEOL Cu CMP process evaluation for advanced technology nodes. J Electrochem Soc, 2013, 160(12): 3247 doi: 10.1149/2.042312jes[3] Nakano H, Itabashi T, Akahoshi H. Electroless deposited cobalt-tungsten-boron capping barrier metal on damascene copper interconnection. J Electrochem Soc, 2015, 152(3): 163[4] Huang Q, Baker-O’Neal B C, Cabral C Jr, et al. Enhanced grain growth of electroplated copper on cobalt -containing seed layer. J Electrochem Soc, 2013, 160(12): 3045 doi: 10.1149/2.008312jes[5] Otake A, Kuroda A, Matsumoto T, et al. BTA removal and prevention of surface oxidation for copper post CMP cleaning. International Conference on Planarization/CMP Technology, 2009: 137[6] Peters S W. Incorporation of Cu passivators in post-CMP cleaners. ECS Trans, 2007, 11(2): 447[7] Zhong M, Venkataraman S S, Lan Y, et al. Role of 1,2,4-triazole as a passivating agent for cobalt during post-chemical mechanical planarization cleaning. J Electrochem Soc, 2014, 161(3): 138[8] He P, Yang G, Xu J B, et al. 1,2,4-triazole as corrosion inhibitor for cobalt CMP in H2O2 based acidic slurry. International Conference on Planarization/CMP Technology, 2016: 320[9] Liu X Y, Liu Y L, Liang Y, et al. Optimization of slurry components for a copper chemical mechanical polishing at low down pressure using response surface methodology. Microelectron Eng, 2011, 88(1): 99 doi: 10.1016/j.mee.2010.09.007[10] Wang C W, Liu Y L, Tian J Y, et al. A study on the comparison of CMP performance between a novel alkaline slurry and a commercial slurry for barrier removal. Microelectron Eng, 2012, 98(5): 29[11] Turk M C, Shi X, Gonyer D A J, et al. Chemical and mechanical aspects of a Co–Cu planarization scheme based on an alkaline slurry formulation. J Solid State Sci Technol, 2016, 5(2): 88 doi: 10.1149/2.0271602jss[12] Jiang L , Lan Y Q, He Y Y , et al. 1,2,4-triazole as a corrosion inhibitor in copper chemical mechanical polishing. Thin Solid Films, 2014, 556: 395 doi: 10.1016/j.tsf.2013.12.047[13] Jiang L, He Y Y, Li Y, et al. Synergetic effect of H2O2 and glycine on cobalt CMP in weakly alkaline slurry. Microelectron Eng, 2014, 122(7): 82[14] Zhang B G, Liu Y L, Wang C W. BTA free alkaline slurries developed for copper and barrier CMP. J Solid State Sci Technol, 2015, 4(11): 5112 doi: 10.1149/2.0171511jss[15] Lu H S, Zeng X, Wang J X, et al. The effect of glycine and benzotriazole on corrosion and polishing properties of cobalt in acid slurry. J Electrochem Soc, 2012, 159(9): 383 doi: 10.1149/2.036209jes -
Proportional views





 DownLoad:
DownLoad: