| Citation: |
Yuanyuan Jin, Huimin Li, Song Liu. Growth of large-scale two-dimensional insulator Na2Ta4O11 through chemical vapor deposition[J]. Journal of Semiconductors, 2020, 41(7): 072901. doi: 10.1088/1674-4926/41/7/072901
****
Y Y Jin, H M Li, S Liu, Growth of large-scale two-dimensional insulator Na2Ta4O11 through chemical vapor deposition[J]. J. Semicond., 2020, 41(7): 072901. doi: 10.1088/1674-4926/41/7/072901.
|
Growth of large-scale two-dimensional insulator Na2Ta4O11 through chemical vapor deposition
DOI: 10.1088/1674-4926/41/7/072901
More Information
-
Abstract
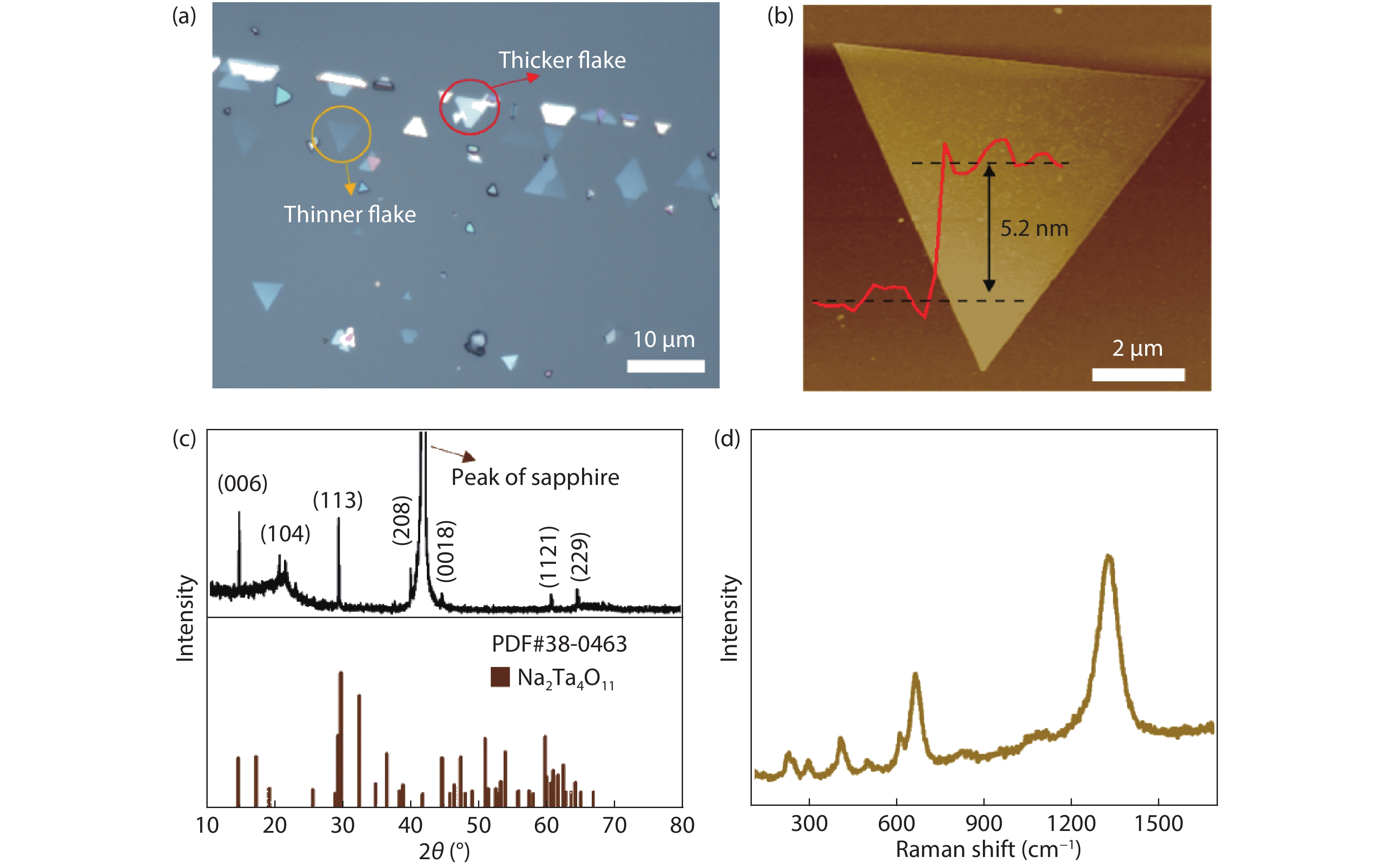
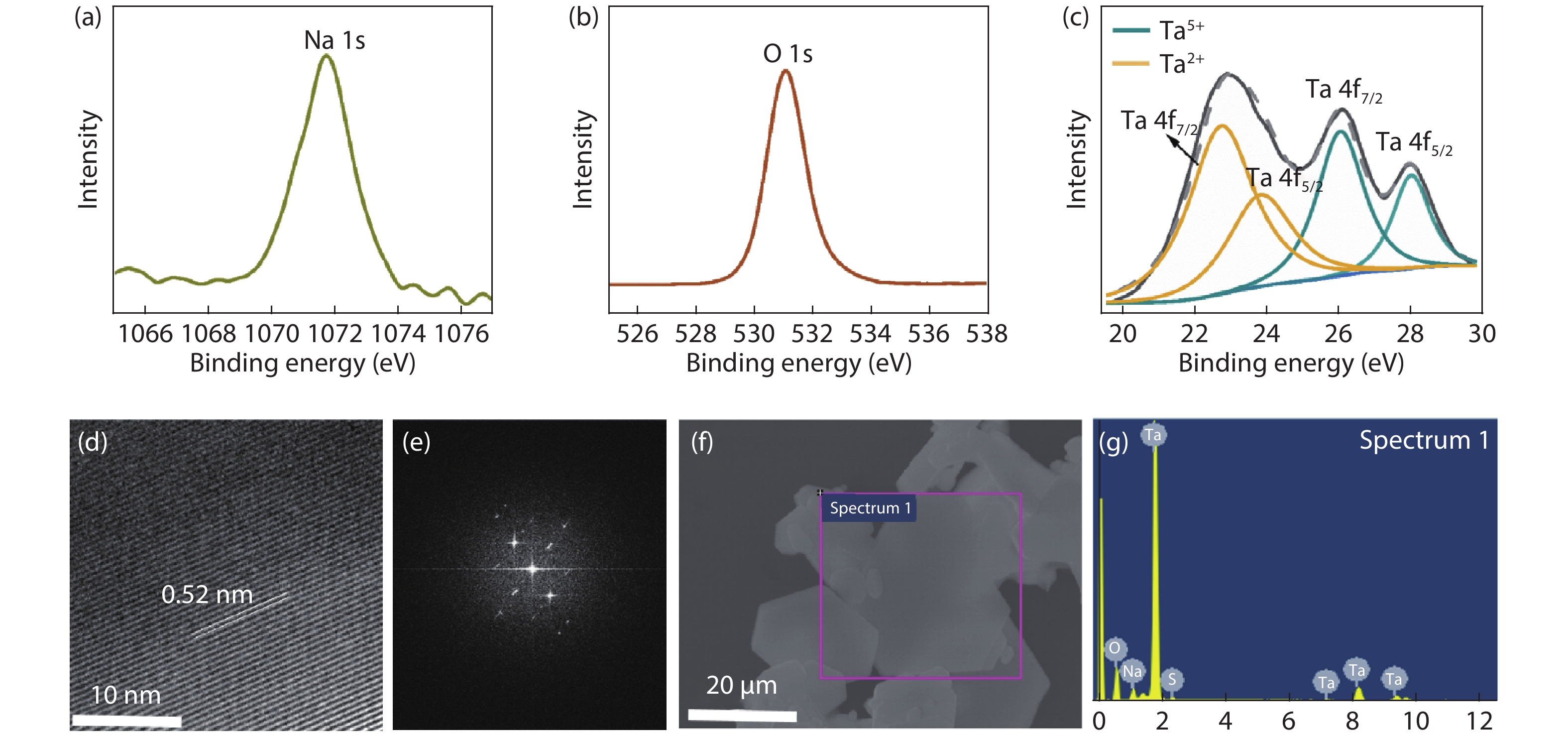
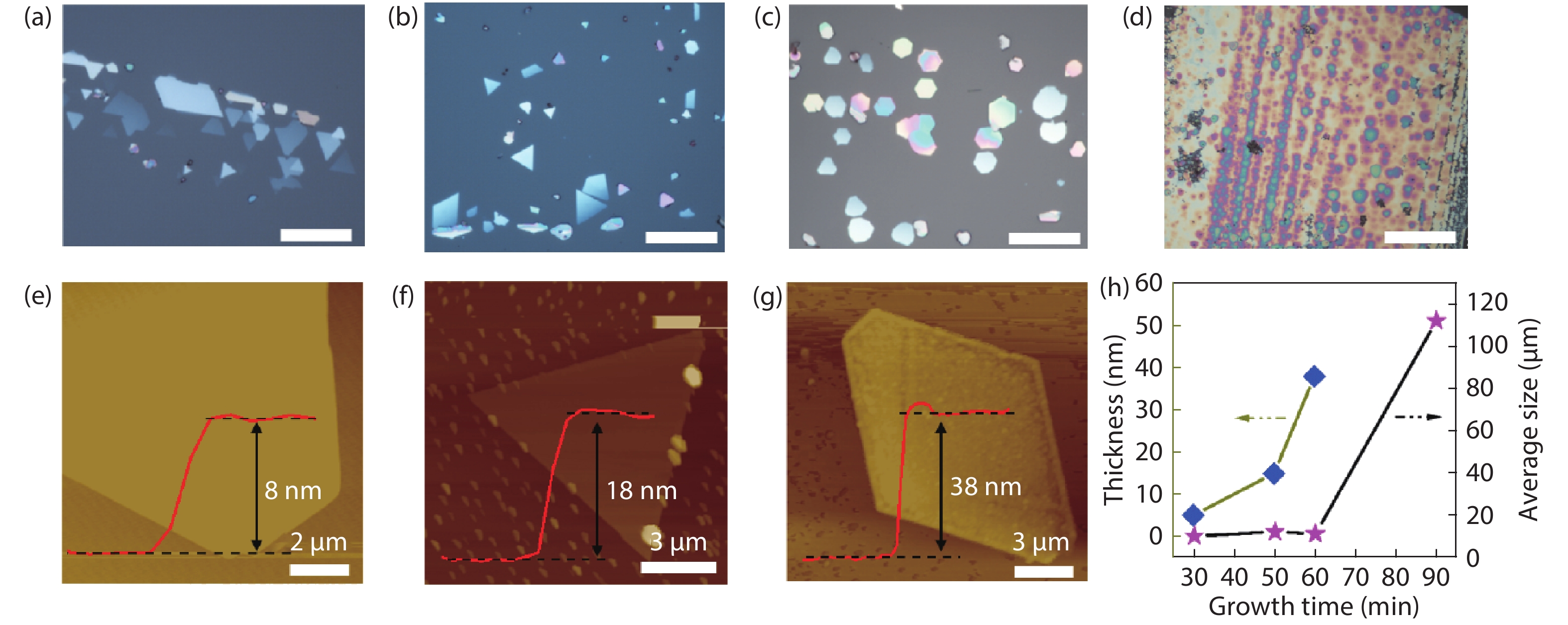
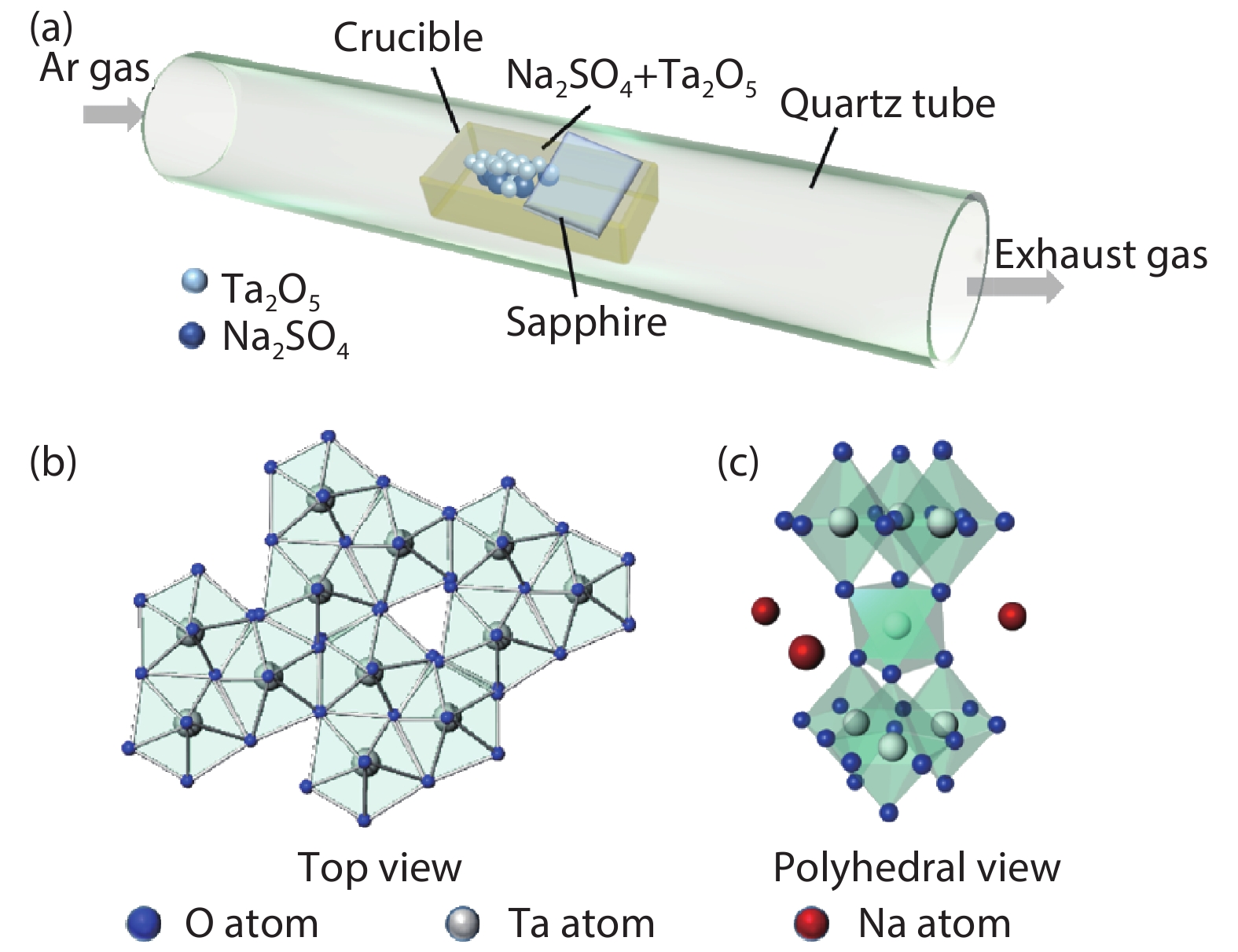
The insulator Na2Ta4O11 has been considered as a potential photocatalyst. However, little attention has been given to the synthesis of Na2Ta4O11 nanoparticles, let alone the growth of two-dimensional (2D) layered Na2Ta4O11 flake, which may bring innovative properties and promising applications. Here, the 2D thin-layer Na2Ta4O11 flake was first produced by chemical vapor deposition (CVD) method, with the smallest thickness reported currently. We have also synthesized 2D Na2Ta4O11 flake over 100 μm, which was the largest value over the 2D level reported to date. Our work proposed novel strategies to synthesize other 2D metal oxide material and endow the Na2Ta4O11 more properties and applications. -
References
[1] Machida M, Yabunaka J I, Kijima T. Efficient photocatalytic decomposition of water with the novel layered tantalate RbNdTa2O7. Chem Commun, 1999, 30(15), 1939 doi: 10.1039/A905246A[2] Tanaka T, Nojima H, Yamamoto T, et al. Structure of surface tantalate species and photo-oxidation of carbon monoxide over silica-supported tantalum oxide. Phys Chem Chem Phys, 1999, 1(22), 5235 doi: 10.1039/a905102c[3] Suzuki S, Saito H, Yubuta K, et al. Growth of millimeter-sized platy single crystals of NaTaO3 from Na2MoO4 flux. Cryst Growth Des, 2019, 19(7), 3607 doi: 10.1021/acs.cgd.9b00526[4] Ivanova I, Kandiel T A, Cho Y J, et al. Mechanisms of photocatalytic molecular hydrogen and molecular oxygen evolution over La-doped NaTaO3 particles: effect of different cocatalysts and their specific activity. ACS Catal, 2018, 8(3), 2313 doi: 10.1021/acscatal.7b04326[5] Sudrajat H, Zhou Y, Sasaki T, et al. The atomic-scale structure of LaCrO3–NaTaO3 solid solution photocatalysts with enhanced electron population. Phys Chem Chem Phys, 2019, 21, 5148 doi: 10.1039/C8CP07688J[6] Kishimoto K, Yoshio M, Mukai T, et al. Nanostructured anisotropic ion-conductive films. J Am Chem Soc, 2003, 125(11), 3196 doi: 10.1021/ja029750u[7] Su Y G, Yang X, Wang T T, et al. Sol-gel synthesis of Na2Ta4O11 nanocrystals showing high efficient photocatalytic performance. Adv Mater Res, 2014, 1058, 35 doi: 10.4028/www.scientific.net/AMR.1058.35[8] Mattes R, Schaper J. Crystal structure of Na2Ta4O11. Revue de Chimie Minerale, 1985, 22(6), 817[9] Ratnamala, A, Suresh, G, Kumari, V, et al. Template synthesized nano-crystalline natrotantite: preparation and photocatalytic activity for water decomposition. Mater Chem Physs, 2008, 110, 176 doi: 10.1016/j.matchemphys.2008.01.039[10] McLamb N, Sahoo P P, Fuoco L, et al. Flux growth of single-crystal Na2Ta4O11 particles and their photocatalytic hydrogen production. Cryst Growth Des, 2013, 13(6), 2322 doi: 10.1021/cg301859d[11] Teshima K, Tomomatsu D, Suzuki T, et al. Growth of Na2Ta4O11 crystals from a Na2Mo2O7 flux. Cryst Growth Des, 2006, 6(1), 18 doi: 10.1021/cg050291t[12] Kim Y, Kim S, Lee W H, et al. Direct transfer of CVD-grown graphene onto eco-friendly cellulose film for highly sensitive gas sensor. Cellulose, 2020, 27(3), 1685 doi: 10.1007/s10570-019-02902-2[13] Kumar D, Ghadai R K, Das S, et al. Effect of nitrogen flow rate on the mechanical properties of CVD-deposited SiCN thin films. Bull Mater Sci, 2019, 42(5), 251 doi: 10.1007/s12034-019-1937-7[14] Jin Y, Zeng Z, Xu Z, et al. Synthesis and transport properties of degenerate p-type Nb-doped WS2 monolayers. Chem Mater, 2019, 31(9), 3534 doi: 10.1021/acs.chemmater.9b00913[15] Kwon K C, Kim C, Le Q V, et al. Synthesis of atomically thin transition metal disulfides for charge transport layers in optoelectronic devices. ACS Nano, 2015, 9(4), 4146 doi: 10.1021/acsnano.5b01504[16] Ko K Y, Lee S, Park K, et al. High-performance gas sensor using a large-area WS2 xSe2–2 x alloy for low-power operation wearable applications. ACS Appl Mater Interfaces, 2018, 10(40), 34163 doi: 10.1021/acsami.8b10455[17] Wang S, Rong Y, Fan Y, et al. Shape evolution of monolayer MoS2 crystals grown by chemical vapor deposition. Chem Mater, 2014, 26(22), 6371 doi: 10.1021/cm5025662[18] Harb M, Masih D, Ould-Chikh S, et al. Determination of the electronic structure and UV–Vis absorption properties of (Na2– xCu x)Ta4O11 from first-principle calculations. J Phys Chem C, 2013, 117(34), 17477 doi: 10.1021/jp405995w[19] Palasyuk O, Palasyuk A, Maggard P A. Site-differentiated solid solution in (Na1− xCu x)2Ta4O11 and its electronic structure and optical properties. Inorg Chem, 2010, 49(22), 10571 doi: 10.1021/ic101529n[20] Mobin M, Malik A. Studies on the interactions of transition metal oxides and sodium sulfate in the temperature range 900–1200 K in oxygen. J Alloy Compd, 1996, 235, 97 doi: 10.1016/0925-8388(95)02125-6[21] Muñoz-Márquez M A, Zarrabeitia M, Castillo-Martínez E, et al. Composition and evolution of the solid-electrolyte interphase in Na2Ti3O7 electrodes for Na-ion batteries: XPS and auger parameter analysis. ACS Appl Mater Interfaces, 2015, 7(14), 7801 doi: 10.1021/acsami.5b01375[22] Kotsis K, Staemmler V. Ab initio calculations of the O1s XPS spectra of ZnO and Zn oxo compounds. Phys Chem Chem Phys, 2006, 8(13), 1490 doi: 10.1039/b515699h[23] Grilli R, Simpson R, Mallinson C, et al. Comparison of Ar+ monoatomic and cluster ion sputtering of Ta2O5 at different ion energies, by XPS: Part 2-cluster ions. Surf Sci Spectra, 2014, 21, 68 doi: 10.1116/11.20140702[24] Van Ngoc H, Qian Y, Han S K, et al. PMMA-etching-free transfer of wafer-scale chemical vapor deposition two-dimensional atomic crystal by a water soluble polyvinyl alcohol polymer method. Sci Rep, 2016, 6(1), 33096 doi: 10.1038/srep33096[25] Ithurria S, Talapin D V. Colloidal atomic layer deposition (c-ALD) using self-limiting reactions at nanocrystal surface coupled to phase transfer between polar and nonpolar media. J Am Chem Soc, 2012, 134(45), 18585 doi: 10.1021/ja308088d -
Proportional views





 DownLoad:
DownLoad: