| Citation: |
Jun Liu, Zhijie Wang, Yong Lei. A close step towards industrialized application of solar water splitting[J]. Journal of Semiconductors, 2020, 41(9): 090401. doi: 10.1088/1674-4926/41/9/090401
****
J Liu, Z J Wang, Y Lei, A close step towards industrialized application of solar water splitting[J]. J. Semicond., 2020, 41(9): 090401. doi: 10.1088/1674-4926/41/9/090401.
|
A close step towards industrialized application of solar water splitting
DOI: 10.1088/1674-4926/41/9/090401
More Information
-
References
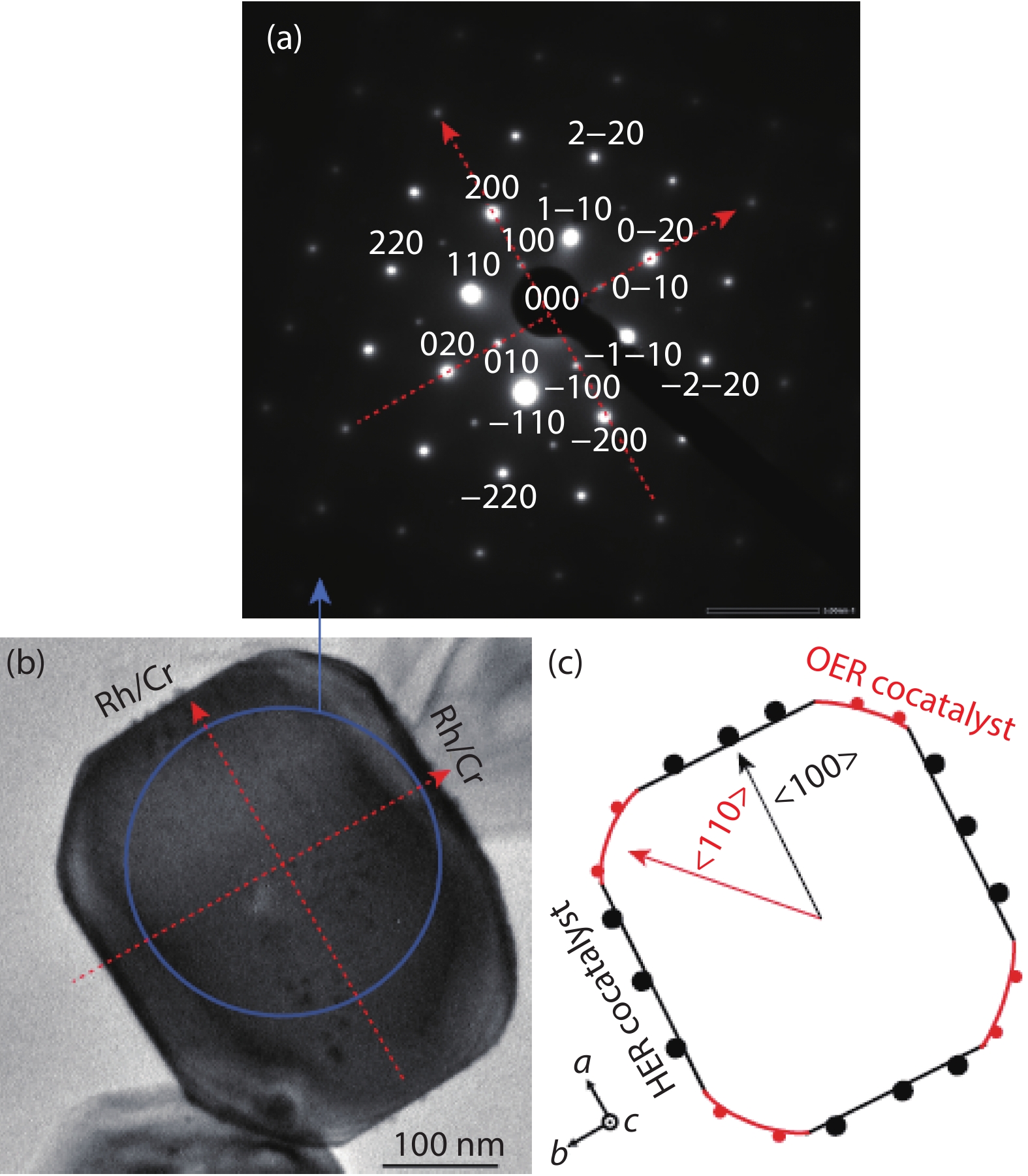
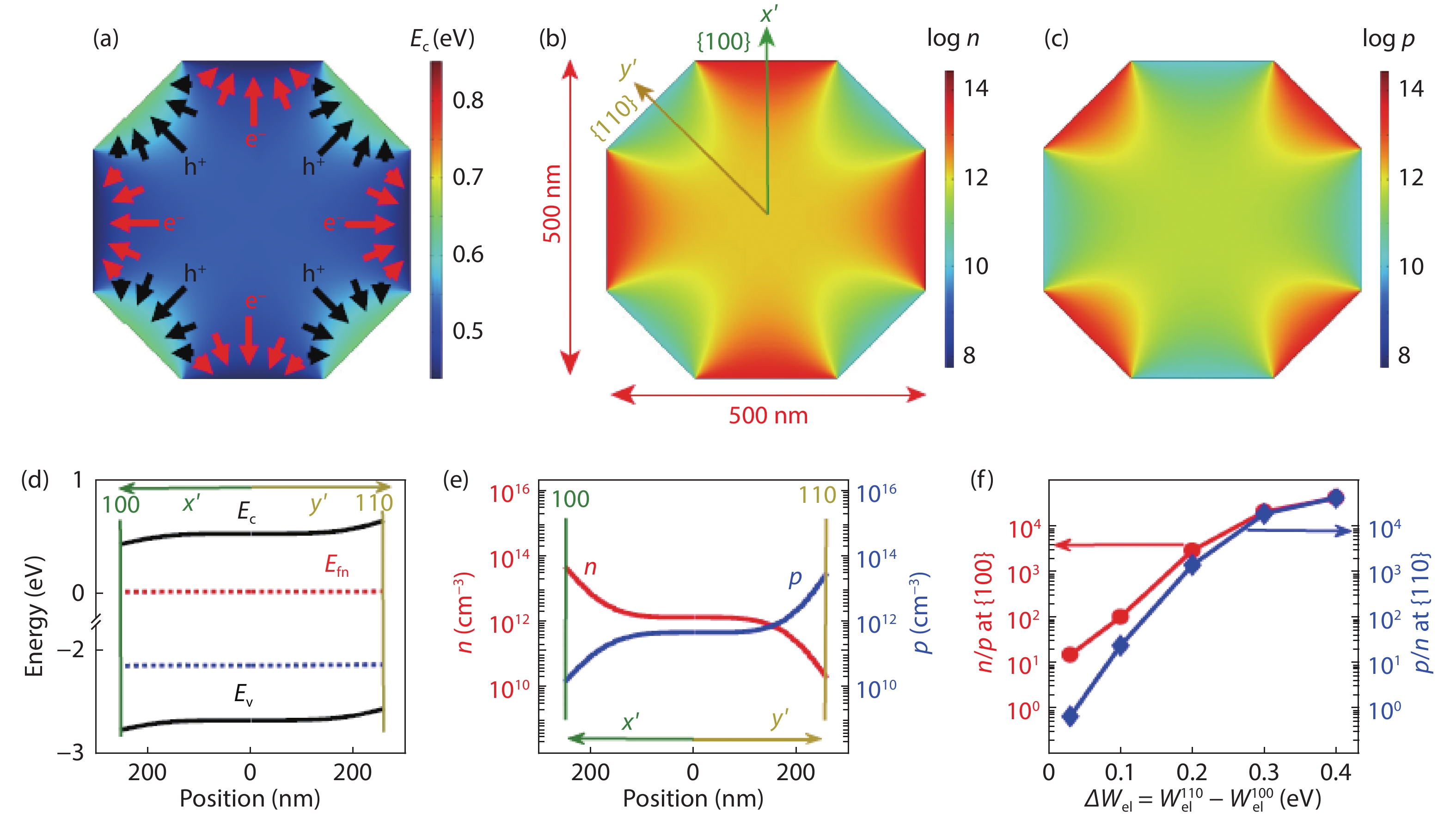
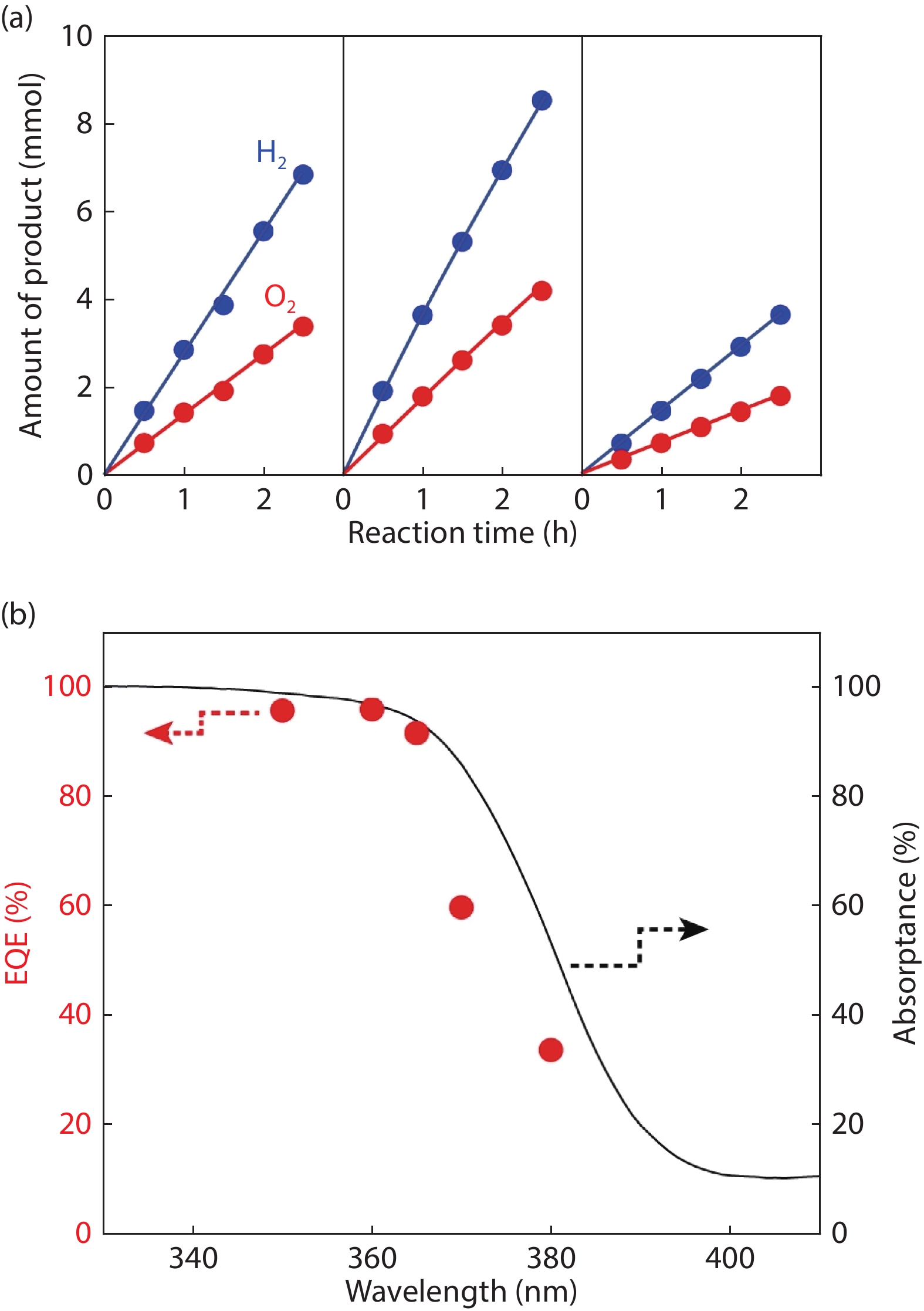
[1] Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature, 1972, 238, 37 doi: 10.1038/238037a0[2] An X, Li T, Wen B, et al. New insights into defect-mediated heterostructures for photoelectrochemical water splitting. Adv Energy Mater, 2016, 6, 1502268 doi: 10.1002/aenm.201502268[3] Wang X D, Xu Y F, Rao H S, et al. Novel porous molybdenum tungsten phosphide hybrid nanosheets on carbon cloth for efficient hydrogen evolution. Energ Environ Sci, 2016, 9, 1468 doi: 10.1039/C5EE03801D[4] Wu B, Liu D, Mubeen S, et al. Anisotropic growth of TiO2 onto gold nanorods for plasmon-enhanced hydrogen production from water reduction. J Am Chem Soc, 2016, 138, 1114 doi: 10.1021/jacs.5b11341[5] Zhang L, Ye X, Boloor M, et al. Significantly enhanced photocurrent for water oxidation in monolithic Mo:BiVO4/SnO2/Si by thermally increasing the minority carrier diffusion length. Energ Environ Sci, 2016, 9, 2044 doi: 10.1039/C6EE00036C[6] Luo J, Steier L, Son M K, et al. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett, 2016, 16, 1848 doi: 10.1021/acs.nanolett.5b04929[7] Domen K, Naito S, Soma M, et al. Photocatalytic decomposition of water vapour on an NiO–SrTiO3 catalyst. J Chem Soc Chem Commun, 1980, 12, 543 doi: 10.1039/c39800000543[8] Zou Z, Ye J, Sayama K, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature, 2001, 414, 625 doi: 10.1038/414625a[9] Maeda K, Takata T, Hara M, et al. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J Am Chem Soc, 2005, 127, 8286 doi: 10.1021/ja0518777[10] Liu J, Liu Y, Liu N, et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science, 2015, 347, 970 doi: 10.1126/science.aaa3145[11] Takata T, Jiang J, Sakata Y, et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature, 2020, 581, 411 doi: 10.1038/s41586-020-2278-9[12] Maeda K, Domen K. Photocatalytic water eplitting: Recent progress and future challenges. J Phys Chem Lett, 2010, 1, 2655 doi: 10.1021/jz1007966[13] Wang Z, Inoue Y, Hisatomi T, et al. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat Catal, 2018, 1, 756 doi: 10.1038/s41929-018-0134-1[14] Wang Q, Nakabayashi M, Hisatomi T, et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting. Nat Mater, 2019, 18, 827 doi: 10.1038/s41563-019-0399-z -
Proportional views





 DownLoad:
DownLoad: