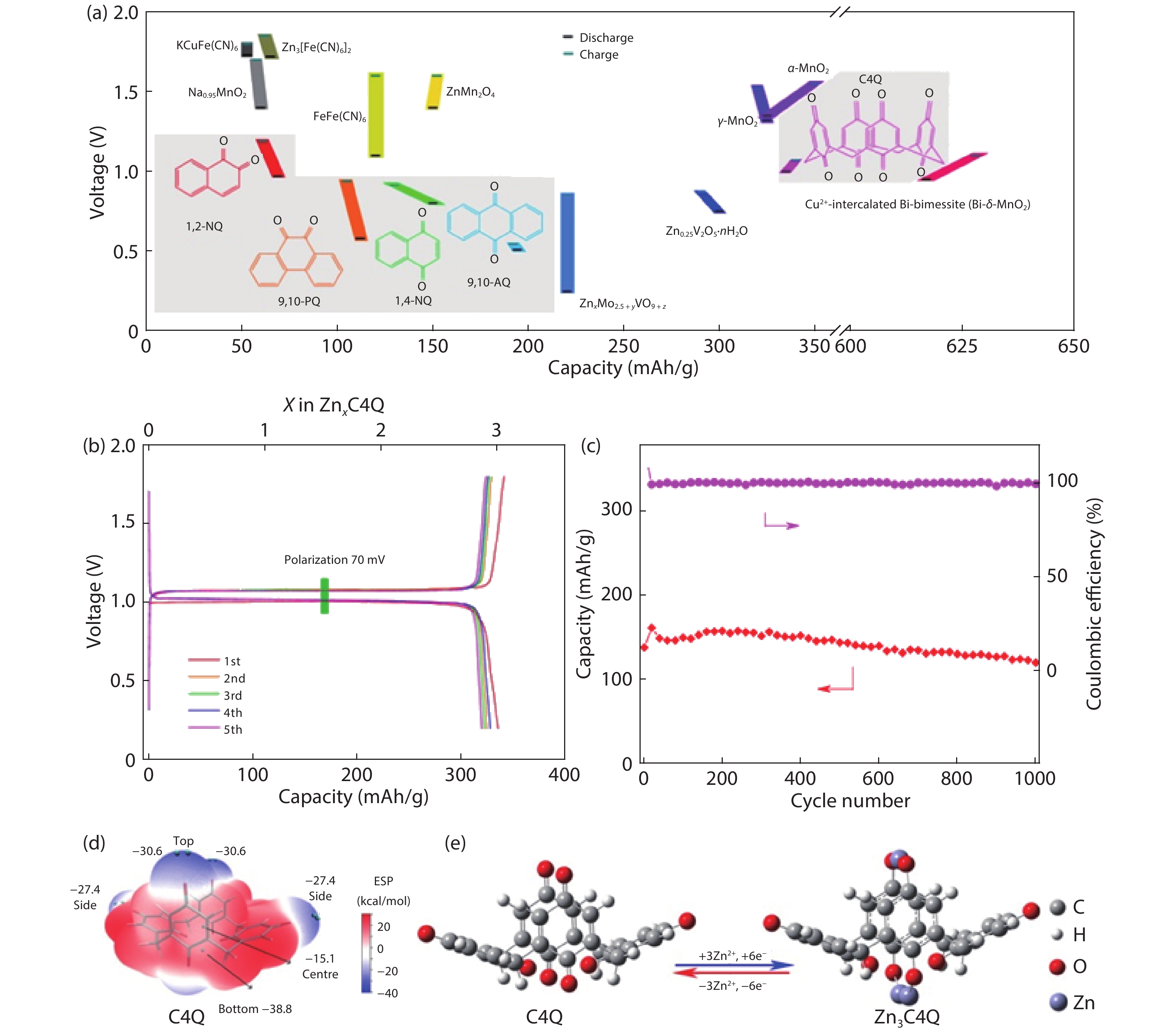
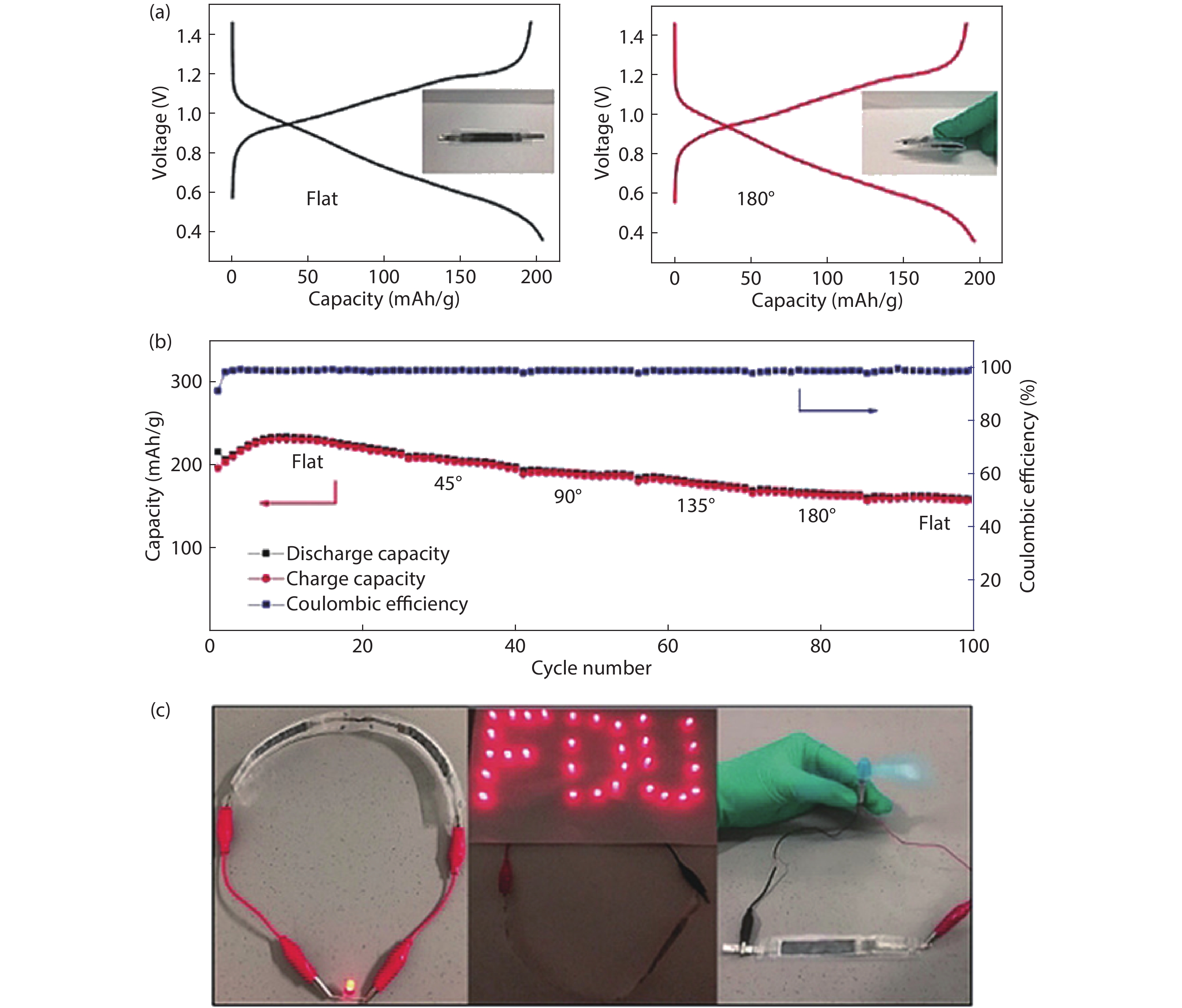
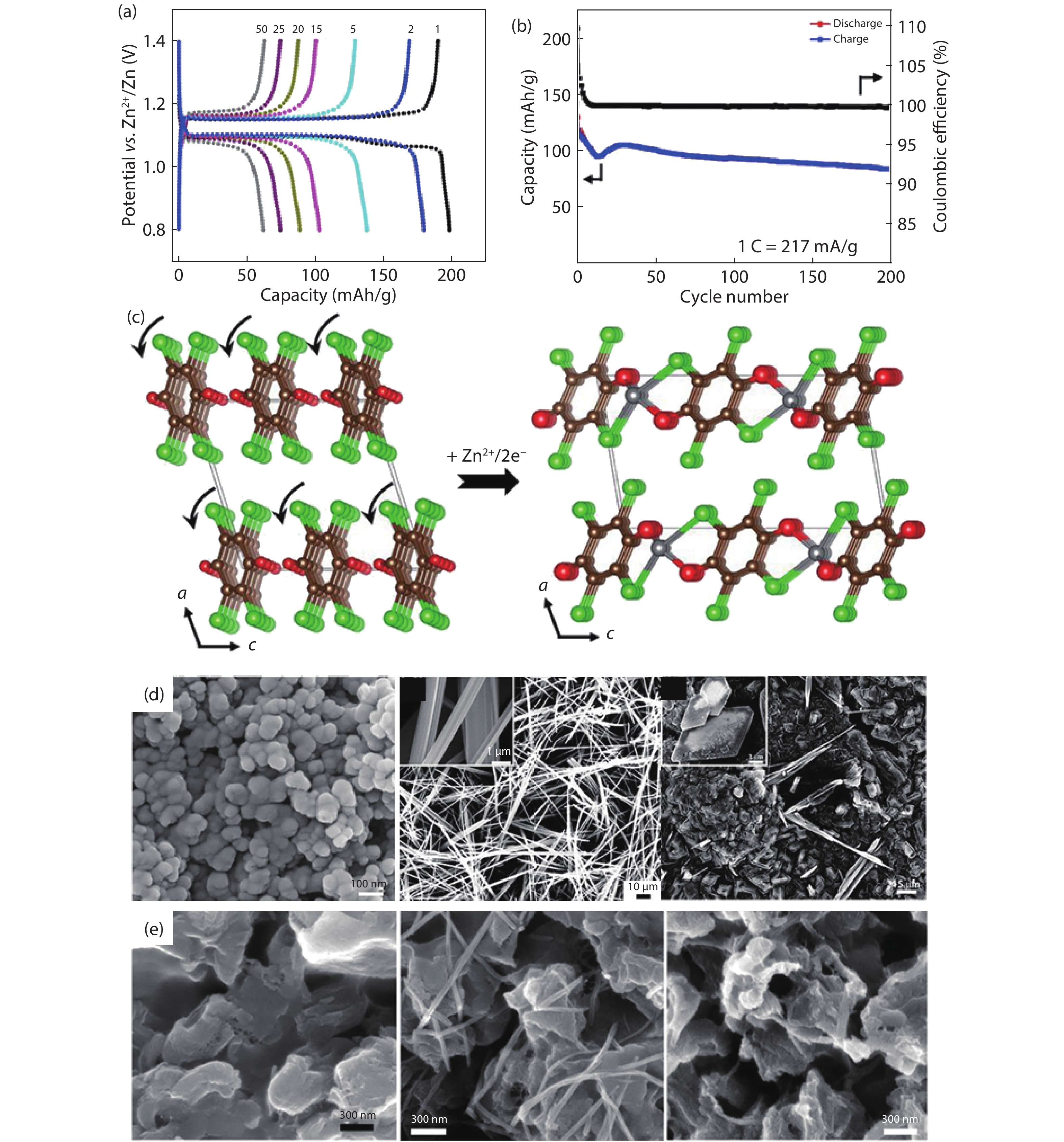
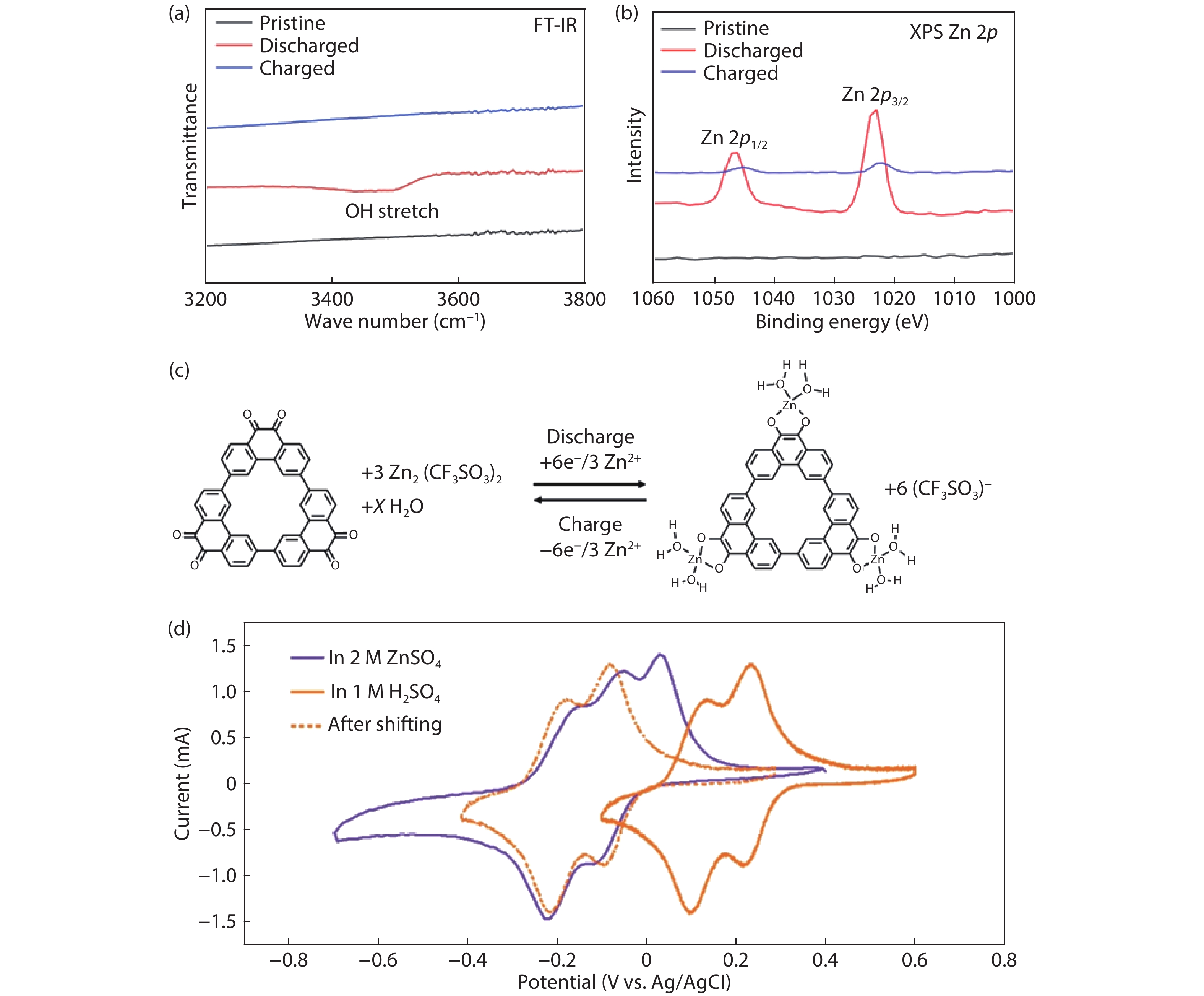
| Citation: |
Shuaifei Xu, Mingxuan Sun, Qian Wang, Chengliang Wang. Recent progress in organic electrodes for zinc-ion batteries[J]. Journal of Semiconductors, 2020, 41(9): 091704. doi: 10.1088/1674-4926/41/9/091704
****
S F Xu, M X Sun, Q Wang, C L Wang, Recent progress in organic electrodes for zinc-ion batteries[J]. J. Semicond., 2020, 41(9): 091704. doi: 10.1088/1674-4926/41/9/091704.
|
Recent progress in organic electrodes for zinc-ion batteries
DOI: 10.1088/1674-4926/41/9/091704
More Information
-
Abstract
Organic zinc-ion batteries (OZIBs) are emerging rechargeable energy storage devices and have attracted increasing attention as one of the promising alternatives of lithium-ion batteries, benefiting from the Zn metal (low cost, safety and small ionic size) and organic electrodes (flexibility, green and designable molecular structure). Organic electrodes have exhibited fine electrochemical performance in ZIBs, but the research is still in infancy and hampered by some issues. Hence, to provide insight into OZIBs, this review summarizes the progress of organic cathode materials for ZIBs and points out the existing challenges and then addresses potential solutions. It is hoped that this review can stimulate the researchers to further develop high-performance OZIBs.-
Keywords:
- organic electrodes,
- zinc-ion batteries,
- redox compounds
-
References
[1] Armand M, Tarascon J M. Building better batteries. Nature, 2008, 451, 652 doi: 10.1038/451652a[2] Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: A battery of choices. Science, 2011, 334, 928 doi: 10.1126/science.1212741[3] Hwang J Y, Myung S T, Sun Y K. Sodium-ion batteries: Present and future. Chem Soc Rev, 2017, 46, 3529 doi: 10.1039/C6CS00776G[4] Hosaka T, Kubota K, Hameed A S, et al. Research development on K-ion batteries. Chem Rev, 2020, 120, 6358 doi: 10.1021/acs.chemrev.9b00463[5] Allegre C, Michard G. Introduction to geochemistry. Boston: D. Reidel, Dordrecht-Holland, 1983[6] Marcus Y. Ionic radii in aqueous solutions. Chem Rev, 1988, 88, 1475 doi: 10.1021/cr00090a003[7] Wang B, Wu Y C, Zhuo S M, et al. Synergistic effect of organic plasticizer and lepidolite filler on polymer electrolytes for all-solid high-voltage Li–metal batteries. J Mater Chem A, 2020, 8, 5968 doi: 10.1039/C9TA14239H[8] Jiang C, Gu Y M, Tang M, et al. Toward stable lithium plating/stripping by successive desolvation and exclusive transport of Li ions. ACS Appl Mater Interfaces, 2020, 12, 10461 doi: 10.1021/acsami.9b21993[9] Liu Y Y, Lin D C, Yuen P Y, et al. An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Adv Mater, 2017, 29, 1605531 doi: 10.1002/adma.201605531[10] Zhang X Q, Cheng X B, Chen X, et al. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv Funct Mater, 2017, 27, 1605989 doi: 10.1002/adfm.201605989[11] Li Q, Zhu S P, Lu Y Y. 3D porous Cu current collector/Li-metal composite anode for stable lithium-metal batteries. Adv Funct Mater, 2017, 27, 1606422 doi: 10.1002/adfm.201606422[12] Zhao Z M, Zhao J W, Hu Z L, et al. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ Sci, 2019, 12, 1938 doi: 10.1039/C9EE00596J[13] Zeng Y X, Zhang X Y, Qin R F, et al. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv Mater, 2019, 31, 1903675 doi: 10.1002/adma.201903675[14] Naveed A, Yang H J, Yang J, et al. Highly reversible and rechargeable safe Zn batteries based on a triethyl phosphate electrolyte. Angew Chem Int Ed, 2019, 58, 2760 doi: 10.1002/anie.201813223[15] Konarov A, Voronina N, Jo J H, et al. Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett, 2018, 3, 2620 doi: 10.1021/acsenergylett.8b01552[16] Chen L N, An Q Y, Mai L Q. Recent advances and prospects of cathode materials for rechargeable aqueous zinc-ion batteries. Adv Mater Interfaces, 2019, 6, 1900387 doi: 10.1002/admi.201900387[17] Cui J, Guo Z W, Yi J, et al. Organic cathode materials for rechargeable zinc batteries: Mechanisms, challenges, and perspectives. ChemSusChem, 2020, 13, 2160 doi: 10.1002/cssc.201903265[18] Yang D, Tan H T, Rui X H, et al. Electrode materials for rechargeable zinc-ion and zinc-air batteries: Current status and future perspectives. Electrochem Energy Rev, 2019, 2, 395 doi: 10.1007/s41918-019-00035-5[19] Selvakumaran D, Pan A Q, Liang S Q, et al. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J Mater Chem A, 2019, 7, 18209 doi: 10.1039/C9TA05053A[20] Chao D L, Zhou W H, Ye C, et al. An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage. Angew Chem Int Ed, 2019, 58, 7823 doi: 10.1002/anie.201904174[21] Pan H, Shao Y, Yan P, et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat Energy, 2016, 1, 16039 doi: 10.1038/nenergy.2016.39[22] Li Z L, Ganapathy S, Xu Y L, et al. Mechanistic insight into the electrochemical performance of Zn/VO2 batteries with an aqueous ZnSO4 electrolyte. Adv Energy Mater, 2019, 9, 1900237 doi: 10.1002/aenm.201900237[23] Wan F, Niu Z Q. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew Chem Int Ed, 2019, 58, 16358 doi: 10.1002/anie.201903941[24] Zhang L Y, Chen L, Zhou X F, et al. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv Energy Mater, 2015, 5, 1400930 doi: 10.1002/aenm.201400930[25] Trócoli R, La Mantia F. An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem, 2015, 8, 481 doi: 10.1002/cssc.201403143[26] Huang J H, Wang Z, Hou M Y, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat Commun, 2018, 9, 2906 doi: 10.1038/s41467-018-04949-4[27] Lee B, Lee H R, Kim H, et al. Elucidating the intercalation mechanism of zinc ions into α-MnO2 for rechargeable zinc batteries. Chem Commun, 2015, 51, 9265 doi: 10.1039/C5CC02585K[28] Wang C, Ren X C, Xu C H, et al. N-type 2D organic single crystals for high-performance organic field-effect transistors and near-infrared phototransistors. Adv Mater, 2018, 30, 1706260 doi: 10.1002/adma.201706260[29] Wang Q Q, Yang F X, Zhang Y, et al. Space-confined strategy toward large-area two-dimensional single crystals of molecular materials. J Am Chem Soc, 2018, 140, 5339 doi: 10.1021/jacs.8b01997[30] Lu Y, Chen J. Prospects of organic electrode materials for practical lithium batteries. Nat Rev Chem, 2020, 4, 127 doi: 10.1038/s41570-020-0160-9[31] Tang M, Jiang C, Liu S Y, et al. Small amount COFs enhancing storage of large anions. Energy Storage Mater, 2020, 27, 35 doi: 10.1016/j.ensm.2020.01.015[32] Wu Y W, Zeng R H, Nan J M, et al. Quinone electrode materials for rechargeable lithium/sodium ion batteries. Adv Energy Mater, 2017, 7, 1700278 doi: 10.1002/aenm.201700278[33] Wang Y R, Wang C X, Ni Z G, et al. Binding zinc ions by carboxyl groups from adjacent molecules toward long-life aqueous zinc-organic batteries. Adv Mater, 2020, 32, 2000338 doi: 10.1002/adma.202000338[34] Zhao Q, Huang W W, Luo Z Q, et al. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci Adv, 2018, 4, eaao1761 doi: 10.1126/sciadv.aao1761[35] Guo Z W, Ma Y Y, Dong X L, et al. An environmentally friendly and flexible aqueous zinc battery using an organic cathode. Angew Chem Int Ed, 2018, 57, 11737 doi: 10.1002/anie.201807121[36] Dawut G, Lu Y, Miao L C, et al. High-performance rechargeable aqueous Zn-ion batteries with a poly(benzoquinonyl sulfide) cathode. Inorg Chem Front, 2018, 5, 1391 doi: 10.1039/C8QI00197A[37] Yue X J, Liu H D, Liu P. Polymer grafted on carbon nanotubes as a flexible cathode for aqueous zinc ion batteries. Chem Commun, 2019, 55, 1647 doi: 10.1039/C8CC10060H[38] Zhang S Q, Zhao W T, Li H, et al. Cross-conjugated polycatechol organic cathode for aqueous zinc-ion storage. ChemSusChem, 2020, 13, 188 doi: 10.1002/cssc.201902697[39] Wang C L, Jiang C, Xu Y, et al. A selectively permeable membrane for enhancing cyclability of organic sodium-ion batteries. Adv Mater, 2016, 28, 9182 doi: 10.1002/adma.201603240[40] Tang M, Zhu S L, Liu Z T, et al. Tailoring π-conjugated systems: From π-π stacking to high-rate-performance organic cathodes. Chem, 2018, 4, 2600 doi: 10.1016/j.chempr.2018.08.014[41] Zhu S L, Tang M, Wu Y C, et al. Free-standing protective films for enhancing the cyclability of organic batteries. Sustain Energy Fuels, 2019, 3, 142 doi: 10.1039/C8SE00477C[42] Chen Y, Tang M, Wu Y C, et al. A one-dimensional π-d conjugated coordination polymer for sodium storage with catalytic activity in Negishi coupling. Angew Chem Int Ed, 2019, 58, 14731 doi: 10.1002/anie.201908274[43] Li H Y, Tang M, Wu Y C, et al. Large π-conjugated porous frameworks as cathodes for sodium-ion batteries. J Phys Chem Lett, 2018, 9, 3205 doi: 10.1021/acs.jpclett.8b01285[44] Wang C L, Dong H L, Hu W P, et al. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem Rev, 2012, 112, 2208 doi: 10.1021/cr100380z[45] Wang C L, Dong H L, Jiang L, et al. Organic semiconductor crystals. Chem Soc Rev, 2018, 47, 422 doi: 10.1039/C7CS00490G[46] Khayum M A, Ghosh M, Vijayakumar V, et al. Zinc ion interactions in a two-dimensional covalent organic framework based aqueous zinc ion battery. Chem Sci, 2019, 10, 8889 doi: 10.1039/C9SC03052B[47] Wang C L. Weak intermolecular interactions for strengthening organic batteries. Energy Environ Mater, 2020 doi: 10.1002/eem2.12076[48] Chen Y, Li H Y, Tang M, et al. Capacitive conjugated ladder polymers for fast-charge and -discharge sodium-ion batteries and hybrid supercapacitors. J Mater Chem A, 2019, 7, 20891 doi: 10.1039/C9TA07546A[49] Wang C L, Xu Y, Fang Y G, et al. Extended π-conjugated system for fast-charge and -discharge sodium-ion batteries. J Am Chem Soc, 2015, 137, 3124 doi: 10.1021/jacs.5b00336[50] Li Z L, Zhao H L. Recent developments of phosphorus-based anodes for sodium ion batteries. J Mater Chem A, 2018, 6, 24013 doi: 10.1039/C8TA08774A[51] Kundu D P, Oberholzer P, Glaros C, et al. Organic cathode for aqueous Zn-ion batteries: Taming a unique phase evolution toward stable electrochemical cycling. Chem Mater, 2018, 30, 3874 doi: 10.1021/acs.chemmater.8b01317[52] Tang M, Wu Y C, Chen Y, et al. An organic cathode with high capacities for fast-charge potassium-ion batteries. J Mater Chem A, 2019, 7, 486 doi: 10.1039/C8TA09960J[53] Nam K W, Kim H, Beldjoudi Y, et al. Redox-active phenanthrenequinone triangles in aqueous rechargeable zinc batteries. J Am Chem Soc, 2020, 142, 2541 doi: 10.1021/jacs.9b12436[54] Wang N, Dong X L, Wang B L, et al. Zinc-organic battery with a wide operation-temperature window from −70 to 150 °C. Angew Chem Int Ed, 2020, 132, 14685 doi: 10.1002/ange.202005603[55] Sun W, Wang F, Hou S, et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J Am Chem Soc, 2017, 139, 9775 doi: 10.1021/jacs.7b04471[56] Shea J J, Luo C. Organic electrode materials for metal ion batteries. ACS Appl Mater Interfaces, 2020, 12, 5361 doi: 10.1021/acsami.9b20384[57] Chen Y, Zhuo S M, Li Z Y, et al. Redox polymers for rechargeable metal-ion batteries. EnergyChem, 2020, 2, 100030 doi: 10.1016/j.enchem.2020.100030[58] Ghanbari K, Mousavi M F, Shamsipur M. Preparation of polyaniline nanofibers and their use as a cathode of aqueous rechargeable batteries. Electrochim Acta, 2006, 52, 1514 doi: 10.1016/j.electacta.2006.02.051[59] Somasiri N L D, MacDiarmid A G. Polyaniline: characterization as a cathode active material in rechargeable batteries in aqueous electrolytes. J Appl Electrochem, 1988, 18, 92 doi: 10.1007/BF01016210[60] Yamamoto K, Yamada M, Nishiumi T. Doping reaction of redox-active dopants into polyaniline. Polym Adv Technol, 2000, 11, 710 doi: 10.1002/1099-1581(200008/12)11:8/12<710::AID-PAT24>3.0.CO;2-K[61] Gospodinova N, Terlemezyan L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog Polym Sci, 1998, 23, 1443 doi: 10.1016/S0079-6700(98)00008-2[62] Jiménez P, Levillain E, Alévêque O, et al. Lithium n-doped polyaniline as a high-performance electroactive material for rechargeable batteries. Angew Chem, 2017, 129, 1575 doi: 10.1002/ange.201607820[63] Karyakin A A, Strakhova A K, Yatsimirsky A K. Self-doped polyanilines electrochemically active in neutral and basic aqueous solutions: Electropolymerization of substituted anilines. J Electroan Chem, 1994, 371, 259 doi: 10.1016/0022-0728(93)03244-J[64] Rahmanifar M S, Mousavi M F, Shamsipur M. Effect of self-doped polyaniline on performance of secondary Zn-polyaniline battery. J Power Sources, 2002, 110, 229 doi: 10.1016/S0378-7753(02)00260-4[65] Shi H Y, Ye Y J, Liu K, et al. A long-cycle-life self-doped polyaniline cathode for rechargeable aqueous zinc batteries. Angew Chem Int Ed, 2018, 57, 16359 doi: 10.1002/anie.201808886[66] Mu S L, Shi Q F. Controllable preparation of poly(aniline-co-5-aminosalicylic acid) nanowires for rechargeable batteries. Synth Met, 2016, 221, 8 doi: 10.1016/j.synthmet.2016.10.008[67] Mu S L. Rechargeable batteries based on poly(aniline-co-o-aminophenol) and the protonation of the copolymer. Synth Met, 2004, 143, 269 doi: 10.1016/j.synthmet.2003.12.009[68] Zhang J, Shan D, Mu S L. A rechargeable Zn- poly(aniline-co-m-aminophenol) battery. J Power Sources, 2006, 161, 685 doi: 10.1016/j.jpowsour.2006.04.077[69] Chen C X, Hong X Z, Xu T T, et al. Electrosynthesis and electrochemical and electrochromic properties of poly(aniline-co-N-methylthionine). J Electrochem Soc, 2015, 162, G54 doi: 10.1149/2.0861508jes[70] Chen C X, Hong X Z, Chen A K, et al. Electrochemical properties of poly(aniline-co-N-methylthionine) for zinc-conducting polymer rechargeable batteries. Electrochim Acta, 2016, 190, 240 doi: 10.1016/j.electacta.2015.12.125[71] Chen C X, Gan Z Y, Xu C, et al. Electrosynthesis of poly(aniline-co-azure B) for aqueous rechargeable zinc-conducting polymer batteries. Electrochim Acta, 2017, 252, 226 doi: 10.1016/j.electacta.2017.08.195[72] Liu Y, Xie L Y, Zhang W, et al. A conjugated system of PEDOT: PSS induced self-doped PANI for flexible zinc-ion batteries with enhanced capacity and cyclability. ACS Appl Mater Interfaces, 2019, 11, 30943 doi: 10.1021/acsami.9b09802[73] Wang Z, Han J J, Zhang N, et al. Synthesis of polyaniline/graphene composite and its application in zinc-rechargeable batteries. J Solid State Electrochem, 2019, 23, 3373 doi: 10.1007/s10008-019-04435-x[74] Du W C, Xiao J F, Geng H B, et al. Rational-design of polyaniline cathode using proton doping strategy by graphene oxide for enhanced aqueous zinc-ion batteries. J Power Sources, 2020, 450, 227716 doi: 10.1016/j.jpowsour.2020.227716[75] Yao H, Li Q J, Zhang M S, et al. Prolonging the cycle life of zinc-ion battery by introduction of [Fe(CN)6]4− to PANI via a simple and scalable synthetic method. Chem Eng J, 2020, 392, 123653 doi: 10.1016/j.cej.2019.123653[76] Li P, Fang Z S, Zhang Y, et al. A high-performance, highly bendable quasi-solid-state zinc –organic battery enabled by intelligent proton-self-buffering copolymer cathodes. J Mater Chem A, 2019, 7, 17292 doi: 10.1039/C9TA04185K[77] Trinidad F. Performance study of Zn/ZnCl2, NH4Cl/polyaniline/carbon battery. J Electrochem Soc, 1991, 138, 3186 doi: 10.1149/1.2085390[78] Ghanbari K, Mousavi M F, Shamsipur M, et al. Synthesis of polyaniline/graphite composite as a cathode of Zn-polyaniline rechargeable battery. J Power Sources, 2007, 170, 513 doi: 10.1016/j.jpowsour.2007.02.090[79] Xia Y, Zhu D R, Si S H, et al. Nickel foam-supported polyaniline cathode prepared with electrophoresis for improvement of rechargeable Zn battery performance. J Power Sources, 2015, 283, 125 doi: 10.1016/j.jpowsour.2015.02.123[80] Wan F, Zhang L L, Wang X Y, et al. An aqueous rechargeable zinc-organic battery with hybrid mechanism. Adv Funct Mater, 2018, 28, 1804975 doi: 10.1002/adfm.201804975[81] Yu H, Liu G, Wang M, et al. Plasma-assisted surface modification on the electrode interface for flexible fiber-shaped Zn–polyaniline batteries. ACS Appl Mater Interfaces, 2020, 12, 5820 doi: 10.1021/acsami.9b19172[82] Xiao X C, Liu W J, Wang K, et al. High-performance solid-state Zn batteries based on a free-standing organic cathode and metal Zn anode with an ordered nano-architecture. Nanoscale Adv, 2020, 2, 296 doi: 10.1039/C9NA00562E[83] Cao H M, Wan F, Zhang L L, et al. Highly compressible zinc-ion batteries with stable performance. J Mater Chem A, 2019, 7, 11734 doi: 10.1039/C9TA02990G[84] Zhang Y, Wang Q R, Bi S S, et al. Flexible all-in-one zinc-ion batteries. Nanoscale, 2019, 11, 17630 doi: 10.1039/C9NR06476A[85] Huang S, Wan F, Bi S S, et al. A self-healing integrated all-in-one zinc-ion battery. Angew Chem, 2019, 131, 4357 doi: 10.1002/ange.201814653[86] Bi S S, Wan F, Huang S, et al. A flexible quasi-solid-state bifunctional device with zinc-ion microbattery and photodetector. ChemElectroChem, 2019, 6, 3933 doi: 10.1002/celc.201900966[87] Zhao Y, Wang Y N, Zhao Z M, et al. Achieving high capacity and long life of aqueous rechargeable zinc battery by using nanoporous-carbon-supported poly(1, 5-naphthalenediamine) nanorods as cathode. Energy Storage Mater, 2020, 28, 64 doi: 10.1016/j.ensm.2020.03.001[88] Wang J Q, Liu J, Hu M M, et al. A flexible, electrochromic, rechargeable Zn//PPy battery with a short circuit chromatic warning function. J Mater Chem A, 2018, 6, 11113 doi: 10.1039/C8TA03143F[89] Li Z X, Huang Y, Zhang J Y, et al. One-step synthesis of MnOx/PPy nanocomposite as a high-performance cathode for a rechargeable zinc-ion battery and insight into its energy storage mechanism. Nanoscale, 2020, 12, 4150 doi: 10.1039/C9NR09870D[90] Li X W, Xie X L, Lv R, et al. Nanostructured polypyrrole composite aerogels for a rechargeable flexible aqueous Zn-ion battery with high rate capabilities. Energy Technol, 2019, 7, 1801092 doi: 10.1002/ente.201801092[91] Li S, Sultana I, Guo Z P, et al. Polypyrrole as cathode materials for Zn-polymer battery with various biocompatible aqueous electrolytes. Electrochim Acta, 2013, 95, 212 doi: 10.1016/j.electacta.2013.02.055[92] Lahiri A, Yang L, Li G Z, et al. Mechanism of Zn-ion intercalation/deintercalation in a Zn-polypyrrole secondary battery in aqueous and bio-ionic liquid electrolytes. ACS Appl Mater Interfaces, 2019, 11, 45098 doi: 10.1021/acsami.9b15340[93] Grgur B N, Gvozdenović M M, Stevanović J, et al. Polypyrrole as possible electrode materials for the aqueous-based rechargeable zinc batteries. Electrochim Acta, 2008, 53, 4627 doi: 10.1016/j.electacta.2008.01.056[94] Cxiricx-Marjanovicx G, Mentus S. Charge-discharge characteristics of polythiopheneas a cathode active material in a rechargeable battery. J Appl Electrochem, 1998, 28, 103 doi: 10.1023/A:1003210220871[95] Simons T J, Salsamendi M, Howlett P C, et al. Rechargeable Zn/PEDOT battery with an imidazolium-based ionic liquid as the electrolyte. ChemElectroChem, 2015, 2, 2071 doi: 10.1002/celc.201500278[96] Fdz de Anastro A, Casado N, Wang X E, et al. Poly(ionic liquid) iongels for all-solid rechargeable zinc/PEDOT batteries. Electrochim Acta, 2018, 278, 271 doi: 10.1016/j.electacta.2018.05.044[97] Pandey P C. Electrochemical synthesis of polyindole and its evaluation for rechargeable battery applications. J Electrochem Soc, 1998, 145, 999 doi: 10.1149/1.1838377[98] Cai Z J, Hou C W. Study on the electrochemical properties of zinc/polyindole secondary battery. J Power Sources, 2011, 196, 10731 doi: 10.1016/j.jpowsour.2011.08.060[99] Liu Z, Prowald A, Höfft O, et al. An ionic liquid-surface functionalized polystyrene spheres hybrid electrolyte for rechargeable zinc/conductive polymer batteries. ChemElectroChem, 2018, 5, 2321 doi: 10.1002/celc.201800805[100] Cai Z J, Guo J, Yang H Z, et al. Electrochemical properties of electrospun poly(5-cyanoindole) submicron-fibrous electrode for zinc/polymer secondary battery. J Power Sources, 2015, 279, 114 doi: 10.1016/j.jpowsour.2014.12.145[101] Nigrey P J. Lightweight rechargeable storage batteries using polyacetylene, (CH)x as the cathode-active material. J Electrochem Soc, 1981, 128, 1651 doi: 10.1149/1.2127704[102] Häupler B, Rössel C, Schwenke A M, et al. Aqueous zinc-organic polymer battery with a high rate performance and long lifetime. NPG Asia Mater, 2016, 8, e283 doi: 10.1038/am.2016.82[103] Wang X S, Chen L, Lu F, et al. Boosting aqueous Zn2+ storage in 1, 4, 5, 8-naphthalenetetracarboxylic dianhydride through nitrogen substitution. ChemElectroChem, 2019, 6, 3644 doi: 10.1002/celc.201900750[104] Peng C, Ning G H, Su J, et al. Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat Energy, 2017, 2, 17074 doi: 10.1038/nenergy.2017.74[105] Kapaev R R, Zhidkov I S, Kurmaev E Z, et al. Hexaazatriphenylene-based polymer cathode for fast and stable lithium-, sodium- and potassium-ion batteries. J Mater Chem A, 2019, 7, 22596 doi: 10.1039/C9TA06430C[106] Tie Z W, Liu L J, Deng S Z, et al. Proton insertion chemistry of a zinc-organic battery. Angew Chem Int Ed, 2020, 59, 4920 doi: 10.1002/anie.201916529[107] Cheng L W, Liang Y H, Zhu Q N, et al. Bio-inspired isoalloxazine redox moieties for rechargeable aqueous zinc-ion batteries. Chem - Asian J, 2020, 15, 1290 doi: 10.1002/asia.202000283[108] Glatz H, Lizundia E, Pacifico F, et al. An organic cathode based dual-ion aqueous zinc battery enabled by a cellulose membrane. ACS Appl Energy Mater, 2019, 2, 1288 doi: 10.1021/acsaem.8b01851[109] Koshika K, Sano N, Oyaizu K, et al. An aqueous, electrolyte-type, rechargeable device utilizing a hydrophilic radical polymer-cathode. Macromol Chem Phys, 2009, 210, 1989 doi: 10.1002/macp.200900257[110] Luo Y W, Zheng F P, Liu L J, et al. A high-power aqueous zinc-organic radical battery with tunable operating voltage triggered by selected anions. ChemSusChem, 2020, 13, 2239 doi: 10.1002/cssc.201903083[111] Jiang C, Tang M, Zhu S L, et al. Constructing universal ionic sieves via alignment of two-dimensional covalent organic frameworks (COFs). Angew Chem Int Ed, 2018, 57, 16072 doi: 10.1002/anie.201809907[112] Jiang C, Wang C L. 2D materials as ionic sieves for inhibiting the shuttle effect in batteries. Chem - Asian J, 2020, 15, 2294 doi: 10.1002/asia.201901475[113] Yan L J, Zhao C X, Sha Y, et al. Electrochemical redox behavior of organic quinone compounds in aqueous metal ion electrolytes. Nano Energy, 2020, 73, 104766 doi: 10.1016/j.nanoen.2020.104766[114] Yan L J, Zeng X M, Li Z H, et al. An innovation: Dendrite free quinone paired with ZnMn2O4 for zinc ion storage. Mater Today Energy, 2019, 13, 323 doi: 10.1016/j.mtener.2019.06.011[115] Guerfi A, Trottier J, Boyano I, et al. High cycling stability of zinc-anode/conducting polymer rechargeable battery with non-aqueous electrolyte. J Power Sources, 2014, 248, 1099 doi: 10.1016/j.jpowsour.2013.09.082[116] Kitani A. Performance study of aqueous polyaniline batteries. J Electrochem Soc, 1986, 133, 1069 doi: 10.1149/1.2108787[117] Liu P F, Lv R, He Y, et al. An integrated, flexible aqueous Zn-ion battery with high energy and power densities. J Power Sources, 2019, 410/411, 137 doi: 10.1016/j.jpowsour.2018.11.017[118] Shan D, Mu S L. Electrochemical characteristics of polyaniline synthesized in the presence of ferrocenesulfonic acid. Synth Met, 2002, 126, 225 doi: 10.1016/S0379-6779(01)00571-9[119] Karami H, Mousavi M F, Shamsipur M. A new design for dry polyaniline rechargeable batteries. J Power Sources, 2003, 117, 255 doi: 10.1016/S0378-7753(03)00168-X -
Proportional views





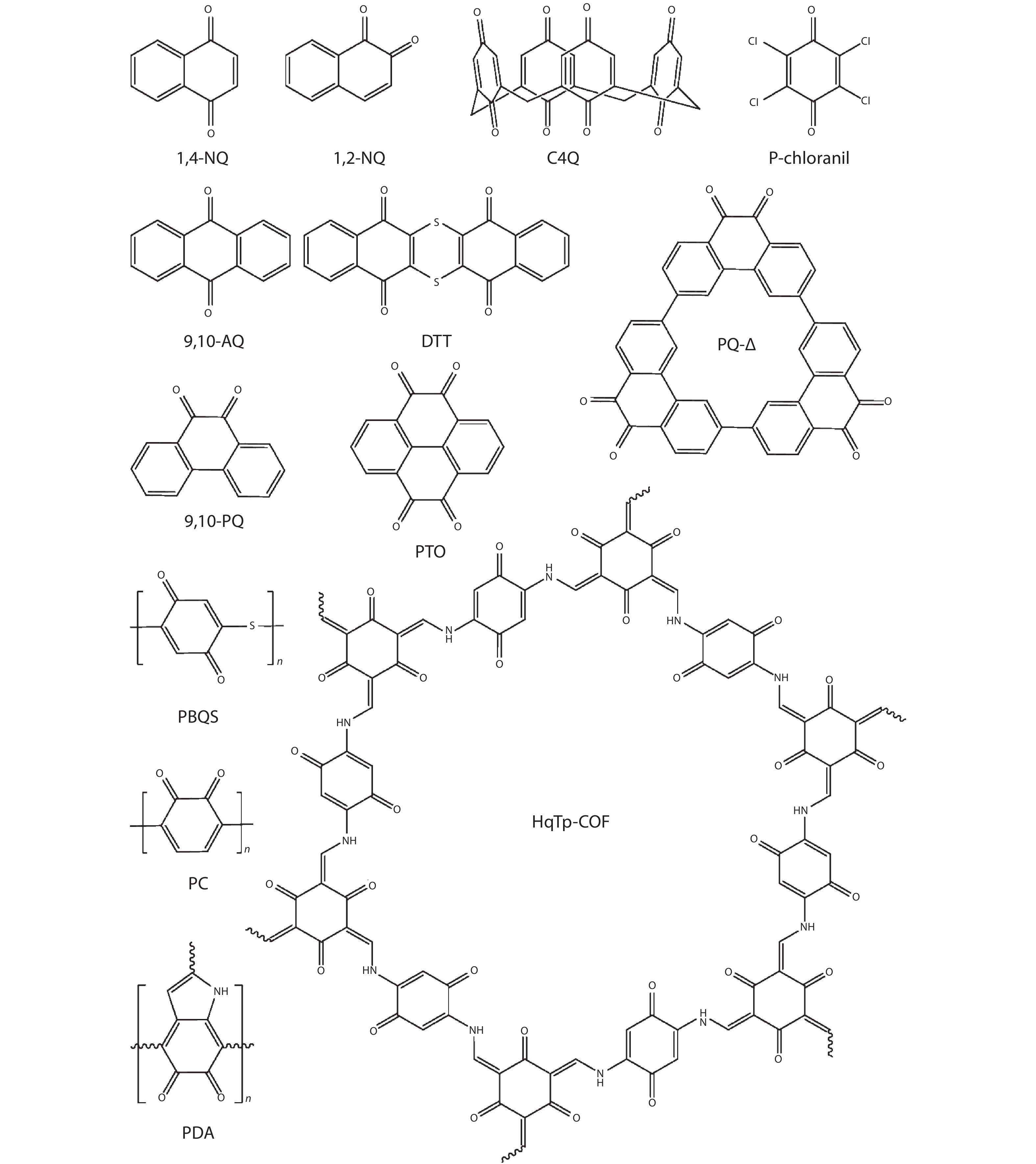
 DownLoad:
DownLoad: