| Citation: |
Anxin Sun, Jingui Xu, Guanhua Zong, Zuo Xiao, Yong Hua, Bin Zhang, Liming Ding. A wide-bandgap copolymer donor with a 5-methyl-4H-dithieno[3,2-e:2',3'-g]isoindole-4,6(5H)-dione unit[J]. Journal of Semiconductors, 2021, 42(10): 100502. doi: 10.1088/1674-4926/42/10/100502
****
A X Sun, J G Xu, G H Zong, Z Xiao, Y Hua, B Zhang, L M Ding, A wide-bandgap copolymer donor with a 5-methyl-4H-dithieno[3,2-e:2\',3\'-g]isoindole-4,6(5H)-dione unit[J]. J. Semicond., 2021, 42(10): 100502. doi: 10.1088/1674-4926/42/10/100502.
|
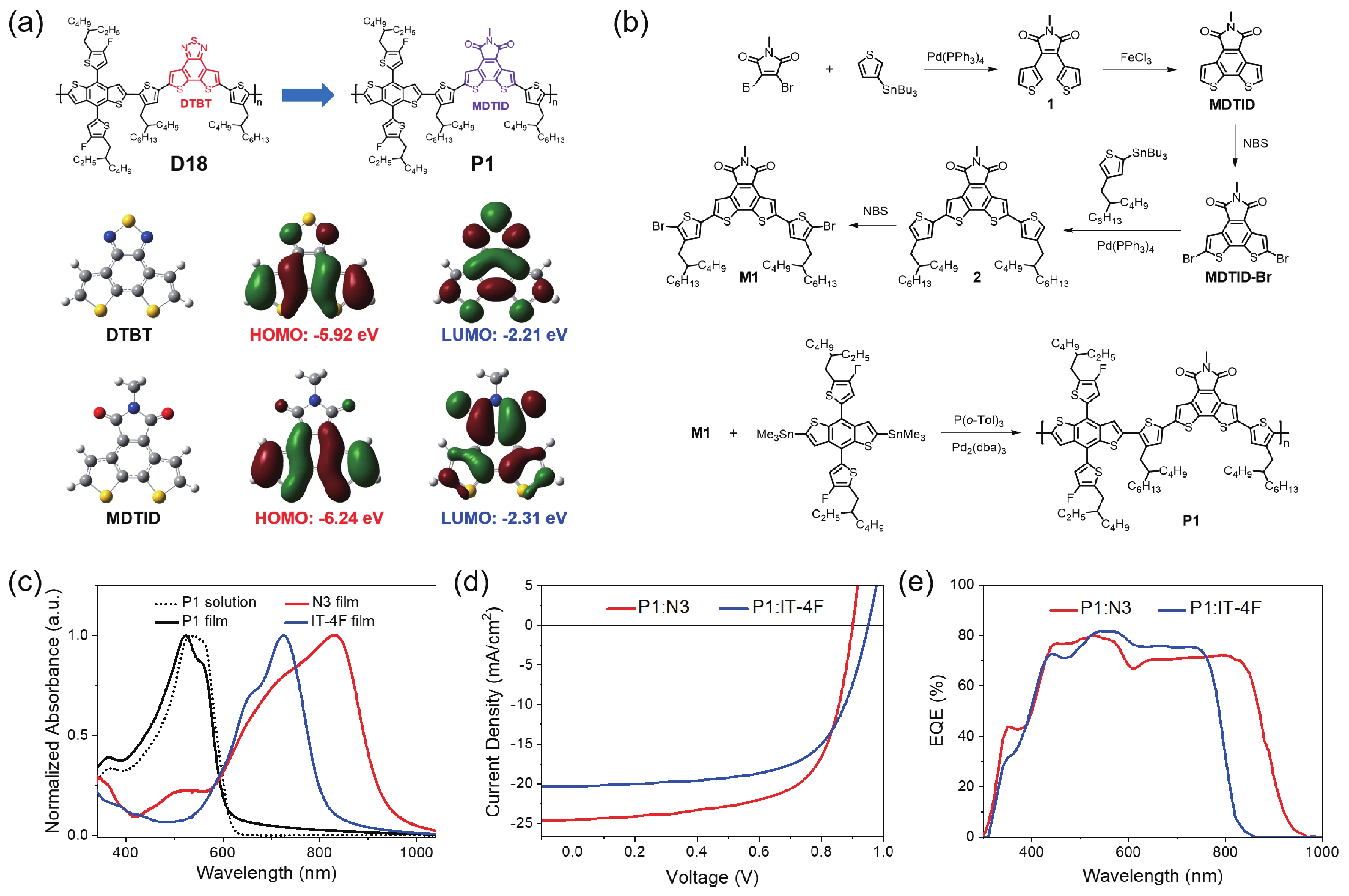
A wide-bandgap copolymer donor with a 5-methyl-4H-dithieno[3,2-e:2',3'-g]isoindole-4,6(5H)-dione unit
DOI: 10.1088/1674-4926/42/10/100502
More Information
-
References
[1] Tong Y, Xiao Z, Du X, et al. Progress of the key materials for organic solar cells. Sci China Chem, 2020, 63, 758 doi: 10.1007/s11426-020-9726-0[2] Duan C, Ding L. The new era for organic solar cells: polymer donors. Sci Bull, 2020, 65, 1422 doi: 10.1016/j.scib.2020.04.044[3] Jin K, Xiao Z, Ding L. D18, an eximious solar polymer!. J Semicond, 2021, 42, 010502 doi: 10.1088/1674-4926/42/1/010502[4] Armin A, Li W, Sandberg O J, et al. A history and perspective of non-fullerene electron acceptors for organic solar cells. Adv Energy Mater, 2021, 11, 20003570 doi: 10.1002/aenm.202003570[5] Zhang M, Guo X, Ma W, et al. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv Mater, 2015, 27, 4655 doi: 10.1002/adma.201502110[6] Zhang S, Qin Y, Zhu J, et al. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv Mater, 2018, 30, 1800868 doi: 10.1002/adma.201800868[7] Xu Y, Cui Y, Yao H, et al. A new conjugated polymer that enables the integration of photovoltaic and light-emitting functions in one device. Adv Mater, 2021, 33, 2101090 doi: 10.1002/adma.202101090[8] Sun C, Pan F, Bin H, et al. A low cost and high performance polymer donor material for polymer solar cells. Nat Commun, 2018, 9, 743 doi: 10.1038/s41467-018-03207-x[9] Zhu C, Meng L, Zhang J, et al. A quinoxaline-based D-A copolymer donor achieving 17.62% efficiency of organic solar cells. Adv Mater, 2021, 33, 2100474 doi: 10.1002/adma.202100474[10] Fan B, Zhang D, Li M, et al. Achieving over 16% efficiency for single-junction organic solar cells. Sci China Chem, 2019, 62, 746 doi: 10.1007/s11426-019-9457-5[11] Xiong J, Jin K, Jiang Y, et al. Thiolactone copolymer donor gifts organic solar cells a 16.72% efficiency. Sci Bull, 2019, 64, 1573 doi: 10.1016/j.scib.2019.10.002[12] Jiang Y, Jin K, Chen X, et al. Post-sulphuration enhances the performance of a lactone polymer donor. J Semicond, 2021, 42, 070501 doi: 10.1088/1674-4926/42/7/070501[13] Liu Q, Jiang Y, Jin K, et al. 18% efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[14] Qin J, Zhang L, Xiao Z, et al. Over 16% efficiency from thick-film organic solar cells. Sci Bull, 2020, 65, 1979 doi: 10.1016/j.scib.2020.08.027[15] Qin J, Zhang L, Zuo C, et al. A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency. J Semicond, 2021, 42, 010501 doi: 10.1088/1674-4926/42/1/010501[16] Jin K, Xiao Z, Ding L. 18.69% PCE from organic solar cells. J Semicond, 2021, 42, 060502 doi: 10.1088/1674-4926/42/6/060502[17] Grzybowski M, Skonieczny K, Butenschon H, et al. Comparison of oxidative aromatic coupling and the Scholl reaction. Angew Chem Int Ed, 2013, 52, 9900 doi: 10.1002/anie.201210238[18] Ziffer M E, Jo S B, Liu Y, et al. Tuning H- and J-aggregate behavior in π-conjugated polymers via noncovalent interactions. J Phys Chem C, 2018, 122, 18860 doi: 10.1021/acs.jpcc.8b05505[19] Jiang K, Wei Q, Lai J Y L, et al. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells. Joule, 2019, 3, 3020 doi: 10.1016/j.joule.2019.09.010[20] Zhao W, Li S, Yao H, et al. Molecular optimization enables over 13% efficiency in organic solar cells. J Am Chem Soc, 2017, 139, 7148 doi: 10.1021/jacs.7b02677[21] Liu J, Liu L, Zuo C, et al. 5H-dithieno[3,2-b:2',3'-d]pyran-5-one unit yields efficient wide-bandgap polymer donors. Sci Bull, 2019, 64, 1655 doi: 10.1016/j.scib.2019.09.001[22] Xiao Z, Geng X, He D, et al. Development of isomer-free fullerene bisadducts for efficient polymer solar cells. Energy Environ Sci, 2016, 9, 2114 doi: 10.1039/C6EE01026A[23] Xiao Z, Jia X, Ding L, et al. Ternary organic solar cells offer 14% power conversion efficiency. Sci Bull, 2017, 62, 1562 doi: 10.1016/j.scib.2017.11.003[24] Wang T, Qin J, Xiao Z, et al. A 2.16 eV bandgap polymer donor gives 16% power conversion efficiency. Sci Bull, 2020, 65, 179 doi: 10.1016/j.scib.2019.11.030[25] Luo Y, Chen X, Xiao Z, et al. A large-bandgap copolymer donor for efficient ternary organic solar cells. Mater Chem Front, 2021, 5, 6139 doi: 10.1039/D1QM00835H[26] Wang T, Qin J, Xiao Z, et al. Multiple conformation locks gift polymer donor high efficiency. Nano Energy, 2020, 77, 105161 doi: 10.1016/j.nanoen.2020.105161[27] Xiao Z, Jia X, Li D, et al. 26 mA cm–2 Jsc from organic solar cells with a low-bandgap nonfullerene acceptor. Sci Bull, 2017, 62, 1494 doi: 10.1016/j.scib.2017.10.017[28] Liu L, Liu Q, Xiao Z, et al. Induced J-aggregation in acceptor alloy enhances photocurrent. Sci Bull, 2019, 64, 1083 doi: 10.1016/j.scib.2019.06.005[29] Xiao Z, Liu F, Geng X, et al. A carbon-oxygen-bridged ladder-type building block for efficient donor and acceptor materials used in organic solar cells. Sci Bull, 2017, 62, 1331 doi: 10.1016/j.scib.2017.09.017 -
Supplements
 2021-100502suppl.pdf
2021-100502suppl.pdf

-
Proportional views





 DownLoad:
DownLoad:
















