| Citation: |
Xiongfeng Li, Jingui Xu, Zuo Xiao, Xingzhu Wang, Bin Zhang, Liming Ding. Dithieno[3',2':3,4;2'',3'':5,6]benzo[1,2-c][1,2,5]oxadiazole-based polymer donors with deep HOMO levels[J]. Journal of Semiconductors, 2021, 42(6): 060501. doi: 10.1088/1674-4926/42/6/060501
****
X F Li, J G Xu, Z Xiao, X Z Wang, B Zhang, L M Ding, Dithieno[3\',2\':3,4;2\'\',3\'\':5,6]benzo[1,2-c][1,2,5]oxadiazole-based polymer donors with deep HOMO levels[J]. J. Semicond., 2021, 42(6): 060501. doi: 10.1088/1674-4926/42/6/060501.
|
Dithieno[3',2':3,4;2'',3'':5,6]benzo[1,2-c][1,2,5]oxadiazole-based polymer donors with deep HOMO levels
DOI: 10.1088/1674-4926/42/6/060501
More Information
-
References
[1] Lin Y, Wang J, Zhang Z, et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv Mater, 2015, 27, 1170 doi: 10.1002/adma.201404317[2] Lin Y, He Q, Zhao F, et al. A facile planar fused-ring electron acceptor for as-cast polymer solar cells with 8.71% efficiency. J Am Chem Soc, 2016, 138, 2973 doi: 10.1021/jacs.6b00853[3] Holliday S, Ashraf R S, Wadsworth A, et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat Commun, 2016, 7, 11585 doi: 10.1038/ncomms11585[4] Zhao W, Li S, Yao H, et al. Molecular optimization enables over 13% efficiency in organic solar cells. J Am Chem Soc, 2017, 139, 7148 doi: 10.1021/jacs.7b02677[5] Xiao Z, Liu F, Geng X, et al. A carbon-oxygen-bridged ladder-type building block for efficient donor and acceptor materials used in organic solar cells. Sci Bull, 2017, 62, 1331 doi: 10.1016/j.scib.2017.09.017[6] Yuan J, Zhang Y, Zhou L, et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule, 2019, 3, 1140 doi: 10.1016/j.joule.2019.01.004[7] Jin K, Xiao Z, Ding L. D18, an eximious solar polymer!. J Semicond, 2021, 42, 010502 doi: 10.1088/1674-4926/42/1/010502[8] Tong Y, Xiao Z, Du X, et al. Progress of the key materials for organic solar cells. Sci China Chem, 2020, 63, 758 doi: 10.1007/s11426-020-9726-0[9] Zhang M, Guo X, Ma W, et al. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv Mater, 2015, 27, 4655 doi: 10.1002/adma.201502110[10] Cui Y, Yao H, Zhang J, et al. Single-junction organic photovoltaic cells with approaching 18% efficiency. Adv Mater, 2020, 32, 1908205 doi: 10.1002/adma.201908205[11] Sun C, Pan F, Bin H, et al. A low cost and high performance polymer donor material for polymer solar cells. Nat Commun, 2018, 9, 743 doi: 10.1038/s41467-018-03207-x[12] Wu Y, Zheng Y, Yang H, et al. Rationally pairing photoactive materials for high-performance polymer solar cells with efficiency of 16.53%. Sci China Chem, 2020, 63, 265 doi: 10.1007/s11426-019-9599-1[13] Lan L, Chen Z, Hu Q, et al. High-performance polymer solar cells based on a wide-bandgap polymer containing pyrrolo[3,4-f] benzotriazole-5,7-dione with a power conversion efficiency of 8.63%. Adv Sci, 2016, 3, 1600032 doi: 10.1002/advs.201600032[14] Fan B, Zhang D, Li M, et al. Achieving over 16% efficiency for single-junction organic solar cells. Sci China Chem, 2019, 62, 746 doi: 10.1007/s11426-019-9457-5[15] Wang T, Qin J, Xiao Z, et al. A 2.16 eV bandgap polymer donor gives 16% power conversion efficiency. Sci Bull, 2020, 65, 179 doi: 10.1016/j.scib.2019.11.030[16] Wang T, Qin J, Xiao Z, et al. Multiple conformation locks gift polymer donor high efficiency. Nano Energy, 2020, 77, 105161 doi: 10.1016/j.nanoen.2020.105161[17] Liu J, Liu L, Zuo C, et al. 5H-dithieno[3,2-b:2',3'-d]pyran-5-one unit yields efficient wide-bandgap polymer donors. Sci Bull, 2019, 64, 1655 doi: 10.1016/j.scib.2019.09.001[18] Xiong J, Jin K, Jiang Y, et al. Thiolactone copolymer donor gifts organic solar cells a 16.72% efficiency. Sci Bull, 2019, 64, 1573 doi: 10.1016/j.scib.2019.10.002[19] Liu Q, Jiang Y, Jin K, et al. 18% Efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[20] Qin J, Zhang L, Xiao Z, et al. Over 16% efficiency from thick-film organic solar cells. Sci Bull, 2020, 65, 1979 doi: 10.1016/j.scib.2020.08.027[21] Qin J, Zhang L, Zuo C, et al. A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency. J Semicond, 2021, 42, 010501 doi: 10.1088/1674-4926/42/1/010501[22] Lee J, Sin D H, Clement J A, et al. Medium-bandgap conjugated polymers containing fused dithienobenzochalcogenadiazoles: Chalcogen atom effects on organic photovoltaics. Macromolecules, 2016, 49, 9358 doi: 10.1021/acs.macromol.6b01569[23] Rand B P, Burk D P, Forrest S R. Offset energies at organic semiconductor heterojunctions and their influence on the open-circuit voltage of thin-film solar cells. Phys Rev B, 2007, 75, 115327 doi: 10.1103/PhysRevB.75.115327[24] Xiong J, Xu J, Jiang Y, et al. Fused-ring bislactone building blocks for polymer donors. Sci Bull, 2020, 65, 1792 doi: 10.1016/j.scib.2020.07.018[25] Xiao Z, Geng X, He D, et al. Development of isomer-free fullerene bisadducts for efficient polymer solar cells. Energy Environ Sci, 2016, 9, 2114 doi: 10.1039/C6EE01026A[26] Gao Y, Li D, Xiao Z, et al. High-performance wide-bandgap copolymers with dithieno[3,2-b:2',3'-d]pyridin-5(4H)-one units. Mater Chem Front, 2019, 3, 399 doi: 10.1039/C8QM00604K[27] Deng L, Li X, Wang S, et al. Stereomeric effects of bisPC71BM on polymer solar cell performance. Sci Bull, 2016, 61, 132 doi: 10.1007/s11434-015-0979-5[28] Wang J, Gao Y, Xiao Z, et al. A wide-bandgap copolymer donor based on a phenanthridin-6(5H)-one unit. Mater Chem Front, 2019, 3, 2686 doi: 10.1039/C9QM00622B[29] Zhang L, Jin K, Xiao Z, et al. Alkoxythiophene and alkylthiothiophene π-bridges enhance the performance of A-D-A electron acceptors. Mater Chem Front, 2019, 3, 492 doi: 10.1039/C8QM00647D[30] Jin K, Deng C, Zhang L, et al. A heptacyclic carbon-oxygen-bridged ladder-type building block for A-D-A acceptors. Mater Chem Front, 2018, 2, 1716 doi: 10.1039/C8QM00285A[31] Li W, Liu Q, Jin K, et al. Fused-ring phenazine building blocks for efficient copolymer donors. Mater Chem Front, 2020, 4, 1454 doi: 10.1039/D0QM00080A -
Supplements
 21030023suppl.pdf
21030023suppl.pdf
-
Proportional views





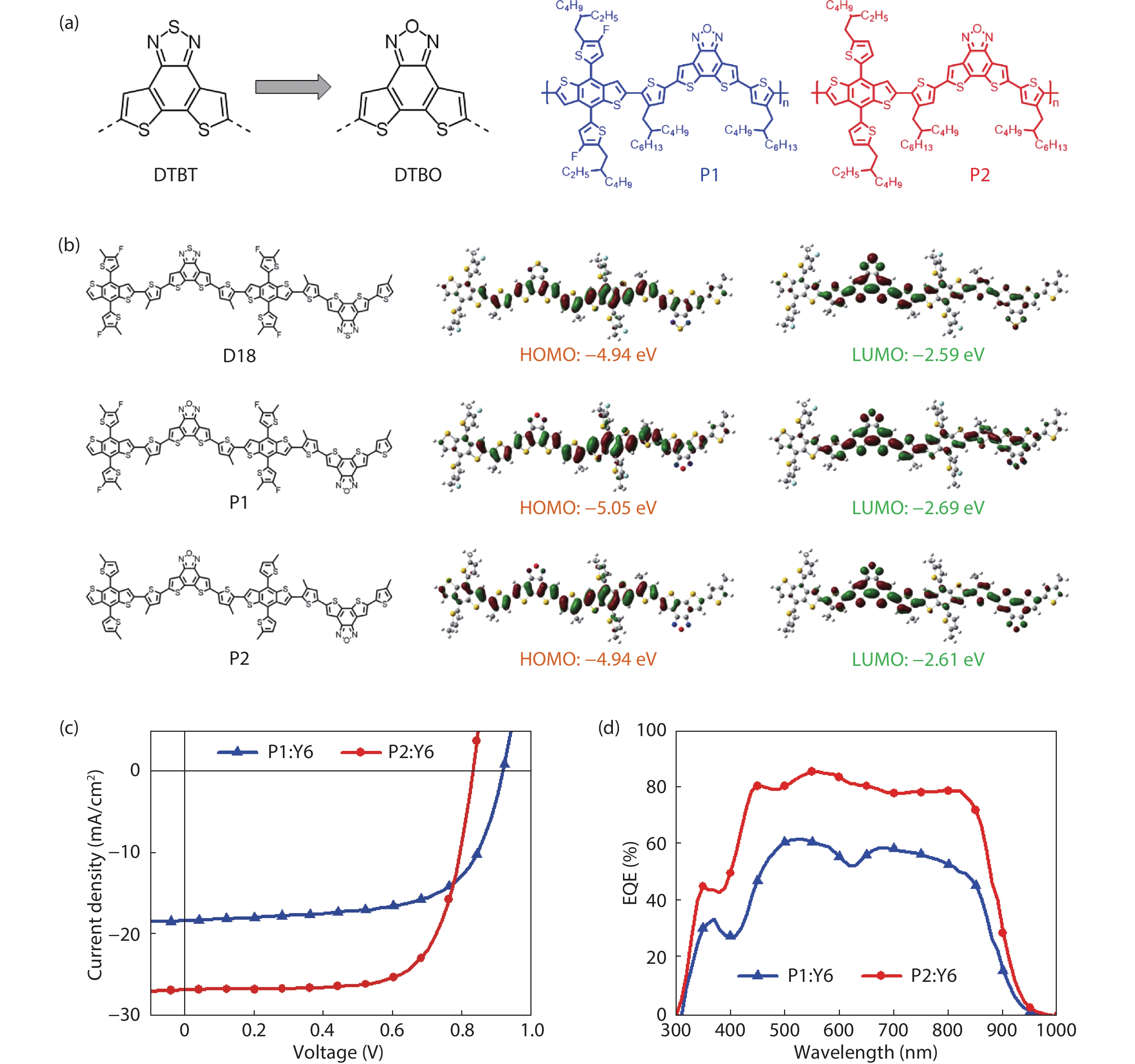
 DownLoad:
DownLoad:
















