| Citation: |
Tian Tian, Meifang Yang, Jianyu Yang, Wuqiang Wu, Liming Ding. Stabilizing black-phase CsPbI3 under over 70% humidity[J]. Journal of Semiconductors, 2022, 43(3): 030501. doi: 10.1088/1674-4926/43/3/030501
****
T Tian, M F Yang, J Y Yang, W Q Wu, L M Ding. Stabilizing black-phase CsPbI3 under over 70% humidity[J]. J. Semicond, 2022, 43(3): 030501. doi: 10.1088/1674-4926/43/3/030501
|
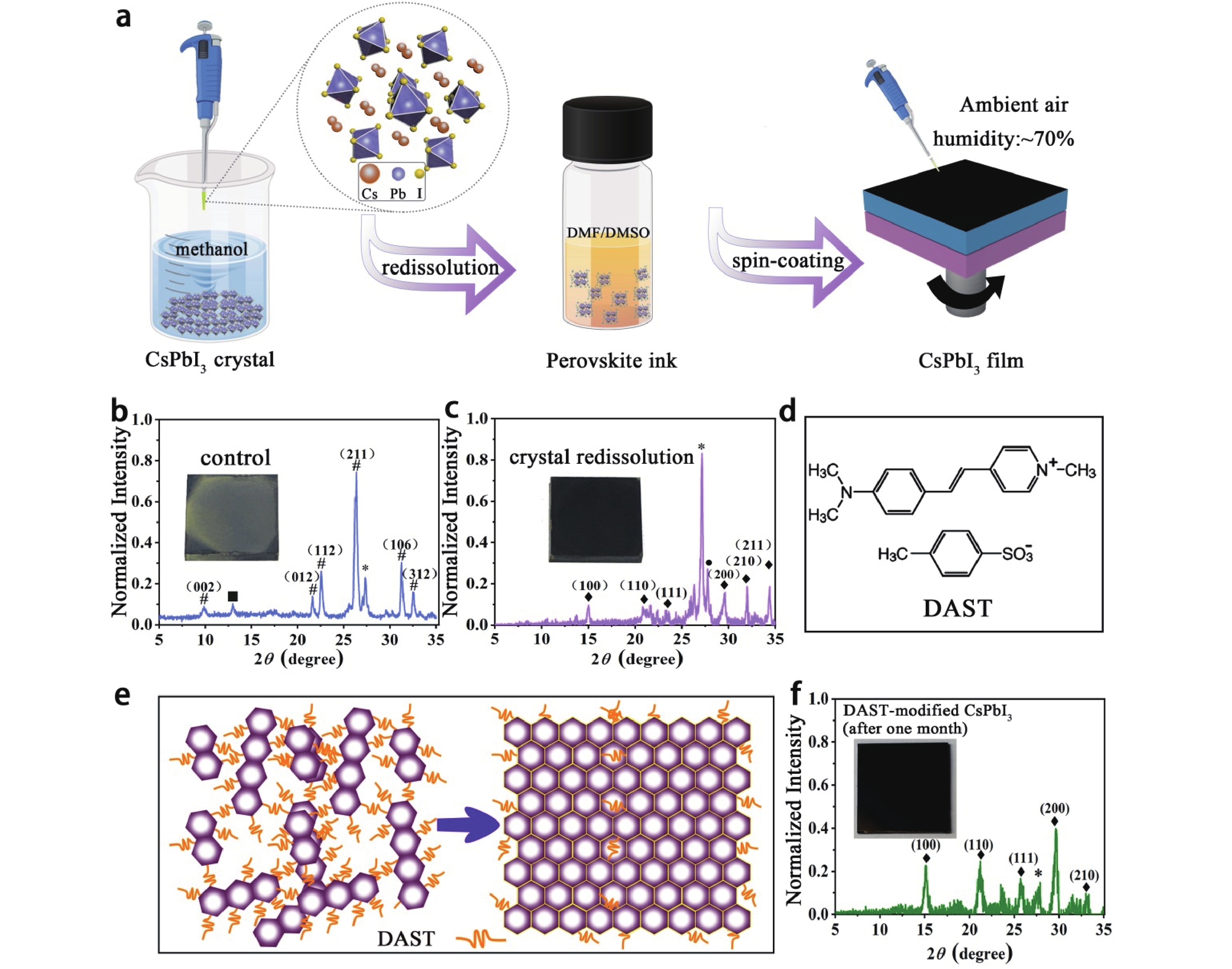
Stabilizing black-phase CsPbI3 under over 70% humidity
DOI: 10.1088/1674-4926/43/3/030501
More Information
-
References
[1] Sutton R J, Eperon G E, Miranda L, et al. Bandgap-tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv Energy Mater, 2016, 6(8), 1502458 doi: 10.1002/aenm.201502458[2] Lin L, Jiang L, Li P, et al. Simulated development and optimized performance of CsPbI3 based all-inorganic perovskite solar cells. Solar Energy, 2020, 198(1), 454 doi: 10.1016/j.solener.2020.01.081[3] Yu B, Zuo C, Shi J, et al. Defect engineering on all-inorganic perovskite solar cells for high efficiency. J Semicond, 2021, 42(5), 050203 doi: 10.1088/1674-4926/42/5/050203[4] Tang Y, Lesage A, Schall P. CsPbI3 nanocrystal films: towards higher stability and efficiency. J Mater Chem C, 2020, 8(48), 17139 doi: 10.1039/D0TC04475J[5] Swarnkar A, Marshall A R, Sanehira E M, et al. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science, 2016, 354(6308), 92 doi: 10.1126/science.aag2700[6] Sutton R J, Filip M R, Haghighirad A A, et al. Cubic or orthorhombic? Revealing the crystal structure of metastable black-phase CsPbI3 by theory and experiment. ACS Energy Lett, 2018, 3(8), 1787 doi: 10.1021/acsenergylett.8b00672[7] Straus D B, Guo S, Abeykoon A M, et al. Understanding the instability of the halide perovskite CsPbI3 through temperature-dependent structural analysis. Adv Mater, 2020, 32(32), 2001069 doi: 10.1002/adma.202001069[8] Li B, Zhang Y, Fu L, et al. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat Commun, 2018, 9(1076), 1076 doi: 10.1038/s41467-018-03169-0[9] Ke F, Wang C, Jia C, et al. Preserving a robust CsPbI3 perovskite phase via pressure-directed octahedral tilt. Nat Commun, 2021, 12(461), 461 doi: 10.1038/s41467-020-20745-5[10] Huang Q, Li F, Wang M, et al. Vapor-deposited CsPbI3 solar cells demonstrate an efficiency of 16%. Sci Bull, 2021, 66(8), 757 doi: 10.1016/j.scib.2020.12.024[11] Wang Q, Zheng X, Deng Y, et al. Stabilizing the α-Phase of CsPbI3 perovskite by sulfobetaine zwitterions in one-step spin-coating films. Joule, 2017, 1(2), 371 doi: 10.1016/j.joule.2017.07.017[12] Wang K, Jin Z, Liang L, et al. All-inorganic cesium lead iodide perovskite solar cells with stabilized efficiency beyond 15%. Nat Commun, 2018, 9, 4544 doi: 10.1038/s41467-018-06915-6[13] Zhang T, Wang F, Chen H, et al. Mediator-antisolvent strategy to stabilize all-inorganic CsPbI3 for perovskite solar cells with efficiency exceeding 16%. ACS Energy Lett, 2020, 5(5), 1619 doi: 10.1021/acsenergylett.0c00497[14] Hu Y, Bai F, Liu X, et al. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett, 2017, 2(10), 2219 doi: 10.1021/acsenergylett.7b00508[15] McMeekin D P, Sadoughi G, Rehman W, et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science, 2016, 351(6269), 151 doi: 10.1126/science.aad5845[16] Beal R E, Slotcavage D J, Leijtens T, et al. Cesium lead halide perovskites with improved stability for tandem solar cells. J Phys Chem Lett, 2016, 7(5), 746 doi: 10.1021/acs.jpclett.6b00002[17] Eperon G E, Paternò G M, Sutton R J, et al. Inorganic caesium lead iodide perovskite solar cells. J Mater Chem A, 2015, 3(39), 19688 doi: 10.1039/C5TA06398A[18] Hutter E M, Sutton R J, Chandrashekar S, et al. Vapour-deposited cesium lead iodide perovskites: microsecond charge carrier lifetimes and enhanced photovoltaic performance. ACS Energy Lett, 2017, 2(8), 1901 doi: 10.1021/acsenergylett.7b00591[19] Wang Y, Zhang T, Kan M, et al. Efficient α-CsPbI3 photovoltaics with surface terminated organic cations. Joule, 2018, 2(10), 2065 doi: 10.1016/j.joule.2018.06.013[20] Xu X, Zhang H, Li E, et al. Electron-enriched thione enables strong Pb-S interaction for stabilizing high quality CsPbI3 perovskite films with low-temperature processing. Chem Sci, 2020, 11(12), 3132 doi: 10.1039/C9SC06574A[21] Yoon S M, Min H, Kim J B, et al. Surface engineering of ambient-air-processed cesium lead triiodide layers for efficient solar cells. Joule, 2021, 5(1), 183 doi: 10.1016/j.joule.2020.11.020[22] Zhang T, Dar M I, Li G, et al. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci Adv, 2017, 3(9), e1700841 doi: 10.1126/sciadv.1700841[23] Zhang J, Liu J, Tan A, et al. Improved stability of β-CsPbI3 inorganic perovskite using π-conjugated bifunctional surface capped organic cations for high performance photovoltaics. Chem Commun, 2020, 56(89), 13816 doi: 10.1039/D0CC05386D[24] Ye T, Pan L, Yang Y, et al. Synthesis of highly-oriented black CsPbI3 microstructures for high-performance solar cells. Chem Mater, 2020, 32(7), 3235 doi: 10.1021/acs.chemmater.0c00427[25] Wang Y, Yuan J, Zhang X, et al. Surface ligand management aided by a secondary amine enables increased synthesis yield of CsPbI3 perovskite quantum dots and high photovoltaic performance. Adv Mater, 2020, 32(32), 2000449 doi: 10.1002/adma.202000449[26] Wang C, Chesman A S R, Jasieniak J J. Stabilizing the cubic perovskite phase of CsPbI3 nanocrystals by using an alkyl phosphinic acid. Chem Commun, 2017, 53(1), 232 doi: 10.1039/C6CC08282C[27] Shi J, Wang Y, Zhao Y. Inorganic CsPbI3 perovskites toward high-efficiency photovoltaics. Energy Environ Mater, 2019, 2(2), 73 doi: 10.1002/eem2.12039[28] Zhang Z, Li J, Fang Z, et al. Adjusting energy level alignment between HTL and CsPbI2Br to improve solar cell efficiency. J Semicond, 2021, 42(3), 030501 doi: 10.1088/1674-4926/42/3/030501 -
Supplements
 短通讯22010037suppl.pdf
短通讯22010037suppl.pdf

-
Proportional views

§‡ These authors contributed equally to this work




 DownLoad:
DownLoad:
















