| Citation: |
Zhen Fang, Yao Liu, Chengyi Song, Peng Tao, Wen Shang, Tao Deng, Xiaoqin Zeng, Jianbo Wu. In-situ monitoring of dynamic behavior of catalyst materials and reaction intermediates in semiconductor catalytic processes[J]. Journal of Semiconductors, 2022, 43(4): 041104. doi: 10.1088/1674-4926/43/4/041104
****
Z Fang, Y Liu, C Y Song, P Tao, W Shang, T Deng, X Q Zeng, J B Wu. In-situ monitoring of dynamic behavior of catalyst materials and reaction intermediates in semiconductor catalytic processes[J]. J. Semicond, 2022, 43(4): 041104. doi: 10.1088/1674-4926/43/4/041104
|
In-situ monitoring of dynamic behavior of catalyst materials and reaction intermediates in semiconductor catalytic processes
DOI: 10.1088/1674-4926/43/4/041104
More Information
-
Abstract
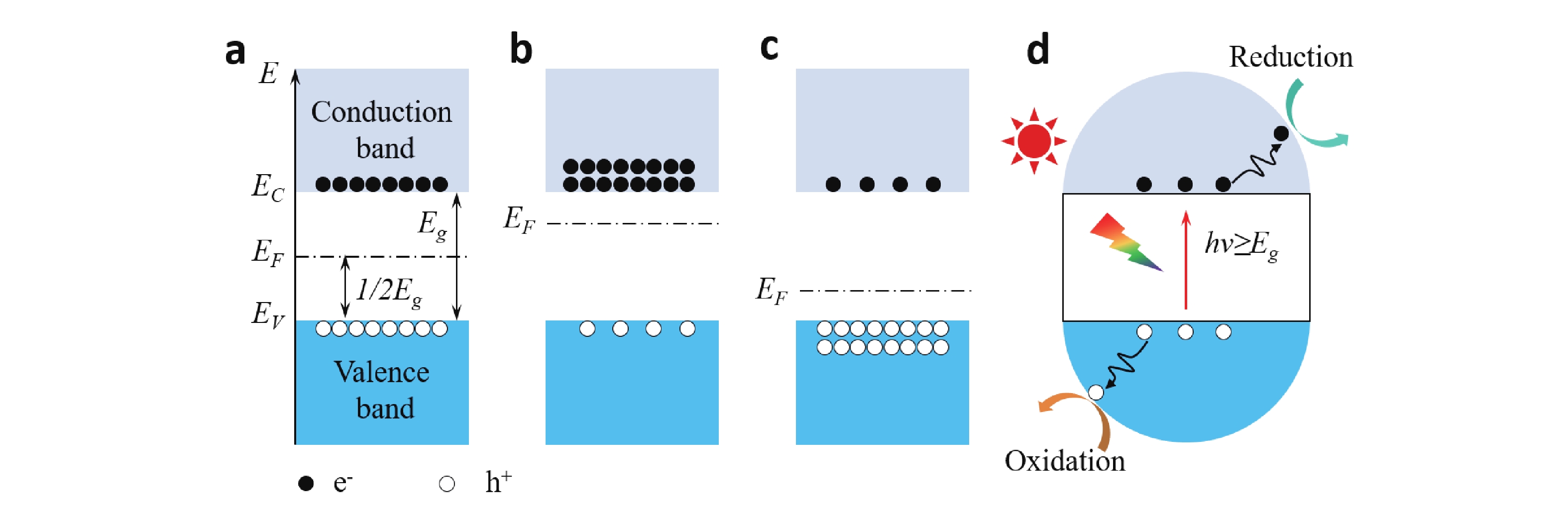
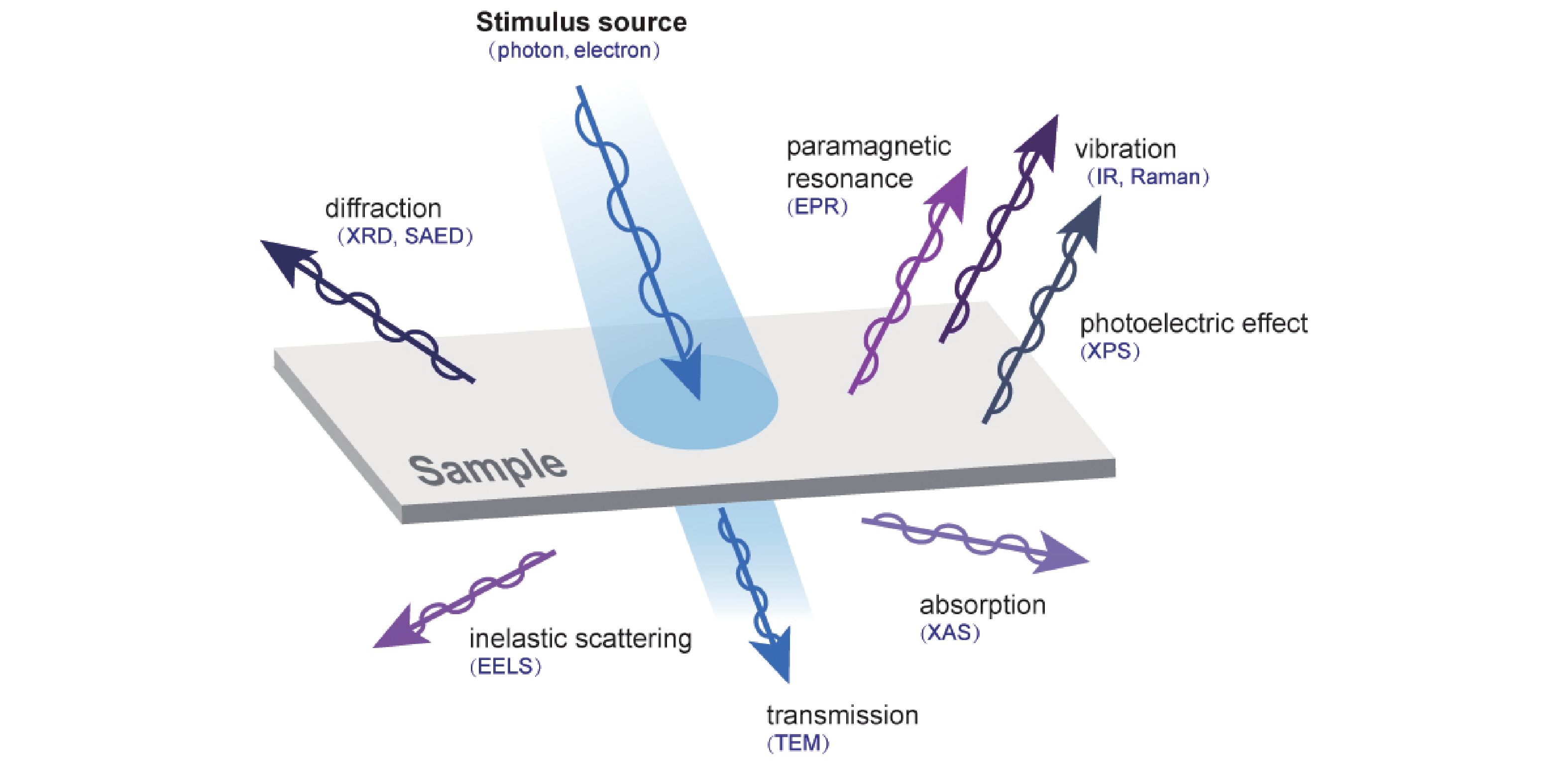
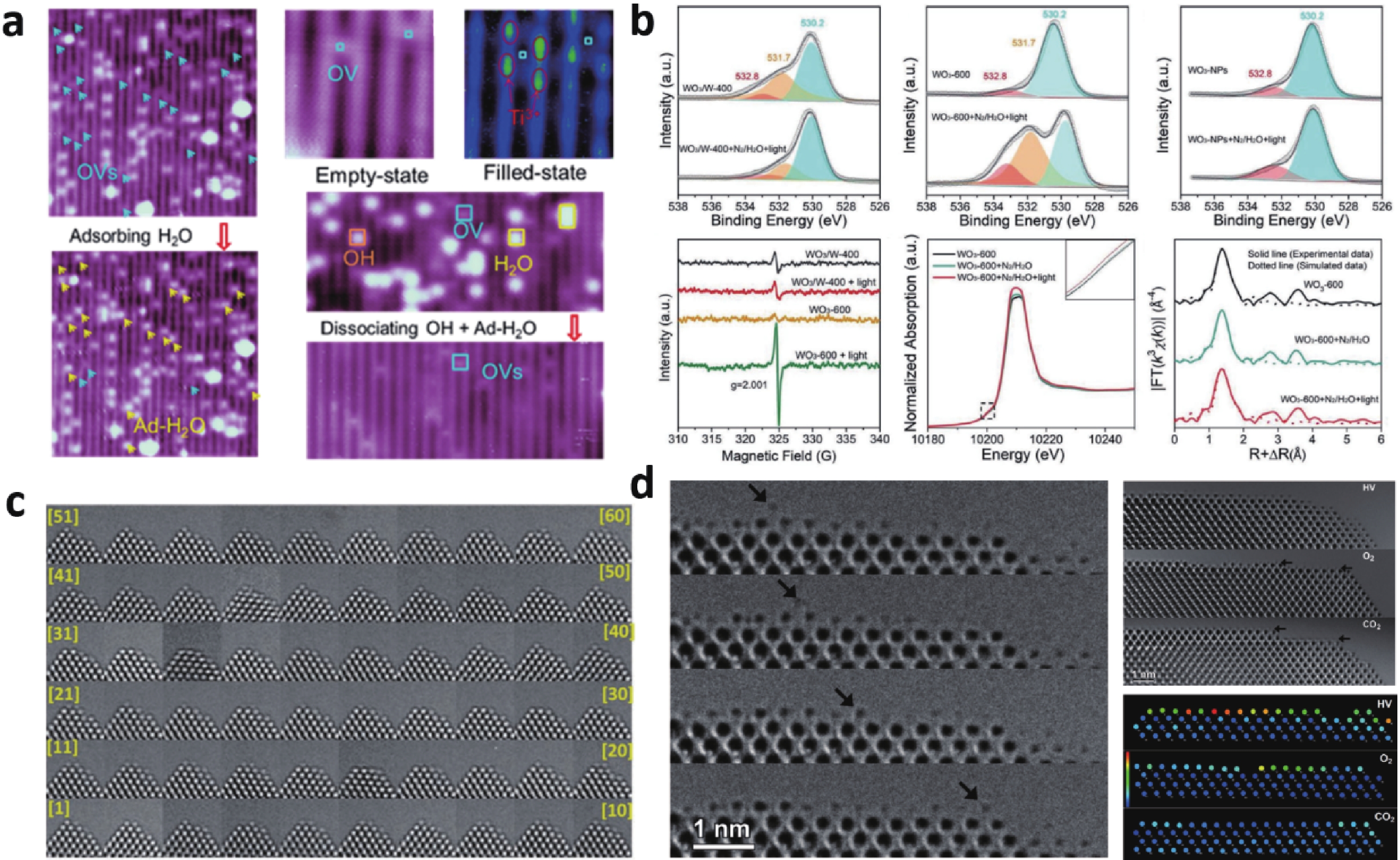
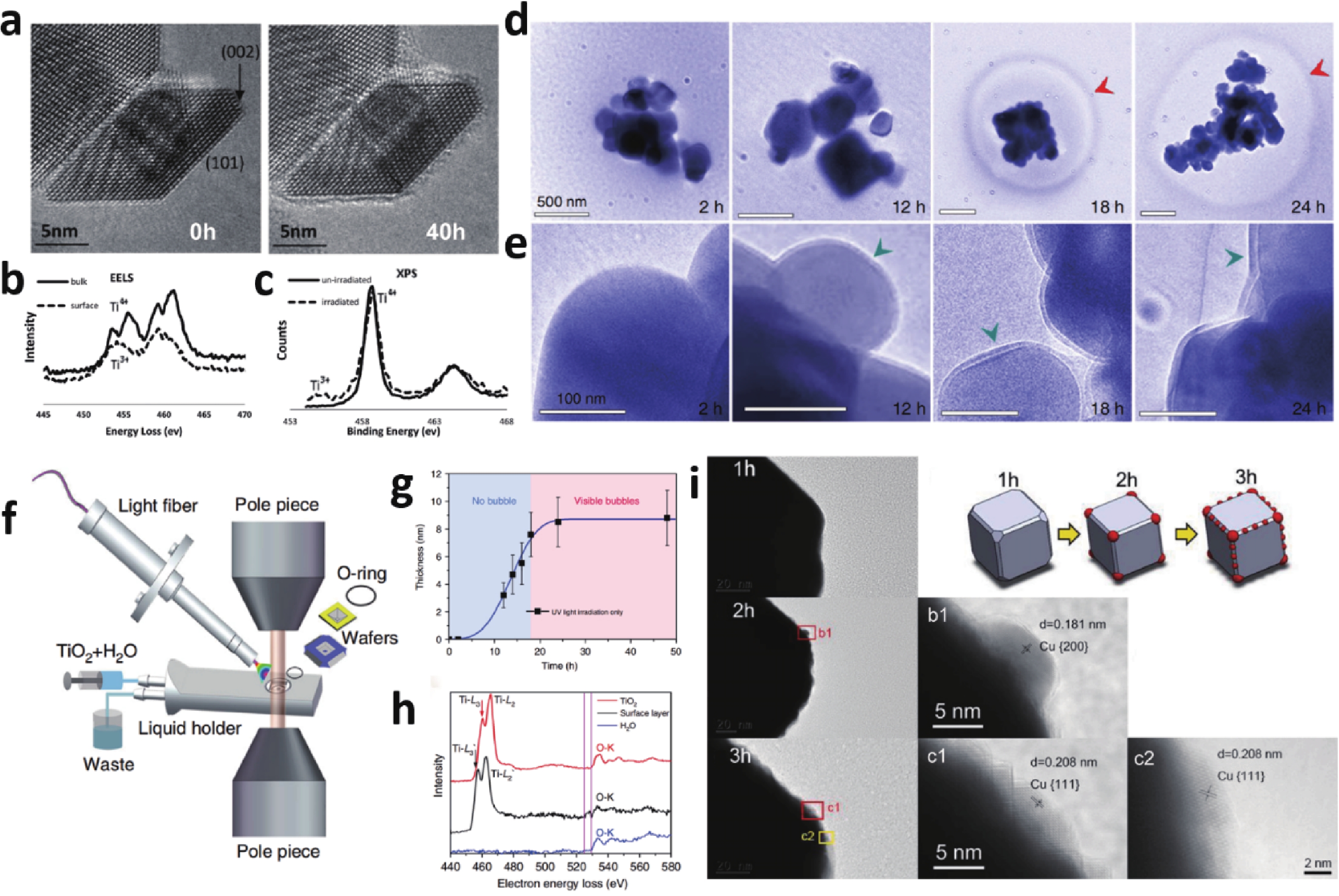
Semiconductor photocatalysis, as a key part of solar energy utilization, has far-reaching implications for industrial, agricultural, and commercial development. Lack of understanding of the catalyst evolution and the reaction mechanism is a critical obstacle for designing efficient and stable photocatalysts. This review summarizes the recent progress of in-situ exploring the dynamic behavior of catalyst materials and reaction intermediates. Semiconductor photocatalytic processes and two major classes of in-situ techniques that include microscopic imaging and spectroscopic characterization are presented. Finally, problems and challenges in in-situ characterization are proposed, geared toward developing more advanced in-situ techniques and monitoring more accurate and realistic reaction processes, to guide designing advanced photocatalysts. -
References
[1] Maeda K, Teramura K, Lu D, et al. Photocatalyst releasing hydrogen from water. Nature, 2006, 440, 295 doi: 10.1038/440295a[2] Zhang Y C, Afzal N, Pan L, et al. Structure-activity relationship of defective metal-based photocatalysts for water splitting: Experimental and theoretical perspectives. Adv Sci, 2019, 6, 1900053 doi: 10.1002/advs.201900053[3] Foster S L, Bakovic S I P, Duda R D, et al. Catalysts for nitrogen reduction to ammonia. Nat Catal, 2018, 1, 490 doi: 10.1038/s41929-018-0092-7[4] Xu Q L, Zhang L Y, Cheng B, et al. S-scheme heterojunction photocatalyst. Chem, 2020, 6, 1543 doi: 10.1016/j.chempr.2020.06.010[5] Chao Y G, Zhou P, Li N, et al. Ultrathin visible-light-driven Mo incorporating In2O3-ZnIn2Se4 Z-scheme nanosheet photocatalysts. Adv Mater, 2019, 31, 1807226 doi: 10.1002/adma.201807226[6] Gu Y, Wu A P, Jiao Y Q, et al. Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen evolution reaction. Angew Chem Int Ed, 2021, 60, 6673 doi: 10.1002/anie.202016102[7] Khan S, Je M, Ton N N T, et al. C-doped ZnS-ZnO/Rh nanosheets as multijunctioned photocatalysts for effective H2 generation from pure water under solar simulating light. Appl Catal B, 2021, 297, 120473 doi: 10.1016/j.apcatb.2021.120473[8] Ran L, Hou J G, Cao S Y, et al. Defect engineering of photocatalysts for solar energy conversion. Sol RRL, 2020, 4, 1900487 doi: 10.1002/solr.201900487[9] Liu M, Chen Y, Su J, et al. Photocatalytic hydrogen production using twinned nanocrystals and an unanchored NiS x co-catalyst. Nat Energy, 2016, 1, 16151 doi: 10.1038/nenergy.2016.151[10] Barawi M, Collado L, Gomez-Mendoza M, et al. Conjugated porous polymers: Ground-breaking materials for solar energy conversion. Adv Energy Mater, 2021, 11, 2101530 doi: 10.1002/aenm.202101530[11] Wang J G, Chen Y J, Zhou W, et al. Cubic quantum dot/hexagonal microsphere ZnIn2S4 heterophase junctions for exceptional visible-light-driven photocatalytic H2 evolution. J Mater Chem A, 2017, 5, 8451 doi: 10.1039/C7TA01914A[12] Yu H B, Huang J H, Jiang L B, et al. Enhanced photocatalytic tetracycline degradation using N-CQDs/OV-BiOBr composites: Unraveling the complementary effects between N-CQDs and oxygen vacancy. Chem Eng J, 2020, 402, 126187 doi: 10.1016/j.cej.2020.126187[13] Gao D D, Wu X H, Wang P, et al. Selenium-enriched amorphous NiSe1+ x nanoclusters as a highly efficient cocatalyst for photocatalytic H2 evolution. Chem Eng J, 2021, 408, 127230 doi: 10.1016/j.cej.2020.127230[14] Bai S, Jiang J, Zhang Q, et al. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem Soc Rev, 2015, 44, 2893 doi: 10.1039/C5CS00064E[15] Chen F Y, Wu Z Y, Adler Z, et al. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule, 2021, 5, 1704 doi: 10.1016/j.joule.2021.05.005[16] Zhang S R, Nguyen L, Zhu Y, et al. In-situ studies of nanocatalysis. Acc Chem Res, 2013, 46, 1731 doi: 10.1021/ar300245g[17] Zaera F. In-situ and operando spectroscopies for the characterization of catalysts and of mechanisms of catalytic reactions. J Catal, 2021, 404, 900 doi: 10.1016/j.jcat.2021.08.013[18] van der Wal L I, Turner S J, Zečević J. Developments and advances in in situ transmission electron microscopy for catalysis research. Catal Sci Technol, 2021, 11, 3634 doi: 10.1039/D1CY00258A[19] Knop-Gericke A, Kleimenov E, Hävecker M, et al. X-ray photoelectron spectroscopy for investigation of heterogeneous catalytic processes. Adv Catal, 2009, 52, 213 doi: 10.1016/B978-0-12-409547-2.12829-X[20] Ahmed M H M, Temperton R H, O'Shea J N. An in situ exploration of subsurface defect migration to a liquid water-exposed rutile TiO2(110) surface by XPS. Surf Interface Anal, 2021, 53, 1013 doi: 10.1002/sia.6906[21] Zhang P, Li Y K, Zhang Y S, et al. Photogenerated electron transfer process in heterojunctions: in situ irradiation XPS. Small Methods, 2020, 4, 2000214 doi: 10.1002/smtd.202000214[22] Bordiga S, Groppo E, Agostini G, et al. Reactivity of surface species in heterogeneous catalysts probed by in situ X-ray absorption techniques. Chem Rev, 2013, 113, 1736 doi: 10.1021/cr2000898[23] Zaera F. New advances in the use of infrared absorption spectroscopy for the characterization of heterogeneous catalytic reactions. Chem Soc Rev, 2014, 43, 7624 doi: 10.1039/C3CS60374A[24] Wachs I E, Roberts C A. Monitoring surface metal oxide catalytic active sites with Raman spectroscopy. Chem Soc Rev, 2010, 39, 5002 doi: 10.1039/c0cs00145g[25] Kim H, Kosuda K M, van Duyne R P, et al. Resonance Raman and surface- and tip-enhanced Raman spectroscopy methods to study solid catalysts and heterogeneous catalytic reactions. Chem Soc Rev, 2010, 39, 4820 doi: 10.1039/c0cs00044b[26] Bakker M G, Fowler B, Bowman M K, et al. Experimental methods in chemical engineering: Electron paramagnetic resonance spectroscopy-EPR/ESR. Can J Chem Eng, 2020, 98, 1668 doi: 10.1002/cjce.23784[27] Wu J B, Shan H, Chen W L, et al. In situ environmental TEM in imaging gas and liquid phase chemical reactions for materials research. Adv Mater, 2016, 28, 9686 doi: 10.1002/adma.201602519[28] Grogger W, Hofer F, Kothleitner G, et al. An introduction to high-resolution EELS in transmission electron microscopy. Top Catal, 2008, 50, 200 doi: 10.1007/s11244-008-9101-4[29] Besenbacher F, Lauritsen J V, Wendt S. STM studies of model catalysts. Nano Today, 2007, 2, 30 doi: 10.1016/S1748-0132(07)70115-9[30] Preet A, Lin T E. A review: Scanning electrochemical microscopy (SECM) for visualizing the real-time local catalytic activity. Catalysts, 2021, 11, 594 doi: 10.3390/catal11050594[31] Zhuang G X, Chen Y W, Zhuang Z Y, et al. Oxygen vacancies in metal oxides: Recent progress towards advanced catalyst design. Sci China Mater, 2020, 63, 2089 doi: 10.1007/s40843-020-1305-6[32] Feng H F, Xu Z F, Ren L, et al. Activating titania for efficient electrocatalysis by vacancy engineering. ACS Catal, 2018, 8, 4288 doi: 10.1021/acscatal.8b00719[33] Hou T T, Xiao Y, Cui P X, et al. Operando oxygen vacancies for enhanced activity and stability toward nitrogen photofixation. Adv Energy Mater, 2019, 9, 1902319 doi: 10.1002/aenm.201902319[34] Kolmakova N, Kolmakov A. Scanning electron microscopy for in situ monitoring of semiconductor−liquid interfacial processes: Electron assisted reduction of Ag ions from aqueous solution on the surface of TiO2 rutile nanowire. J Phys Chem C, 2010, 114, 17233 doi: 10.1021/jp1044546[35] Möbus G, Saghi Z, Sayle D C, et al. Dynamics of polar surfaces on ceria nanoparticles observed in situ with single-atom resolution. Adv Funct Mater, 2011, 21, 1971 doi: 10.1002/adfm.201002135[36] Bugnet M, Overbury S H, Wu Z L, et al. Direct visualization and control of atomic mobility at {100} surfaces of ceria in the environmental transmission electron microscope. Nano Lett, 2017, 17, 7652 doi: 10.1021/acs.nanolett.7b03680[37] Cavalca F, Laursen A B, Kardynal B E, et al. In situ transmission electron microscopy of light-induced photocatalytic reactions. Nanotechnology, 2012, 23, 075705 doi: 10.1088/0957-4484/23/7/075705[38] Zhang L X, Miller B K, Crozier P A. Atomic level in situ observation of surface amorphization in anatase nanocrystals during light irradiation in water vapor. Nano Lett, 2013, 13, 679 doi: 10.1021/nl304333h[39] Lu Y, Yin W J, Peng K L, et al. Self-hydrogenated shell promoting photocatalytic H2 evolution on anatase TiO2. Nat Commun, 2018, 9, 2752 doi: 10.1038/s41467-018-05144-1[40] Yu S H, Jiang Y H, Sun Y, et al. Real time imaging of photocatalytic active site formation during H2 evolution by in situ TEM. Appl Catal B, 2021, 284, 119743 doi: 10.1016/j.apcatb.2020.119743[41] Rycenga M, Cobley C M, Zeng J, et al. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem Rev, 2011, 111, 3669 doi: 10.1021/cr100275d[42] Bamwenda G R, Tsubota S, Nakamura T, et al. Photoassisted hydrogen production from a water-ethanol solution: A comparison of activities of Au–TiO2 and Pt–TiO2. J Photochem Photobiol A, 1995, 89, 177 doi: 10.1016/1010-6030(95)04039-I[43] Priebe J B, Karnahl M, Junge H, et al. Water reduction with visible light: Synergy between optical transitions and electron transfer in Au-TiO2 catalysts visualized by in situ EPR spectroscopy. Angew Chem Int Ed, 2013, 52, 11420 doi: 10.1002/anie.201306504[44] Yang K S, Lu Y R, Hsu Y Y, et al. Plasmon-induced visible-light photocatalytic activity of Au nanoparticle-decorated hollow mesoporous TiO2: A view by X-ray spectroscopy. J Phys Chem C, 2018, 122, 6955 doi: 10.1021/acs.jpcc.8b00205[45] Ekande O S, Kumar M. Review on polyaniline as reductive photocatalyst for the construction of the visible light active heterojunction for the generation of reactive oxygen species. J Environ Chem Eng, 2021, 9, 105725 doi: 10.1016/j.jece.2021.105725[46] Yuan Y, Guo R T, Hong L F, et al. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater Today Energy, 2021, 21, 100829 doi: 10.1016/j.mtener.2021.100829[47] Di T M, Xu Q L, Ho W, et al. Review on metal sulphide-based Z-scheme photocatalysts. ChemCatChem, 2019, 11, 1394 doi: 10.1002/cctc.201802024[48] Sai L M, Kong X Y. Type II hybrid structures of TiO2 nanorods conjugated with CdS quantum dots: Assembly and optical properties. Appl Phys A, 2014, 114, 605 doi: 10.1007/s00339-013-7631-5[49] Zhu Y Y, Liu Y F, Lv Y H, et al. Enhancement of photocatalytic activity for BiPO4 via phase junction. J Mater Chem A, 2014, 2, 13041 doi: 10.1039/C4TA01807A[50] Xue J W, Bao J. Interfacial charge transfer of heterojunction photocatalysts: Characterization and calculation. Surf Interfaces, 2021, 25, 101265 doi: 10.1016/j.surfin.2021.101265[51] Yang H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater Res Bull, 2021, 142, 111406 doi: 10.1016/j.materresbull.2021.111406[52] Wang L B, Cheng B, Zhang L Y, et al. In situ irradiated XPS investigation on S-scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small, 2021, 17, 2103447 doi: 10.1002/smll.202103447[53] Beck A, Huang X, Artiglia L, et al. The dynamics of overlayer formation on catalyst nanoparticles and strong metal-support interaction. Nat Commun, 2020, 11, 3220 doi: 10.1038/s41467-020-17070-2[54] Vincent J L, Crozier P A. Atomic level fluxional behavior and activity of CeO2-supported Pt catalysts for CO oxidation. Nat Commun, 2021, 12, 5789 doi: 10.1038/s41467-021-26047-8[55] Simpson B H, Rodríguez-López J. Emerging techniques for the in situ analysis of reaction intermediates on photo-electrochemical interfaces. Anal Methods, 2015, 7, 7029 doi: 10.1039/C5AY00503E[56] Nosaka Y, Nosaka A Y. Generation and detection of reactive oxygen species in photocatalysis. Chem Rev, 2017, 117, 11302 doi: 10.1021/acs.chemrev.7b00161[57] Liu J Y, Wei Z D, Shangguan W F. Defects engineering in photocatalytic water splitting materials. ChemCatChem, 2019, 11, 6177 doi: 10.1002/cctc.201901579[58] Connor P A, Dobson K D, McQuillan A J. Infrared spectroscopy of the TiO2/aqueous solution interface. Langmuir, 1999, 15, 2402 doi: 10.1021/la980855d[59] Haschke S, Mader M, Schlicht S, et al. Direct oxygen isotope effect identifies the rate-determining step of electrocatalytic OER at an oxidic surface. Nat Commun, 2018, 9, 4565 doi: 10.1038/s41467-018-07031-1[60] Lin W Y, Frei H. Photochemical and FT-IR probing of the active site of hydrogen peroxide in Ti silicalite sieve. J Am Chem Soc, 2002, 124, 9292 doi: 10.1021/ja012477w[61] Rong X, Parolin J, Kolpak A M. A fundamental relationship between reaction mechanism and stability in metal oxide catalysts for oxygen evolution. ACS Catal, 2016, 6, 1153 doi: 10.1021/acscatal.5b02432[62] Zandi O, Hamann T W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat Chem, 2016, 8, 778 doi: 10.1038/nchem.2557[63] Zou Z, Ye J, Sayama K, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature, 2001, 414, 625 doi: 10.1038/414625a[64] Guo S H, Li Y H, Tang S W, et al. Monitoring hydrogen evolution reaction intermediates of transition metal dichalcogenides via operando Raman spectroscopy. Adv Funct Mater, 2020, 30, 2003035 doi: 10.1002/adfm.202003035[65] Peng Y H, Geng M J, Yu J Q, et al. Vacancy-induced 2H@1T MoS2 phase-incorporation on ZnIn2S4 for boosting photocatalytic hydrogen evolution. Appl Catal B, 2021, 298, 120570 doi: 10.1016/j.apcatb.2021.120570[66] Ye L Q, Ma Z Y, Deng Y, et al. Robust and efficient photocatalytic hydrogen generation of ReS2/CdS and mechanistic study by on-line mass spectrometry and in situ infrared spectroscopy. Appl Catal B, 2019, 257, 117897 doi: 10.1016/j.apcatb.2019.117897[67] Wang X, Wang X, Huang J, et al. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat Commun, 2021, 12, 4112 doi: 10.1038/s41467-021-24511-z[68] Nakamura R, Nakato Y. Primary intermediates of oxygen photoevolution reaction on TiO2 (Rutile) particles, revealed by in situ FTIR absorption and photoluminescence measurements. J Am Chem Soc, 2004, 126, 1290 doi: 10.1021/ja0388764[69] Zhang M, de Respinis M, Frei H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat Chem, 2014, 6, 362 doi: 10.1038/nchem.1874[70] Ding Q, Liu Y, Chen T, et al. Unravelling the water oxidation mechanism on NaTaO3-based photocatalysts. J Mater Chem A, 2020, 8, 6812 doi: 10.1039/C9TA14235E[71] Fresno F, Galdón S, Barawi M, et al. Selectivity in UV photocatalytic CO2 conversion over bare and silver-decorated niobium-tantalum perovskites. Catal Today, 2021, 361, 85 doi: 10.1016/j.cattod.2020.01.013[72] Halmann M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature, 1978, 275, 115 doi: 10.1038/275115a0[73] Marszewski M, Cao S W, Yu J G, et al. Semiconductor-based photocatalytic CO2 conversion. Mater Horiz, 2015, 2, 261 doi: 10.1039/C4MH00176A[74] Rao H, Schmidt L C, Bonin J, et al. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature, 2017, 548, 74 doi: 10.1038/nature23016[75] Schreier M, Héroguel F, Steier L, et al. Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat Energy, 2017, 2, 17087 doi: 10.1038/nenergy.2017.87[76] Wang Y, Shang X, Shen J, et al. Direct and indirect Z-scheme heterostructure-coupled photosystem enabling cooperation of CO2 reduction and H2O oxidation. Nat Commun, 2020, 11, 3043 doi: 10.1038/s41467-020-16742-3[77] Kou Y, Nabetani Y, Dai M S, et al. Direct detection of key reaction intermediates in photochemical CO2 reduction sensitized by a rhenium bipyridine complex. J Am Chem Soc, 2014, 136, 6021 doi: 10.1021/ja500403e[78] Liu L J, Li Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review. Aerosol Air Qual Res, 2014, 14, 453 doi: 10.4209/aaqr.2013.06.0186[79] Liu L J, Zhao C Y, Miller J T, et al. Mechanistic study of CO2 photoreduction with H2O on Cu/TiO2 nanocomposites by in situ X-ray absorption and infrared spectroscopies. J Phys Chem C, 2017, 121, 490 doi: 10.1021/acs.jpcc.6b10835[80] Wu J, Li X D, Shi W, et al. Efficient visible-light-driven CO2 reduction mediated by defect-engineered BiOBr atomic layers. Angew Chem Int Ed, 2018, 57, 8719 doi: 10.1002/anie.201803514[81] Chen S, Wang H, Kang Z, et al. Oxygen vacancy associated single-electron transfer for photofixation of CO2 to long-chain chemicals. Nat Commun, 2019, 10, 788 doi: 10.1038/s41467-019-08697-x[82] Zhu J C, Shao W W, Li X D, et al. Asymmetric triple-atom sites confined in ternary oxide enabling selective CO2 photothermal reduction to acetate. J Am Chem Soc, 2021, 143, 18233 doi: 10.1021/jacs.1c08033[83] Ren X J, Gao M C, Zhang Y F, et al. Photocatalytic reduction of CO2 on BiOX: Effect of halogen element type and surface oxygen vacancy mediated mechanism. Appl Catal B, 2020, 274, 119063 doi: 10.1016/j.apcatb.2020.119063[84] MacKay B A, Fryzuk M D. Dinitrogen coordination chemistry: The biomimetic borderlands. ChemInform, 2004, 35, 703 doi: 10.1002/chin.200430145[85] Shen H D, Yang M M, Hao L D, et al. Photocatalytic nitrogen reduction to ammonia: Insights into the role of defect engineering in photocatalysts. Nano Res, 2021, 275, 115 doi: 10.1007/s12274-021-3725-0[86] Guo J P, Chen P. Catalyst: NH3 as an energy carrier. Chem, 2017, 3, 709 doi: 10.1016/j.chempr.2017.10.004[87] Medford A J, Hatzell M C. Photon-driven nitrogen fixation: Current progress, thermodynamic considerations, and future outlook. ACS Catal, 2017, 7, 2624 doi: 10.1021/acscatal.7b00439[88] Hoffman B M, Lukoyanov D, Yang Z Y, et al. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chem Rev, 2014, 114, 4041 doi: 10.1021/cr400641x[89] Jia H P, Quadrelli E A. Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen. Chem Soc Rev, 2014, 43, 547 doi: 10.1039/C3CS60206K[90] Yuzawa H, Mori T, Itoh H, et al. Reaction mechanism of ammonia decomposition to nitrogen and hydrogen over metal loaded titanium oxide photocatalyst. J Phys Chem C, 2012, 116, 4126 doi: 10.1021/jp209795t[91] Hirakawa H, Hashimoto M, Shiraishi Y, et al. Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide. J Am Chem Soc, 2017, 139, 10929 doi: 10.1021/jacs.7b06634[92] Li C C, Wang T, Zhao Z J, et al. Promoted fixation of molecular nitrogen with surface oxygen vacancies on plasmon-enhanced TiO2 photoelectrodes. Angew Chem, 2018, 130, 5376 doi: 10.1002/ange.201713229[93] Li H, Shang J, Ai Z H, et al. Efficient visible light nitrogen fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} facets. J Am Chem Soc, 2015, 137, 6393 doi: 10.1021/jacs.5b03105[94] Li P S, Zhou Z A, Wang Q, et al. Visible-light-driven nitrogen fixation catalyzed by Bi5O7Br nanostructures: Enhanced performance by oxygen vacancies. J Am Chem Soc, 2020, 142, 12430 doi: 10.1021/jacs.0c05097[95] Wang S Y, Hai X, Ding X, et al. Light-switchable oxygen vacancies in ultrafine Bi5O7Br nanotubes for boosting solar-driven nitrogen fixation in pure water. Adv Mater, 2017, 29, 1701774 doi: 10.1002/adma.201701774[96] Yang J H, Guo Y Z, Jiang R B, et al. High-efficiency “working-in-tandem” nitrogen photofixation achieved by assembling plasmonic gold nanocrystals on ultrathin titania nanosheets. J Am Chem Soc, 2018, 140, 8497 doi: 10.1021/jacs.8b03537[97] Rao F, Zhu G Q, Zhang W B, et al. In-situ generation of oxygen vacancies and metallic bismuth from (BiO)2CO3 via N2-assisted thermal-treatment for efficient selective photocatalytic NO removal. Appl Catal B, 2021, 281, 119481 doi: 10.1016/j.apcatb.2020.119481[98] Shang H, Li M Q, Li H, et al. Oxygen vacancies promoted the selective photocatalytic removal of NO with blue TiO2 via simultaneous molecular oxygen activation and photogenerated hole annihilation. Environ Sci Technol, 2019, 53, 6444 doi: 10.1021/acs.est.8b07322[99] Jin H, You R, Zhou S, et al. In-situ DRIFTS and XANES identification of copper species in the ternary composite oxide catalysts CuMnCeO during CO preferential oxidation. Int J Hydrog Energy, 2015, 40, 3919 doi: 10.1016/j.ijhydene.2015.01.086[100] Zigah D, Rodríguez-López J, Bard A J. Quantification of photoelectrogenerated hydroxyl radical on TiO2 by surface interrogation scanning electrochemical microscopy. Phys Chem Chem Phys, 2012, 14, 12764 doi: 10.1039/c2cp40907k[101] Kreuzer L B, Patel C K. Nitric oxide air pollution: Detection by optoacoustic spectroscopy. Science, 1971, 173, 45 doi: 10.1126/science.173.3991.45[102] Jin S, Dong G H, Luo J M, et al. Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst. Appl Catal B, 2018, 227, 24 doi: 10.1016/j.apcatb.2018.01.020[103] Lu Y F, Huang Y, Zhang Y F, et al. Oxygen vacancy engineering of Bi2O3/Bi2O2CO3 heterojunctions: Implications of the interfacial charge transfer, NO adsorption and removal. Appl Catal B, 2018, 231, 357 doi: 10.1016/j.apcatb.2018.01.008[104] Nakamura R, Imanishi A, Murakoshi K, et al. In situ FTIR studies of primary intermediates of photocatalytic reactions on nanocrystalline TiO2 films in contact with aqueous solutions. J Am Chem Soc, 2003, 125, 7443 doi: 10.1021/ja029503q -
Proportional views






 DownLoad:
DownLoad: