| Citation: |
Peng Zhang. Electronic origin of structural degradation in Li-rich transition metal oxides: The case of Li2MnO3 and Li2RuO3[J]. Journal of Semiconductors, 2024, 45(4): 042801. doi: 10.1088/1674-4926/45/4/042801
****
P Zhang. Electronic origin of structural degradation in Li-rich transition metal oxides: The case of Li2MnO3 and Li2RuO3[J]. J. Semicond, 2024, 45(4): 042801. doi: 10.1088/1674-4926/45/4/042801
|
Electronic origin of structural degradation in Li-rich transition metal oxides: The case of Li2MnO3 and Li2RuO3
DOI: 10.1088/1674-4926/45/4/042801
More Information
-
Abstract
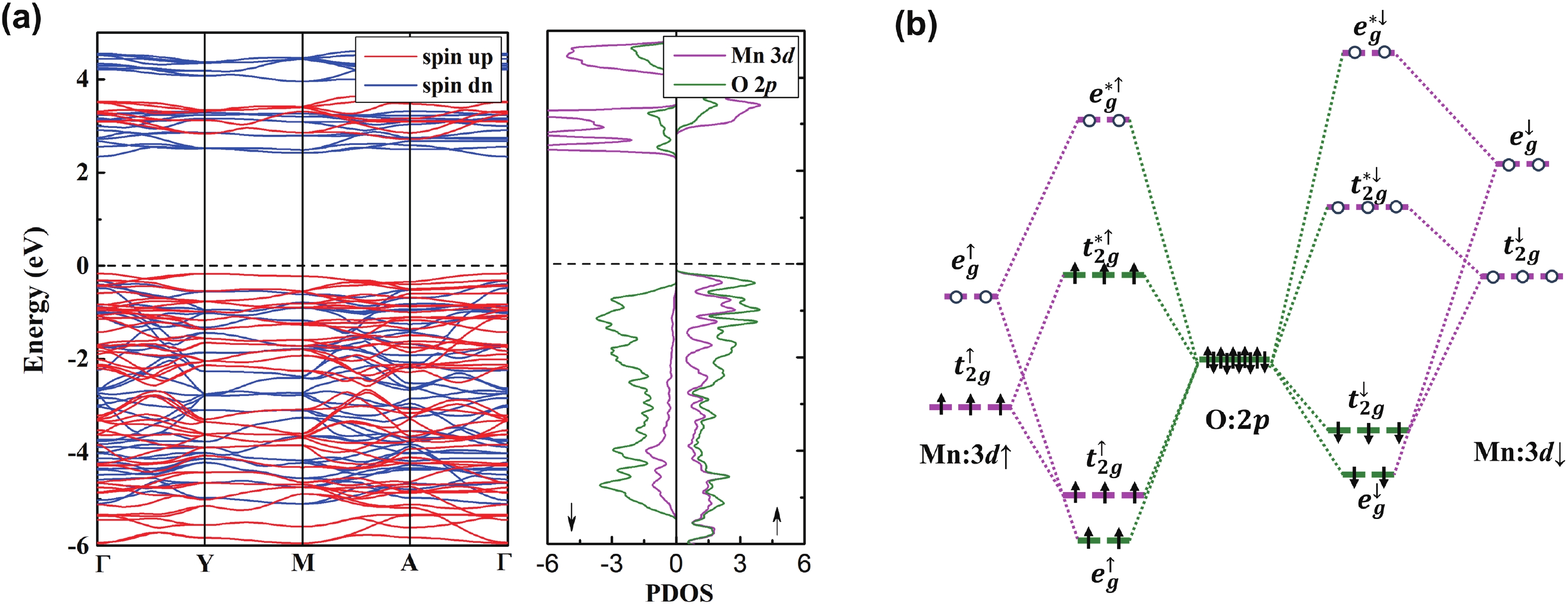
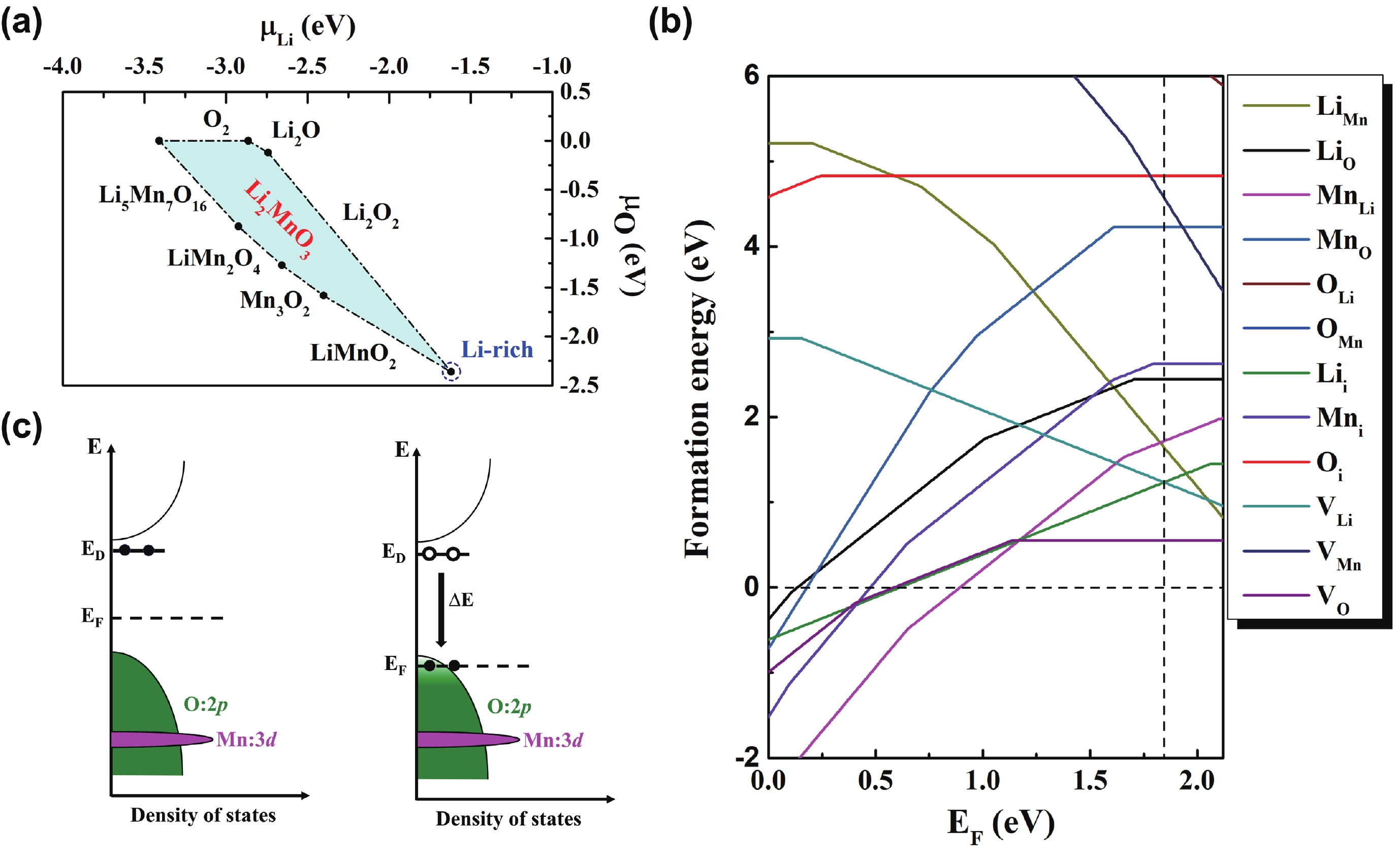
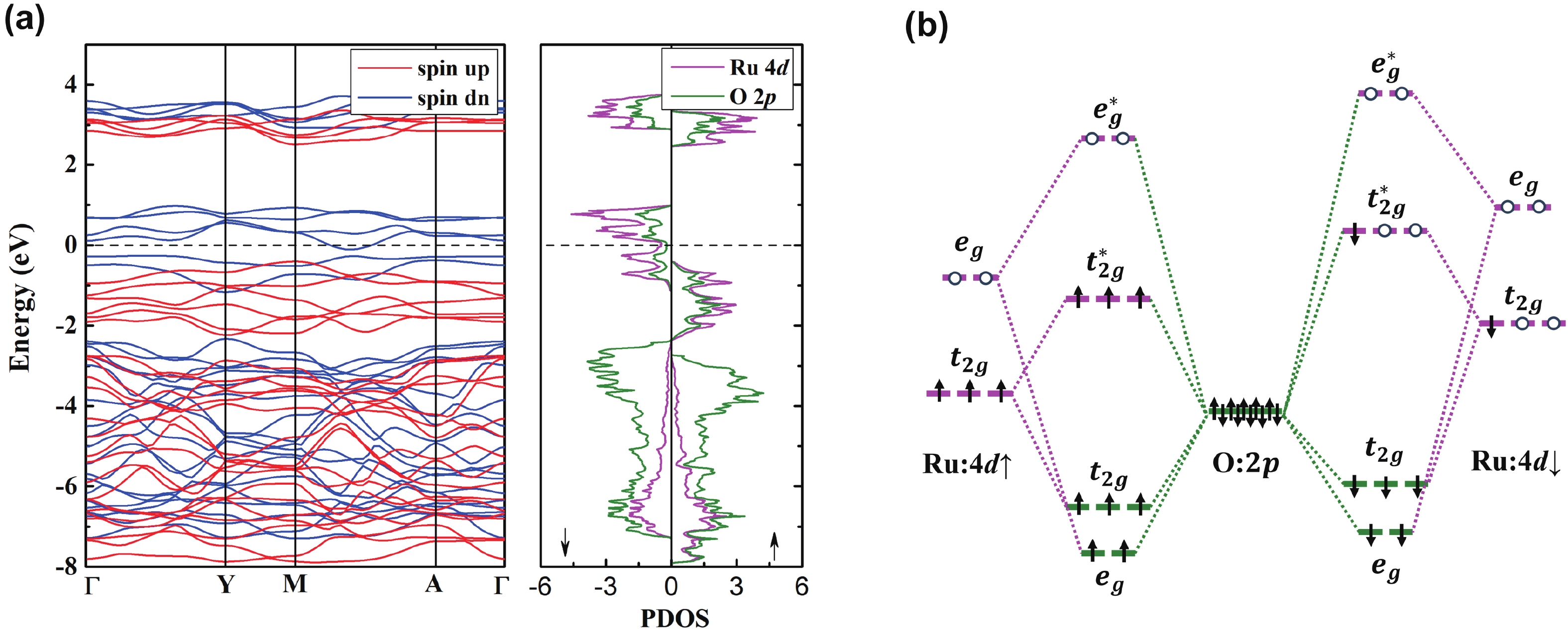
Li2MnO3 and Li2RuO3 represent two prototype Li-rich transition metal (TM) oxides as high-capacity cathodes for Li-ion batteries, which have similar crystal structures but show quite different cycling performances. Here, based on the first-principles calculations, we systematically studied the electronic structures and defect properties of these two Li-rich cathodes, in order to get more understanding on the structural degradation mechanism in Li-rich TM oxides. Our calculations indicated that the structural and cycling stability of Li2MnO3 and Li2RuO3 depend closely on their electronic structures, especially the energy of their highest occupied electronic states (HOS), as it largely determines the defect properties of these cathodes. For Li2MnO3 with low-energy HOS, we found that, due to the defect charge transfer mechanism, various defects can form spontaneously in its host structure as Li ions are extracted upon delithiation, which seriously deteriorates its structural and cycling stability. While for Li2RuO3, on the other hand, we identified that the high-energy HOS prevents it from the defect formation upon delithiation and thus preserve its cycling reversibility. Our studies thus illustrated an electronic origin of the structural degradation in Li-rich TM oxides and implied that it is possible to improve their cycling performances by carefully adjusting their TM components.-
Keywords:
- defects,
- Li-ion batteries,
- first-principles calculations
-
References
[1] Goodenough J B, Park K S. The Li-ion rechargeable battery: a perspective. J Am Chem Soc, 2013, 135, 1167 doi: 10.1021/ja3091438[2] Larcher D, Tarascon J M. Towards greener and more sustainable batteries for electrical energy storage. Nat Chem, 2015, 7, 19 doi: 10.1038/nchem.2085[3] Melot B C, Tarascon J M. Design and preparation of materials for advanced electrochemical storage. Acc Chem Res, 2013, 46, 1226 doi: 10.1021/ar300088q[4] Li W, Erickson E M, Manthiram A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat Energy, 2020, 5, 26[5] Whittingham M S. Ultimate limits to intercalation reactions for lithium batteries. Chem Rev, 2014, 114, 11414 doi: 10.1021/cr5003003[6] Mizushima K, Jones P, Wiseman P, et al. Li xCoO2 (0<x<-1): A new cathode material for batteries of high energy density. MRS Bull, 1980, 15, 783 doi: 10.1016/0025-5408(80)90012-4[7] Padhi A K, Nanjundaswamy K S, Goodenough J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc, 1997, 144, 1188 doi: 10.1149/1.1837571[8] Lu Z, MacNeil D, Dahn J. Layered cathode materials Li[Ni xLi(1/3−2 x/3)Mn (2/3− x/3)]O2 for lithium-ion batteries. Electrochem Solid-State Lett, 2001, 4, A191 doi: 10.1149/1.1407994[9] Robertson A D, Bruce P G. Mechanism of electrochemical activity in Li2MnO3. Chem Mater, 2003, 15, 1984 doi: 10.1021/cm030047u[10] Koga H, Croguennec L, Menetrier M, et al. Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J Electrochem Soc, 2013, 160, A786 doi: 10.1149/2.038306jes[11] Rozier P, Tarascon J M. Review—Li-rich layered oxide cathodes for next-generation Li-ion batteries: chances and challenges. J Electrochem Soc, 2015, 162, A2490 doi: 10.1149/2.0111514jes[12] Assat G, Tarascon J M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat Energy, 2018, 3, 373 doi: 10.1038/s41560-018-0097-0[13] House R A, Marie J J, Pérez-Osorio M A, et al. The role of O2 in O-redox cathodes for Li-ion batteries. Nat Energy, 2021, 6, 781 doi: 10.1038/s41560-021-00780-2[14] Zhang M, Kitchaev D A, Lebens-Higgins Z, et al. Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Nat Rev Mater, 2022, 7, 522 doi: 10.1038/s41578-022-00416-1[15] Sathiya M, Abakumov A M, Foix D, et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat Mater, 2015, 14, 230 doi: 10.1038/nmat4137[16] Hong J, Gent W E, Xiao P, et al. Metal-oxygen decoordination stabilizes anion redox in Li-rich oxides. Nat Mater, 2019, 18, 256 doi: 10.1038/s41563-018-0276-1[17] House R A, Maitra U, Perez-Osorio M A, et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature, 2020, 577, 502 doi: 10.1038/s41586-019-1854-3[18] Liu T, Liu J, Li L, et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature, 2022, 606, 305 doi: 10.1038/s41586-022-04689-y[19] Liu X, Xu G L, Kolluru V S C, et al. Origin and regulation of oxygen redox instability in high-voltage battery cathodes. Nat Energy, 2022, 7, 808 doi: 10.1038/s41560-022-01036-3[20] Luo K, Roberts M R, Hao R, et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat Chem, 2016, 8, 684 doi: 10.1038/nchem.2471[21] Gent W E, Lim K, Liang Y, et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat Commun, 2017, 8, 2091 doi: 10.1038/s41467-017-02041-x[22] Sathiya M, Rousse G, Ramesha K, et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat Mater, 2013, 12, 827 doi: 10.1038/nmat3699[23] Sathiya M, Ramesha K, Rousse G, et al. High performance Li2Ru1– yMn yO3 (0.2 ≤y≤0.8) cathode materials for rechargeable Lithium-ion batteries: their understanding. Chem Mater, 2013, 25, 1121 doi: 10.1021/cm400193m[24] McCalla E, Abakumov A M, Saubanère M, et al. Visualization of O-O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science, 2015, 350, 1516 doi: 10.1126/science.aac8260[25] Wu Y, Zhou K, Ren F, et al. Highly reversible Li2RuO3 cathodes in sulfide-based all solid-state lithium batteries. Energy Environ Sci, 2022, 15, 3470 doi: 10.1039/D2EE01067D[26] Blochl P E. Projector augmented-wave method. Phys Rev B, 1994, 50, 17953 doi: 10.1103/PhysRevB.50.17953[27] Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B, 1996, 54, 11169 doi: 10.1103/PhysRevB.54.11169[28] Sun J, Ruzsinszky A, Perdew J P. Strongly constrained and appropriately normed semilocal density functional. Phys Rev Lett, 2015, 115, 036402 doi: 10.1103/PhysRevLett.115.036402[29] Huang M, Zheng Z, Dai Z, et al. DASP: Defect and dopant ab-initio simulation package. J Semicond, 2022, 43, 042101 doi: 10.1088/1674-4926/43/4/042101[30] Strobel P, Lambert-Andron B. Crystallographic and magnetic structure of Li2MnO3. J Solid State Chem, 1988, 75, 90 doi: 10.1016/0022-4596(88)90305-2[31] James A, Goodenough J B. Structure and bonding in lithium ruthenate, Li2RuO3. J Solid State Chem, 1988, 74, 287 doi: 10.1016/0022-4596(88)90357-X[32] Xiao R, Li H, Chen L. Density functional investigation on Li2MnO3. Chem Mater, 2012, 24, 4242 doi: 10.1021/cm3027219[33] Park C H, Zhang S B, Wei S H. Origin of p-type doping difficulty in ZnO: The impurity perspective. Phys Rev B, 2002, 66, 073202 doi: 10.1103/PhysRevB.66.073202[34] Wei S H. Overcoming the doping bottleneck in semiconductors. Comput Mater Sci, 2004, 30, 337 doi: 10.1016/j.commatsci.2004.02.024 -
Proportional views





 Peng Zhang received his doctoral degree from Xiamen University, Xiamen, China, in 2014 and become a postdoctoral researcher in Korea Advanced Institute of Science and Technology (KAIST) in 2014 and Beijing Computational Science Research Center (CSRC) in 2016. He is currently an assistant professor in the college of physics and optoelectronic engineering of Shenzhen University, Shenzhen, China. His research interests include the computational materials science, optoelectronic materials, energy conservation and storage materials, and semiconductors.
Peng Zhang received his doctoral degree from Xiamen University, Xiamen, China, in 2014 and become a postdoctoral researcher in Korea Advanced Institute of Science and Technology (KAIST) in 2014 and Beijing Computational Science Research Center (CSRC) in 2016. He is currently an assistant professor in the college of physics and optoelectronic engineering of Shenzhen University, Shenzhen, China. His research interests include the computational materials science, optoelectronic materials, energy conservation and storage materials, and semiconductors.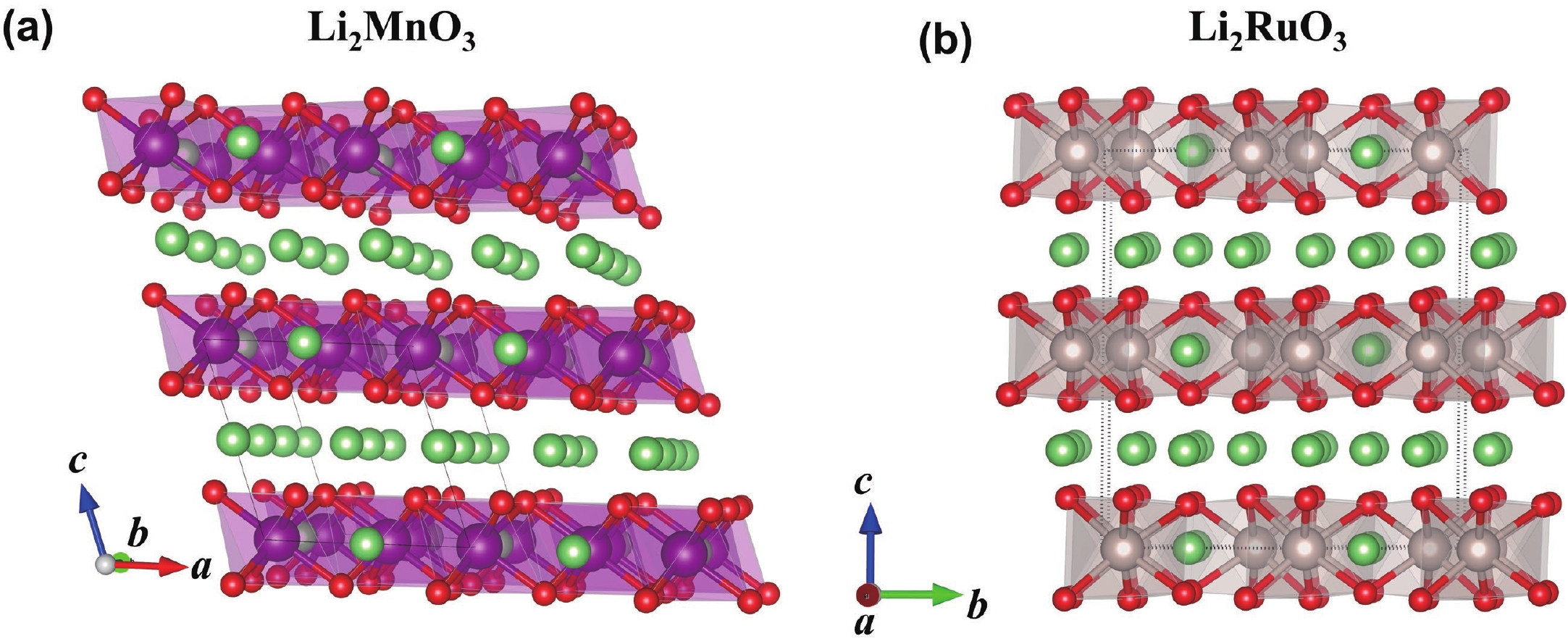
 DownLoad:
DownLoad: