| Citation: |
Yanbin Huang, Jun Liu, Yanchun Deng, Yuanyuan Qian, Xiaohao Jia, Mengmeng Ma, Cheng Yang, Kong Liu, Zhijie Wang, Shengchun Qu, Zhanguo Wang. The application of perovskite materials in solar water splitting[J]. Journal of Semiconductors, 2020, 41(1): 011701. doi: 10.1088/1674-4926/41/1/011701
****
Y B Huang, J Liu, Y C Deng, Y Y Qian, X H Jia, M M Ma, C Yang, K Liu, Z J Wang, S C Qu, Z G Wang, The application of perovskite materials in solar water splitting[J]. J. Semicond., 2020, 41(1): 011701. doi: 10.1088/1674-4926/41/1/011701.
|
The application of perovskite materials in solar water splitting
DOI: 10.1088/1674-4926/41/1/011701
More Information
-
Abstract
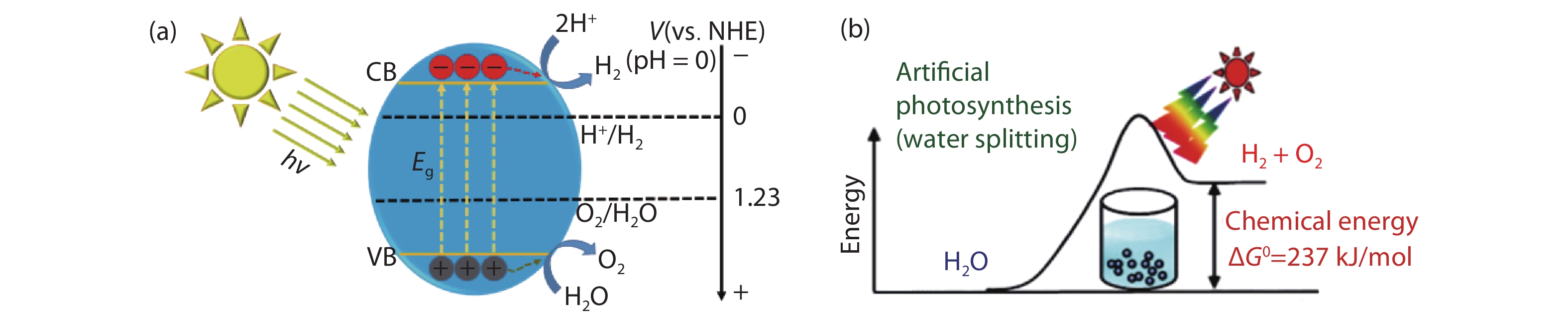
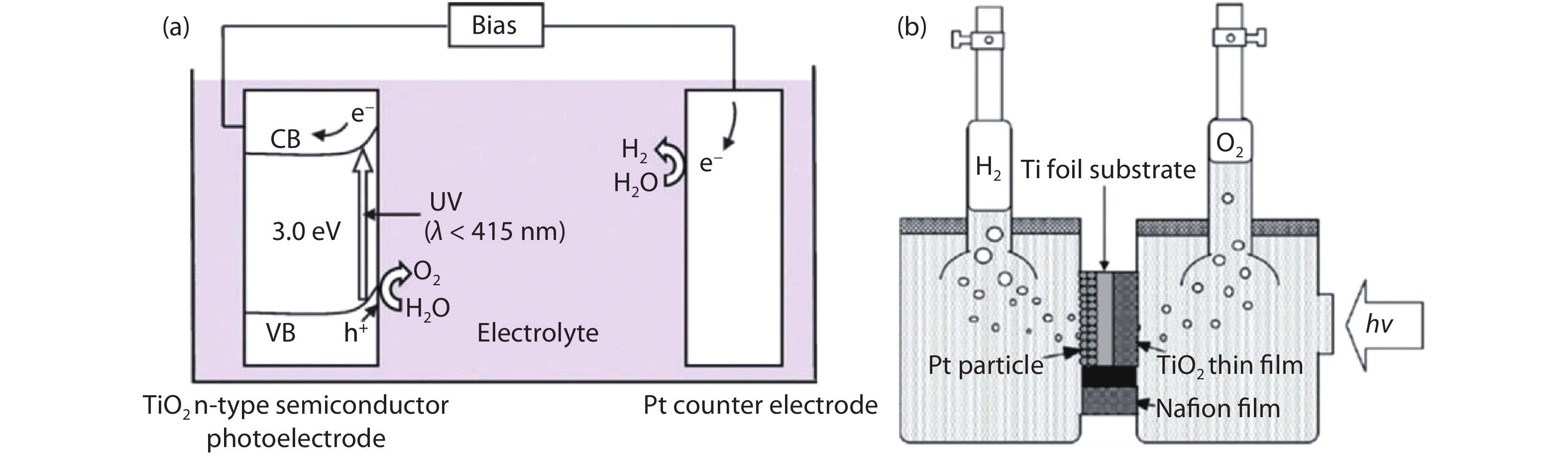
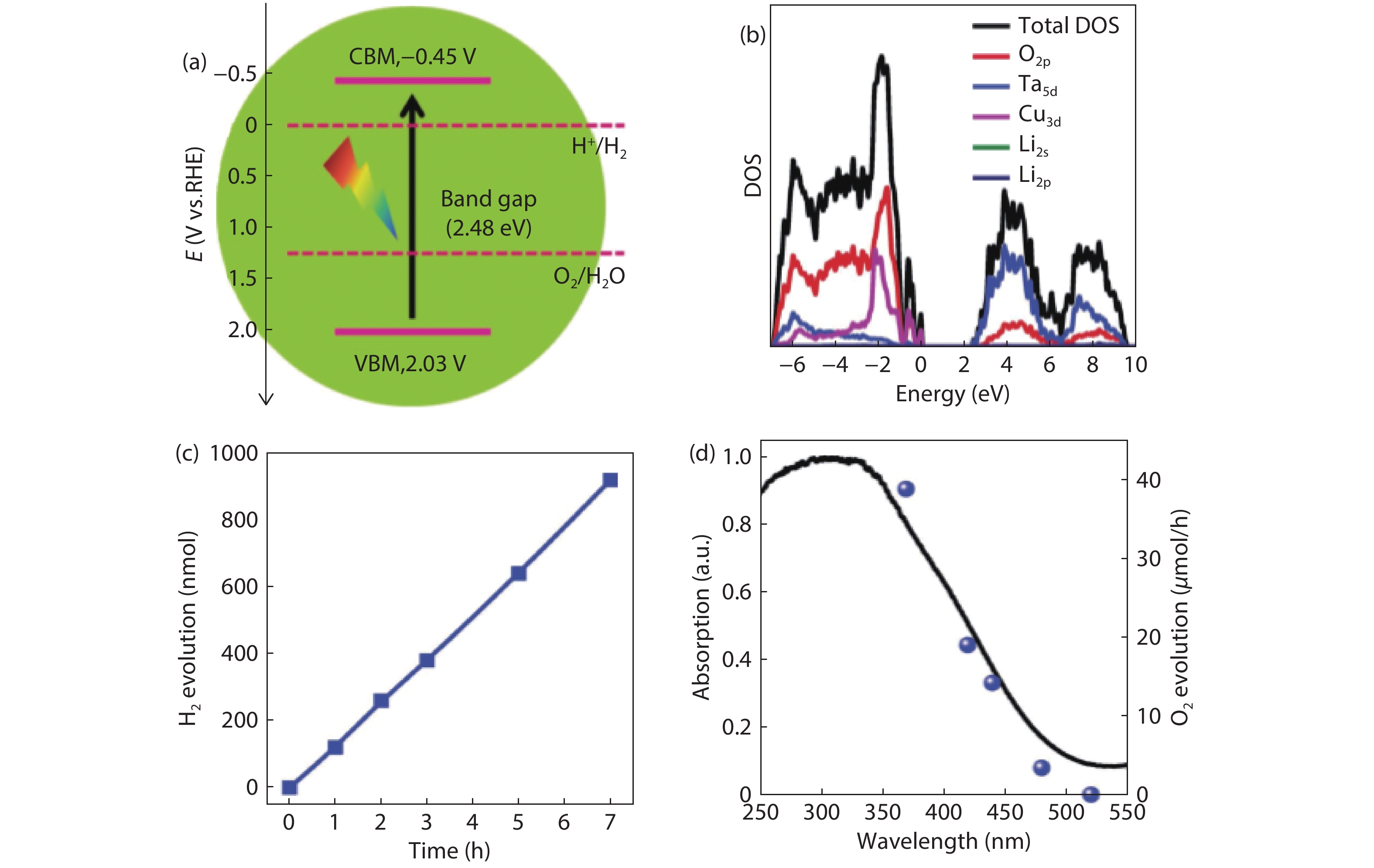
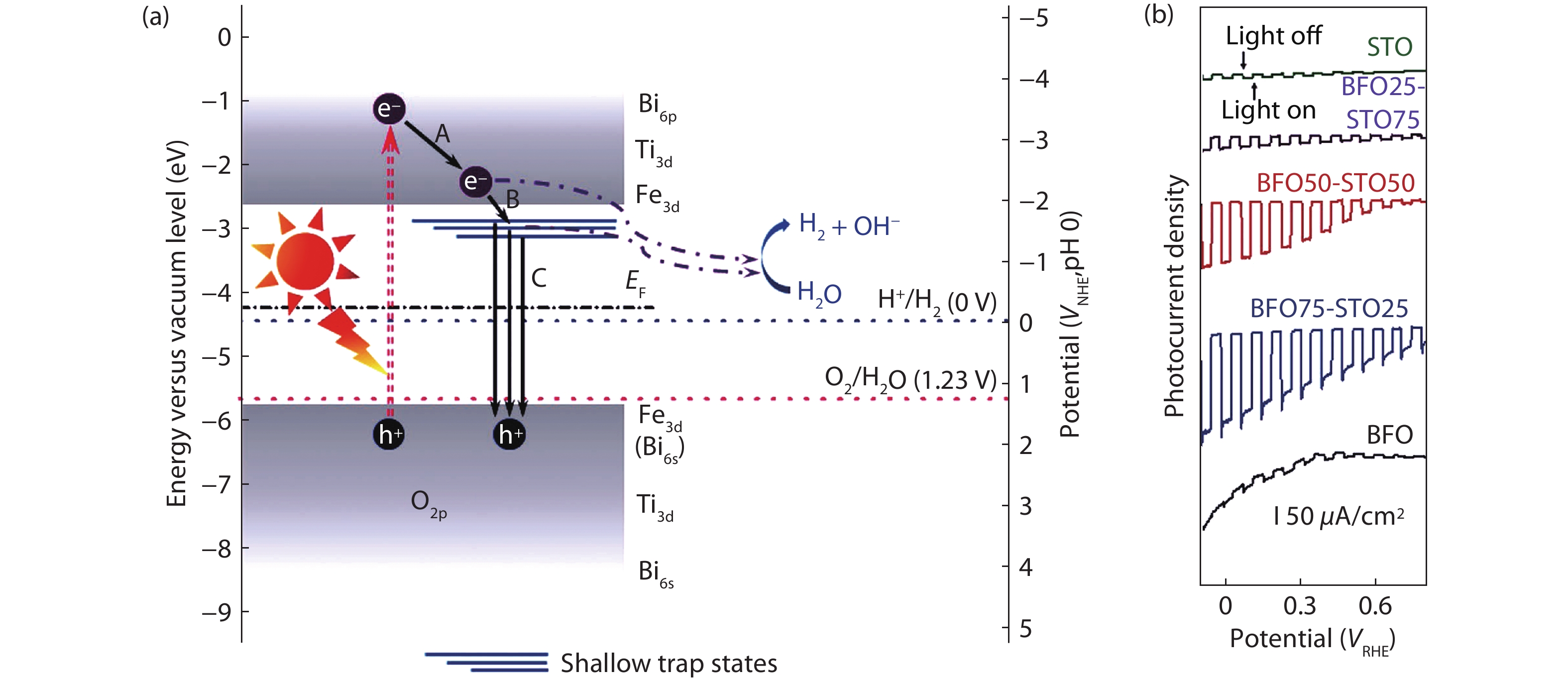
Solar water splitting is a promising strategy for sustainable production of renewable hydrogen, and solving the crisis of energy and environment in the world. However, large-scale application of this method is hampered by the efficiency and the expense of the solar water splitting systems. Searching for non-toxic, low-cost, efficient and stable photocatalysts is an important way for solar water splitting. Due to the simplicity of structure and the flexibility of composition, perovskite based photocatalysts have recently attracted widespread attention for application in solar water splitting. In this review, the recent developments of perovskite based photocatalysts for water splitting are summarized. An introduction including the structures and properties of perovskite materials, and the fundamentals of solar water splitting is first provided. Then, it specifically focuses on the strategies for designing and modulating perovskite materials to improve their photocatalytic performance for solar water splitting. The current challenges and perspectives of perovskite materials in solar water splitting are also reviewed. The aim of this review is to summarize recent findings and developments of perovskite based photocatalysts and provide some useful guidance for the future research on the design and development of highly efficient perovskite based photocatalysts and the relevant systems for water splitting.-
Keywords:
- solar water splitting,
- perovskite materials,
- photocatalyst
-
References
[1] Hosseini S E, Wahid M A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew Sust Energ Rev, 2016, 57, 850 doi: 10.1016/j.rser.2015.12.112[2] Vita A, Italiano C, Pino L, et al. Hydrogen-rich gas production by steam reforming of n-dodecane. Part II: Stability, regenerability and sulfur poisoning of low loading Rh-based catalyst. Appl Catal B, 2017, 218, 317 doi: 10.1016/j.apcatb.2017.06.059[3] Hisatomi T, Domen K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat Catal, 2019, 2(5), 387 doi: 10.1038/s41929-019-0242-6[4] Wang W, Xu M, Xu X, et al. Perovskite oxide-based electrodes for high-performance photoelectrochemical water splitting. Angew Chem Int Ed Engl, 2019, 58, 2 doi: 10.1002/anie.201900292[5] Chen G, Hu Z, Zhu Y, et al. A universal strategy to design superior water-splitting electrocatalysts based on fast in situ reconstruction of amorphous nanofilm precursors. Adv Mater, 2018, 30(43), 1804333 doi: 10.1002/adma.201804333[6] Zhang G, Liu G, Wang L Z, et al. Inorganic perovskite photocatalysts for solar energy utilization. Chem Soc Rev, 2016, 45(21), 5951 doi: 10.1039/C5CS00769K[7] Sheng X, Xu T, Feng X J. Rational design of photoelectrodes with rapid charge transport for photoelectrochemical applications. Adv Mater, 2019, 31(11), 1805132 doi: 10.1002/adma.201805132[8] Chen W J, Wang T T, Xue J W, et al. Cobalt-nickel layered double hydroxides modified on TiO2 nanotube arrays for highly efficient and stable PEC water splitting. Small, 2017, 13(10), 1602420 doi: 10.1002/smll.201602420[9] Faraji M, Yousefi M, Yousefzadeh S, et al. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energ Environ Sci, 2019, 12(1), 59 doi: 10.1039/C8EE00886H[10] Huang X, Qi X Y, Boey F, et al. Graphene-based composites. Chem Soc Rev, 2012, 41(2), 666 doi: 10.1039/C1CS15078B[11] Li X, Liu S W, Fan K, et al. MOF-based transparent passivation layer modified ZnO nanorod arrays for enhanced photo-electrochemical water splitting. Adv Energy Mater, 2018, 8(18), 1800101 doi: 10.1002/aenm.201800101[12] Ong W J, Tan L L, Ng Y H, et al. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability. Chem Rev, 2016, 116(12), 7159 doi: 10.1021/acs.chemrev.6b00075[13] Wang S C, Chen P, Bai Y, et al. New BiVO4 dual photoanodes with enriched oxygen vacancies for efficient solar-driven water splitting. Adv Mater, 2018, 30(20), 1800486 doi: 10.1002/adma.201800486[14] Xu Y F, Rao H S, Chen B X, et al. Achieving highly efficient photoelectrochemical water oxidation with a TiCl4 treated 3D antimony-doped SnO2 macropore/branched alpha-Fe2O3 nanorod heterojunction photoanode. Adv Sci, 2015, 2(7), 1500049 doi: 10.1002/advs.201500049[15] Yeh T F, Teng C Y, Chen L C, et al. Graphene oxide-based nanomaterials for efficient photoenergy conversion. J Mater Chem A, 2016, 4(6), 2014 doi: 10.1039/C5TA07780J[16] Zhang Z M, Gao C T, W u Z M, et al. Toward efficient photoelectrochemical water-splitting by using screw-like SnO2 nanostructures as photoanode after being decorated with CdS quantum dots. Nano Energy, 2016, 19, 318 doi: 10.1016/j.nanoen.2015.11.011[17] Zhou Y E, Zhang L Y, Lin L H, et al. Highly efficient photoelectrochemical water splitting from hierarchical WO3/BiVO4 nanoporous sphere arrays. Nano Lett, 2017, 17(12), 8012 doi: 10.1021/acs.nanolett.7b04626[18] Pinaud B A, Benck J D, Seitz L C, et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energ Environ Sci, 2013, 6(7), 1983 doi: 10.1039/c3ee40831k[19] Tan P, Liu M L, Shao Z P, et al. Recent advances in perovskite oxides as electrode materials for nonaqueous lithium-oxygen batteries. Adv Energy Mater, 2017, 7(13), 1602674 doi: 10.1002/aenm.201602674[20] Wang W, Tade M O, Shao Z P. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog Mater Sci, 2018, 92, 33 doi: 10.1016/j.pmatsci.2017.09.002[21] Pena M A, Fierro J L. Chemical structures and performance of perovskite oxides. Chem Rev, 2001, 101(7), 1981 doi: 10.1021/cr980129f[22] Suntivich J, May K J, Gasteiger H A, et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science, 2011, 334(6061), 1383 doi: 10.1126/science.1212858[23] Hwang J, Rao R R, Giordano L, et al. Perovskites in catalysis and electrocatalysis. Science, 2017, 358(6364), 751 doi: 10.1126/science.aam7092[24] Cheng Z Y, Lin J. Layered organic-inorganic hybrid perovskites: structure, optical properties, film preparation, patterning and templating engineering. CrystEngComm, 2010, 12(10), 2646 doi: 10.1039/c001929a[25] Green M A, Ho-Baillie A, Snaith H J. The emergence of perovskite solar cells. Nat Photonics, 2014, 8(7), 506 doi: 10.1038/nphoton.2014.134[26] Goldschmidt V M. Crystal structure and chemical correlation. Ber Dtsch Chem Ges, 1927, 60, 1263 doi: 10.1002/cber.19270600550[27] Wang W, Tade M O, Shao Z P. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem Soc Rev, 2015, 44(15), 5371 doi: 10.1039/C5CS00113G[28] Li C, Soh K C K, Wu P. Formability of ABO3 perovskites. J Alloy Compd, 2004, 372(1/2), 40 doi: 10.1016/S0925-8388(03)01146-0[29] Li C H, Lu X G, Ding W Z, et al. Formability of ABX3 (X = F, Cl, Br, I) halide perovskites. Acta Crystallogr B, 2008, 64, 702 doi: 10.1107/S0108768108032734[30] Hu C C, Lee Y L, Teng H S. Efficient water splitting over Na1– xKxTaO3 photocatalysts with cubic perovskite structure. J Mater Chem, 2011, 21(11), 3824 doi: 10.1039/c0jm03451g[31] Li P, Ouyang S X, Xi G C, et al. The effects of crystal structure and electronic structure on photocatalytic H2 evolution and CO2 reduction over two phases of perovskite-structured NaNbO3. J Phys Chem C, 2012, 116(14), 7621 doi: 10.1021/jp210106b[32] Cohen R E. Origin of ferroelectricity in perovskite oxides. Nature, 1992, 358(6382), 136 doi: 10.1038/358136a0[33] Ohtomo A, Hwang H Y. A high-mobility electron gas at the LaAlO3/SrTiO3 heterointerface. Nature, 2004, 427(6973), 423 doi: 10.1038/nature02308[34] Reyren N, Thiel S, Caviglia A D, et al. Superconducting interfaces between insulating oxides. Science, 2007, 317(5842), 1196 doi: 10.1126/science.1146006[35] Jin S, Tiefel T H, McCormack M, et al. Thousandfold change in resistivity in magnetoresistive La–Ca–Mn–O films. Science, 1994, 264(5157), 413 doi: 10.1126/science.264.5157.413[36] Huang K, Tichy R S, Goodenough J B. Superior perovskite oxide-Ion conductor; strontium- and magnesium-doped LaGaO3:I, phase relationships and electrical properties. J Am Ceram Soc, 1998, 81(10), 2565 doi: 10.1111/j.1151-2916.1998.tb02662.x[37] Ibarra J. Influence of composition on the structure and conductivity of the fast ionic conductors La2/3− xLi3 xTiO3 (0.03 ≤ x ≤ 0.167). Solid State Ionics, 2000, 134(3/4), 219 doi: 10.1016/S0167-2738(00)00761-X[38] Chan K S, Ma J, Jaenicke S, et al. Catalytic carbon-monoxide oxidation over strontium, cerium and copper-substituted lanthanum manganates and cobaltates. Appl Catal A, 1994, 107(2), 201 doi: 10.1016/0926-860X(94)85156-5[39] Royer S, Duprez D, Can F, et al. Perovskites as substitutes of noble metals for heterogeneous catalysis: dream or reality. Chem Rev, 2014, 114(20), 10292 doi: 10.1021/cr500032a[40] Yin W J, Weng B, Ge J, et al. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energ Environ Sci, 2019, 12(2), 442 doi: 10.1039/C8EE01574K[41] Mignard D, Batik R C, Bharadwaj A S, et al. Revisiting strontium-doped lanthanum cuprate perovskite for the electrochemical reduction of CO2. J CO2 Util, 2014, 5, 53 doi: 10.1016/j.jcou.2013.12.006[42] Suntivich J, Gasteiger H A, Yabuuchi N, et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat Chem, 2011, 3(7), 546 doi: 10.1038/nchem.1069[43] Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature, 1972, 238(5358), 37 doi: 10.1038/238037a0[44] Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev, 2009, 38(1), 253 doi: 10.1039/B800489G[45] Chen S S, Takata T, Domen K. Particulate photocatalysts for overall water splitting. Nat Rev Mater, 2017, 2(10), 17050 doi: 10.1038/natrevmats.2017.50[46] Kim J H, Hansora D, Sharma P, et al. Toward practical solar hydrogen production-an artificial photosynthetic leaf-to-farm challenge. Chem Soc Rev, 2019, 48(7), 1908 doi: 10.1039/C8CS00699G[47] Yang M Q, Gao M M, Hong M H, et al. Visible-to-NIR photon harvesting: progressive engineering of catalysts for solar-powered environmental purification and fuel production. Adv Mater, 2018, 30(47), 1802894 doi: 10.1002/adma.201802894[48] Li X B, Tung C H, Wu L Z. Semiconducting quantum dots for artificial photosynthesis. Nat Rev Chem, 2018, 2(8), 160 doi: 10.1038/s41570-018-0024-8[49] Maeda K, Domen K. New non-oxide photocatalysts designed for overall water splitting under visible light. J Phys Chem C, 2007, 111(22), 7851 doi: 10.1021/jp070911w[50] Yerga R M N, Galvan M C A, del Valle F, et al. Water splitting on semiconductor catalysts under visible-light irradiation. ChemSusChem, 2009, 2(6), 471 doi: 10.1002/cssc.200900018[51] Pushkarev A P, Bochkarev M N. Organic electroluminescent materials and devices emitting in UV and NIR regions. Russ Chem Rev, 2016, 85(12), 1338 doi: 10.1070/RCR4665[52] Kang Z, Si H N, Zhang S C, et al. Interface engineering for modulation of charge carrier behavior in ZnO photoelectrochemical water splitting. Adv Funct Mater, 2019, 29(15), 1808032 doi: 10.1002/adfm.201808032[53] Jiang C R, Moniz S J A, Wang A Q, et al. Photoelectrochemical devices for solar water splitting-materials and challenges. Chem Soc Rev, 2017, 46(15), 4645 doi: 10.1039/C6CS00306K[54] Kitano M, Takeuchi M, Matsuoka M, et al. Photocatalytic water splitting using Pt-loaded visible light-responsive TiO2 thin film photocatalysts. Catal Today, 2007, 120(2), 133 doi: 10.1016/j.cattod.2006.07.043[55] Suen N T, Hung S F, Quan Q, et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem Soc Rev, 2017, 46(2), 337 doi: 10.1039/C6CS00328A[56] Kanhere P, Chen Z. A review on visible light active perovskite-based photocatalysts. Molecules, 2014, 19(12), 19995 doi: 10.3390/molecules191219995[57] Moniruddin M, Ilyassov B, Zhao X, et al. Recent progress on perovskite materials in photovoltaic and water splitting applications. Mater Today Energy, 2018, 7, 246 doi: 10.1016/j.mtener.2017.10.005[58] Khan M A, Nadeem M A, Idrissn H. Ferroelectric polarization effect on surface chemistry and photo-catalytic activity: A review. Surf Sci Rep, 2016, 71(1), 1 doi: 10.1016/j.surfrep.2016.01.001[59] Konta R, Ishii T, Kato H, et al. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J Phys Chem B, 2004, 108(26), 8992 doi: 10.1021/jp049556p[60] Ohno T, Tsubota T, Nakamura Y, et al. Preparation of S, C cation-codoped SrTiO3 and its photocatalytic activity under visible light. Appl Catal A, 2005, 288(1/2), 74 doi: 10.1016/j.apcata.2005.04.035[61] Grabowska E. Selected perovskite oxides: characterization, preparation and photocatalytic properties-A review. Appl Catal B, 2016, 186, 97 doi: 10.1016/j.apcatb.2015.12.035[62] Kawasaki M, Takahashi K, Maeda T, et al. Atomic control of the SrTiO3 crystal surface. Science, 1994, 266(5190), 1540 doi: 10.1126/science.266.5190.1540[63] Iwashina K, Kudo A. Rh-doped SrTiO3 photocatalyst electrode showing cathodic photocurrent for water splitting under visible-light irradiation. J Am Chem Soc, 2011, 133(34), 13272 doi: 10.1021/ja2050315[64] Shenoy U S, Bantawal H, Bhat D K. Band engineering of SrTiO3: effect of synthetic technique and site occupancy of doped rhodium. J Phys Chem C, 2018, 122(48), 27567 doi: 10.1021/acs.jpcc.8b10083[65] Umebayashi T, Yamaki T, Itoh H, et al. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J Phys Chem Solids, 2002, 63(10), 1909 doi: 10.1016/S0022-3697(02)00177-4[66] Zou J P, Zhang L Z, Luo S L, et al. Preparation and photocatalytic activities of two new Zn-doped SrTiO3 and BaTiO3 photocatalysts for hydrogen production from water without cocatalysts loading. Int J Hydrogen Energ, 2012, 37(22), 17068 doi: 10.1016/j.ijhydene.2012.08.133[67] Machida M, Miyazaki K, Matsushima S, et al. Photocatalytic properties of layered perovskite tantalates, MLnTa2O7 (M = Cs, Rb, Na, and H; Ln = La, Pr, Nd, and Sm). J Mater Chem, 2003, 13(6), 1433 doi: 10.1039/b301938c[68] Yin J, Zou Z, Ye J. Photophysical and photocatalytic properties of MIn0.5Nb0.5O3 (M = Ca, Sr, and Ba). J Phys Chem B, 2003, 107(1), 61 doi: 10.1021/jp026403y[69] Dong B B, Cui J Y, Liu T F, et al. Development of novel perovskite-like oxide photocatalyst LiCuTa3O9 with dual functions of water reduction and oxidation under visible light irradiation. Adv Energy Mater, 2018, 8(35), 1801660 doi: 10.1002/aenm.201801660[70] Wang B, Kanhere P D, Chen Z, et al. Anion-doped NaTaO3 for visible light photocatalysis. J Phys Chem C, 2013, 117(44), 22518 doi: 10.1021/jp407025r[71] Li F F, Liu D R, Gao G M, et al. Improved visible-light photocatalytic activity of NaTaO3 with perovskite-like structure via sulfur anion doping. Appl Catal B, 2015, 166/167, 104 doi: 10.1016/j.apcatb.2014.10.049[72] Yu H, Wang J J, Yan S C, et al. Elements doping to expand the light response of SrTiO3. J Photoch Photobio A, 2014, 275, 65 doi: 10.1016/j.jphotochem.2013.10.014[73] Humayun M, Xu L, Zhou L, et al. Exceptional co-catalyst free photocatalytic activities of B and Fe co-doped SrTiO3 for CO2 conversion and H2 evolution. Nano Res, 2018, 11(12), 6391 doi: 10.1007/s12274-018-2164-z[74] Pan C, Takata T, Kumamoto K, et al. Band engineering of perovskite-type transition metal oxynitrides for photocatalytic overall water splitting. J Mater Chem A, 2016, 4(12), 4544 doi: 10.1039/C5TA10612E[75] Shi J, Ye J, Zhou Z, et al. Hydrothermal synthesis of Na0.5La0.5TiO3-LaCrO3 solid-solution single-crystal nanocubes for visible-light-driven photocatalytic H2 evolution. Chem-Eur J, 2011, 17(28), 7858 doi: 10.1002/chem.201003755[76] Wang D, Kako T, Ye J. Efficient photocatalytic decomposition of acetaldehyde over a solid-solution perovskite (Ag0.75Sr0.25)(Nb0.75Ti0.25)O3 under visible-light irradiation. J Am Chem Soc, 2008, 130(9), 2724 doi: 10.1021/ja710805x[77] Luo W, Li Z, Jiang X, et al. Correlation between the band positions of (SrTiO3)1− x·(LaTiO2N)x solid solutions and photocatalytic properties under visible light irradiation. Phys Chem Chem Phys, 2008, 10(44), 6717 doi: 10.1039/b803996h[78] Cho S, Jang J W, Zhang W, et al. Single-crystalline thin films for studying intrinsic properties of BiFeO3-SrTiO3 solid solution photoelectrodes in solar energy conversion. Chem Mater, 2015, 27(19), 6635 doi: 10.1021/acs.chemmater.5b02394[79] Lu L, Lv M, Wang D, et al. Efficient photocatalytic hydrogen production over solid solutions Sr1– xBi xTi1– xFexO3 (0 ≤ x ≤ 0.5). Appl Catal B, 2017, 200, 412 doi: 10.1016/j.apcatb.2016.07.035[80] Zhang G, Sun S, Jiang W, et al. A novel perovskite SrTiO3-Ba2FeNbO6 solid solution for visible light photocatalytic hydrogen production. Adv Energy Mater, 2017, 7(2), 1600932 doi: 10.1002/aenm.201600932[81] Li W, Jiang K, Li Z, et al. Origin of improved photoelectrochemical water splitting in mixed perovskite oxides. Adv Mater, 2018, 8(31), 1801972 doi: 10.1002/aenm.201801972[82] Martin D J, Umezawa N, Chen X, et al. Facet engineered Ag3PO4 for efficient water photooxidation. Energ Environ Sci, 2013, 6(11), 3380 doi: 10.1039/c3ee42260g[83] Martin D J, Qiu K, Shevlin S A, et al. Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew Chem Int Ed, 2014, 53(35), 9240 doi: 10.1002/anie.201403375[84] Ham Y, Hisatomi T, Goto Y, et al. Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting. J Mater Chem A, 2016, 4(8), 3027 doi: 10.1039/C5TA04843E[85] Mu L, Zhao Y, Li A, et al. Enhancing charge separation on high symmetry SrTiO3 exposed with anisotropic facets for photocatalytic water splitting. Energ Environ Sci, 2016, 9(7), 2463 doi: 10.1039/C6EE00526H[86] Zhong D L, Liu W W, Tan P F, et al. Insights into the synergy effect of anisotropic {001} and {230} facets of BaTiO3 nanocubes sensitized with CdSe quantum dots for photocatalytic water reduction. Appl Catal B, 2018, 227, 1 doi: 10.1016/j.apcatb.2018.01.009[87] Qiao M, Liu J, Wang Y, et al. PdSeO3 monolayer: promising inorganic 2D photocatalyst for direct overall water splitting without using sacrificial reagents and cocatalysts. J Am Chem Soc, 2018, 140(38), 12256 doi: 10.1021/jacs.8b07855[88] Chandrasekaran S, Kim E J, Chung J S, et al. Structurally tuned lead magnesium titanate perovskite as a photoelectrode material for enhanced photoelectrochemical water splitting. Chem Eng J, 2017, 309, 682 doi: 10.1016/j.cej.2016.10.087[89] Parida K M, Reddy K H, Martha S, et al. Fabrication of nanocrystalline LaFeO3: An efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int J Hydrogen Energ, 2010, 35(22), 12161 doi: 10.1016/j.ijhydene.2010.08.029[90] Tijare S N, Joshi M V, Padole P S, et al. Photocatalytic hydrogen generation through water splitting on nano-crystalline LaFeO3 perovskite. Int J Hydrogen Energ, 2012, 37(13), 10451 doi: 10.1016/j.ijhydene.2012.01.120[91] Lee C W, Kim D W, Cho I S, et al. Simple synthesis and characterization of SrSnO3 nanoparticles with enhanced photocatalytic activity. Int J Hydrogen Energ, 2012, 37(14), 10557 doi: 10.1016/j.ijhydene.2012.04.063[92] Klusackova M, Nebel R, Macounova K M, et al. Size control of the photo-electrochemical water splitting activity of SrTiO3 nano-cubes. Electrochimica Acta, 2019, 297, 215 doi: 10.1016/j.electacta.2018.11.185[93] Kudo A, Tanaka A, Domen K, et al. The effects of the calcination temperature of SrTiO3 powder on photocatalytic activities. J Catal, 1988, 111(2), 296 doi: 10.1016/0021-9517(88)90088-7[94] Grinberg I, West D V, Torres M, et al. Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature, 2013, 503(7477), 509 doi: 10.1038/nature12622[95] Yi H T, Choi T, Choi S G, et al. Mechanism of the switchable photovoltaic effect in ferroelectric BiFeO3. Adv Mater, 2011, 23(30), 3403 doi: 10.1002/adma.201100805[96] Bhatnagar A, Chaudhuri A R, Kim Y H, et al. Role of domain walls in the abnormal photovoltaic effect in BiFeO3. Nat Commun, 2013, 4, 2835 doi: 10.1038/ncomms3835[97] Cao D, Xu J, Fang L, et al. Interface effect on the photocurrent: A comparative study on Pt sandwiched (Bi3.7Nd0.3)Ti3O12 and Pb(Zr0.2Ti0.8)O3 films. Appl Phys Lett, 2010, 96(19), 192101 doi: 10.1063/1.3427500[98] Wang C, Cao D, Zheng F, et al. Photocathodic behavior of ferroelectric Pb(Zr,Ti)O3 films decorated with silver nanoparticles. Chem Commun, 2013, 49(36), 3769 doi: 10.1039/c3cc38545k[99] Cao D, Wang Z, Nasori, et al. Switchable charge-transfer in the photoelectrochemical energy-conversion process of ferroelectric BiFeO3 photoelectrodes. Angew Chem Int Ed, 2014, 53(41), 11027 doi: 10.1002/anie.201406044[100] Song J, Kim T L, Lee J, et al. Domain-engineered BiFeO3 thin-film photoanodes for highly enhanced ferroelectric solar water splitting. Nano Res, 2018, 11(2), 642 doi: 10.1007/s12274-017-1669-1[101] Wang Z, Cao D, Wen L, et al. Manipulation of charge transfer and transport in plasmonic-ferroelectric hybrids for photoelectrochemical applications. Nat Commun, 2016, 7, 10348 doi: 10.1038/ncomms10348[102] Shi J, Zhao P, Wang X. Piezoelectric-polarization-enhanced photovoltaic performance in depleted-heterojunction quantum-dot solar cells. Adv Mater, 2013, 25(6), 916 doi: 10.1002/adma.201203021[103] Huang X, Wang K, Wang Y, et al. Enhanced charge carrier separation to improve hydrogen production efficiency by ferroelectric spontaneous polarization electric field. Appl Catal B, 2018, 227, 322 doi: 10.1016/j.apcatb.2018.01.036[104] Yang W, Yu Y, Starr M B, et al. Ferroelectric polarization-enhanced photoelectrochemical water splitting in TiO2-BaTiO3 core-shell nanowire photoanodes. Nano Lett, 2015, 15(11), 7574 doi: 10.1021/acs.nanolett.5b03988[105] Li W, Wang F, Li M, et al. Polarization-dependent epitaxial growth and photocatalytic performance of ferroelectric oxide heterostructures. Nano Energy, 2018, 45, 304 doi: 10.1016/j.nanoen.2018.01.002[106] Iyer A A, Ertekin E. Asymmetric response of ferroelectric/metal oxide heterojunctions for catalysis arising from interfacial chemistry. Phys Chem Chem Phys, 2017, 19(8), 5870 doi: 10.1039/C6CP06700J[107] Lee J H, Selloni A. TiO2/ferroelectric heterostructures as dynamic polarization-promoted catalysts for photochemical and electrochemical oxidation of water. Phys Rev Lett, 2014, 112(19), 196102 doi: 10.1103/PhysRevLett.112.196102[108] Xie J, Guo C, Yang P, et al. Bi-functional ferroelectric BiFeO3 passivated BiVO4 photoanode for efficient and stable solar water oxidation. Nano Energy, 2017, 31, 28 doi: 10.1016/j.nanoen.2016.10.048[109] Low J, Yu J, Jaroniec M, et al. Heterojunction photocatalysts. Adv Mater, 2017, 29(20), 1601694 doi: 10.1002/adma.201601694[110] Li H, Zhou Y, Tu W, et al. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv Funct Mater, 2015, 25(7), 998 doi: 10.1002/adfm.201401636[111] Nashim A, Parida K. n-La2Ti2O7/p-LaCrO3: a novel heterojunction based composite photocatalyst with enhanced photoactivity towards hydrogen production. J Mater Chem A, 2014, 2(43), 18405 doi: 10.1039/C4TA02401J[112] Xu X, Liu G, Randorn C, et al. g-C3N4 coated SrTiO3 as an efficient photocatalyst for H2 production in aqueous solution under visible light irradiation. Int J Hydrogen Energ, 2011, 36(21), 13501 doi: 10.1016/j.ijhydene.2011.08.052[113] Kang H W, Lim S N, Song D, et al. Organic-inorganic composite of g-C3N4-SrTiO3:Rh photocatalyst for improved H2 evolution under visible light irradiation. Int J Hydrogen Energ, 2012, 37(16), 11602 doi: 10.1016/j.ijhydene.2012.05.020[114] Opoku F, Govender K K, van Sittert C G C E, et al. Tuning the electronic structures, work functions, optical properties and stability of bifunctional hybrid graphene oxide/V-doped NaNbO3 type-II heterostructures: A promising photocatalyst for H2 production. Carbon, 2018, 136, 187 doi: 10.1016/j.carbon.2018.04.076[115] Jia Q, Iwase A, Kudo A. BiVO4-Ru/SrTiO3:Rh composite Z-scheme photocatalyst for solar water splitting. Chem Sci, 2014, 5(4), 1513 doi: 10.1039/c3sc52810c[116] Dong C, Lu S, Yao S, et al. Colloidal synthesis of ultrathin monoclinic BiVO4 nanosheets for Z-scheme overall water splitting under visible light. ACS Catal, 2018, 8(9), 8649 doi: 10.1021/acscatal.8b01645[117] Ma Z, Li Y, Lv Y, et al. Synergistic effect of doping and compositing on photocatalytic efficiency: a case study of La2Ti2O7. ACS Appl Mater Inter, 2018, 10(45), 39327 doi: 10.1021/acsami.8b12178[118] Wei Y, Wang J, Yu R, et al. Constructing SrTiO3-TiO2 heterogeneous hollow multi-shelled structures for enhanced solar water splitting. Angew Chem Int Ed, 2019, 131(5), 1436 doi: 10.1002/ange.201812364[119] Chang Y, Yu K, Zhang C, et al. Ternary CdS/Au/3DOM-SrTiO3 composites with synergistic enhancement for hydrogen production from visible-light photocatalytic water splitting. Appl Catal B, 2017, 215, 74 doi: 10.1016/j.apcatb.2017.05.054[120] Valenti M, Jonsson M P, Biskos G, et al. Plasmonic nanoparticle-semiconductor composites for efficient solar water splitting. J Mater Chem A, 2016, 4(46), 17891 doi: 10.1039/C6TA06405A[121] Zhang P, Wang T, Gong J. Mechanistic understanding of the plasmonic enhancement for solar water splitting. Adv Mater, 2015, 27(36), 5328 doi: 10.1002/adma.201500888[122] Xu D, Yang S, Jin Y, et al. Ag-decorated ATaO3 (A = K, Na) nanocube plasmonic photocatalysts with enhanced photocatalytic water-splitting properties. Langmuir, 2015, 31(35), 9694 doi: 10.1021/acs.langmuir.5b01294[123] Liu J, Sun Y, Li Z, et al. Photocatalytic hydrogen production from water/methanol solutions over highly ordered Ag-SrTiO3 nanotube arrays. Int J Hydrogen Energ, 2011, 36(10), 5811 doi: 10.1016/j.ijhydene.2011.01.117[124] Lu D, Ouyang S, Xu H, et al. Designing Au surface-modified nanoporous-single-crystalline SrTiO3 to optimize diffusion of surface plasmon resonance-induce photoelectron toward enhanced visible-light photoactivity. ACS Appl Mater Inter, 2016, 8(14), 9506 doi: 10.1021/acsami.6b00889[125] Zhang B T, Liu J, Yue S, et al. Hot electron injection: an efficacious approach to charge LaCoO3 for improving the water splitting efficiency. Appl Catal B, 2017, 219, 432 doi: 10.1016/j.apcatb.2017.07.033[126] Huang Y B, Liu J, Cao D W, et al. Separation of hot electrons and holes in Au/LaFeO3 to boost the photocatalytic activities both for water reduction and oxidation. Int J Hydrogen Energ, 2019, 44(26), 13242 doi: 10.1016/j.ijhydene.2019.03.182[127] Cai X, Zhu M, Elbanna O A, et al. Au nanorod photosensitized La2Ti2O7 nanosteps: successive surface heterojunctions boosting visible to near-infrared photocatalytic H2 evolution. ACS Catal, 2018, 8(1), 122 doi: 10.1021/acscatal.7b02972[128] Shi L, Zhou W, Li Z, et al. Periodically ordered nanoporous perovskite photoelectrode for efficient photoelectrochemical water splitting. ACS Nano, 2018, 12(6), 6335 doi: 10.1021/acsnano.8b03940[129] Zhong Y, Ueno K, Mori Y, et al. Plasmon-assisted water splitting using two sides of the same SrTiO3 single-crystal substrate: conversion of visible light to chemical energy. Angew Chem Int Ed, 2014, 53(39), 10350 doi: 10.1002/anie.201404926[130] Liu Q, Zhou Y, You L, et al. Enhanced ferroelectric photoelectrochemical properties of polycrystalline BiFeO3 film by decorating with Ag nanoparticles. Appl Phys Lett, 2016, 108(2), 022902 doi: 10.1063/1.4939747[131] Huang Y L, Chang W S, Van C N, et al. Tunable photoelectrochemical performance of Au/BiFeO3 heterostructure. Nanoscale, 2016, 8(34), 15795 doi: 10.1039/C6NR04997D[132] Zhu M, Cai X, Fujitsuka M, et al. Au/La2Ti2O7 nanostructures sensitized with black phosphorus for plasmon-enhanced photocatalytic hydrogen production in visible and near-infrared light. Angew Chem Int Ed, 2017, 56(8), 2064 doi: 10.1002/anie.201612315[133] Liu J, Liu Y, Liu N, et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science, 2015, 347(6225), 970 doi: 10.1126/science.aaa3145 -
Proportional views





 DownLoad:
DownLoad: