| Citation: |
Liu Ye, Weiyu Ye, Shiming Zhang. Recent advances and prospects of asymmetric non-fullerene small molecule acceptors for polymer solar cells[J]. Journal of Semiconductors, 2021, 42(10): 101607. doi: 10.1088/1674-4926/42/10/101607
****
L Ye, W Y Ye, S M Zhang, Recent advances and prospects of asymmetric non-fullerene small molecule acceptors for polymer solar cells[J]. J. Semicond., 2021, 42(10): 101607. doi: 10.1088/1674-4926/42/10/101607.
|
Recent advances and prospects of asymmetric non-fullerene small molecule acceptors for polymer solar cells
DOI: 10.1088/1674-4926/42/10/101607
More Information
-
Abstract
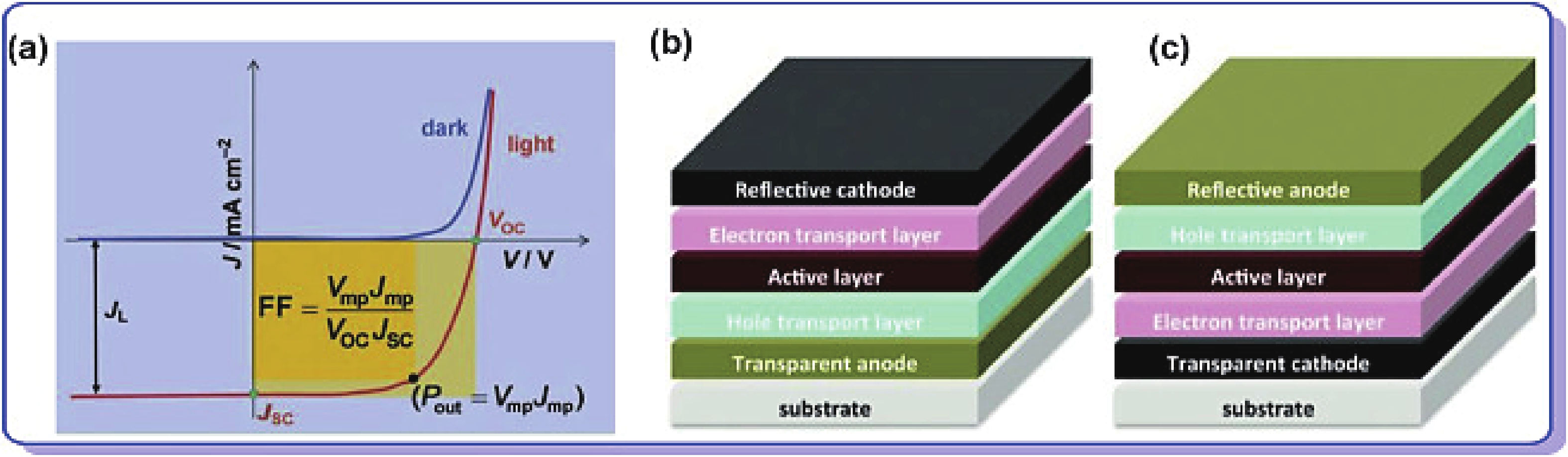
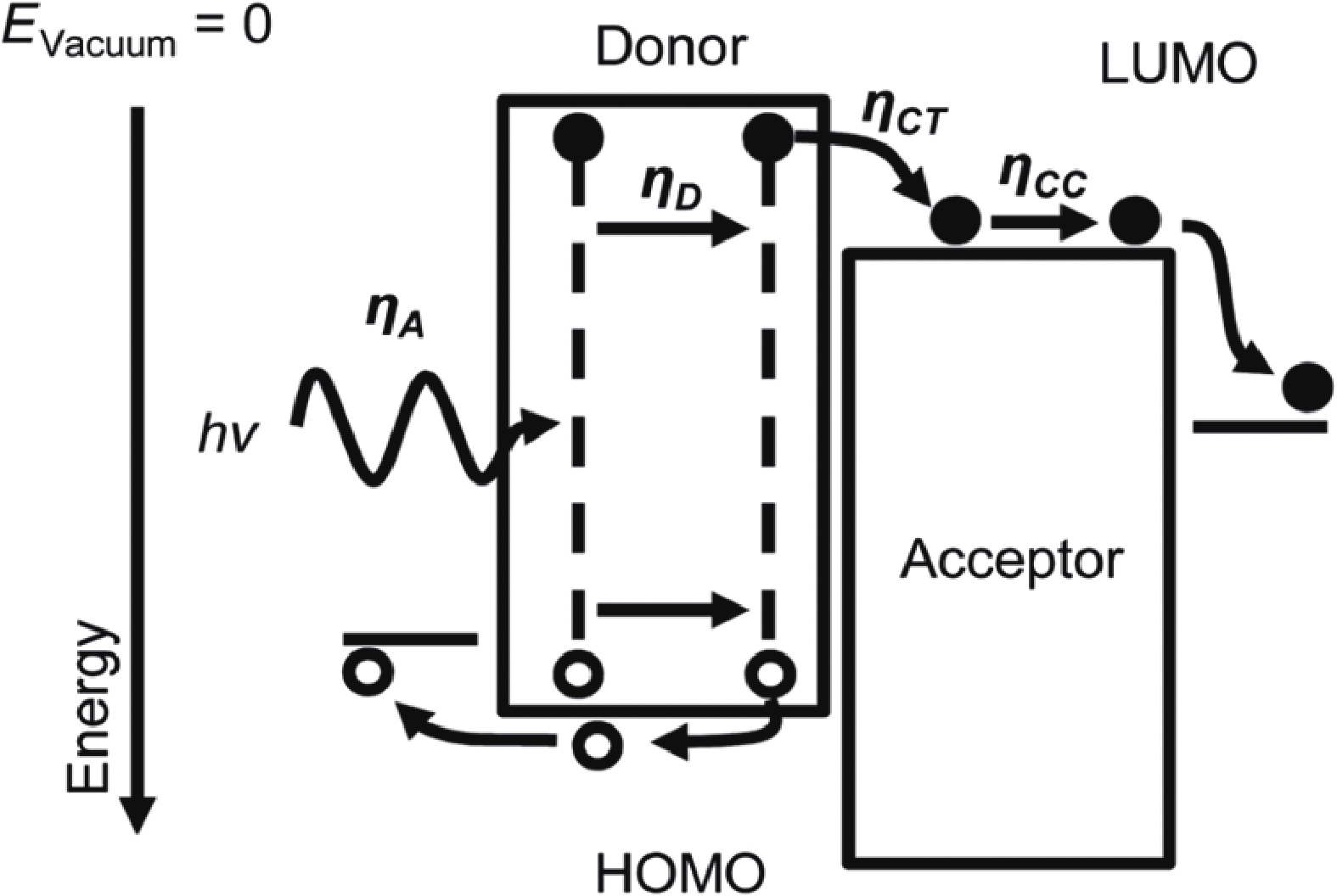
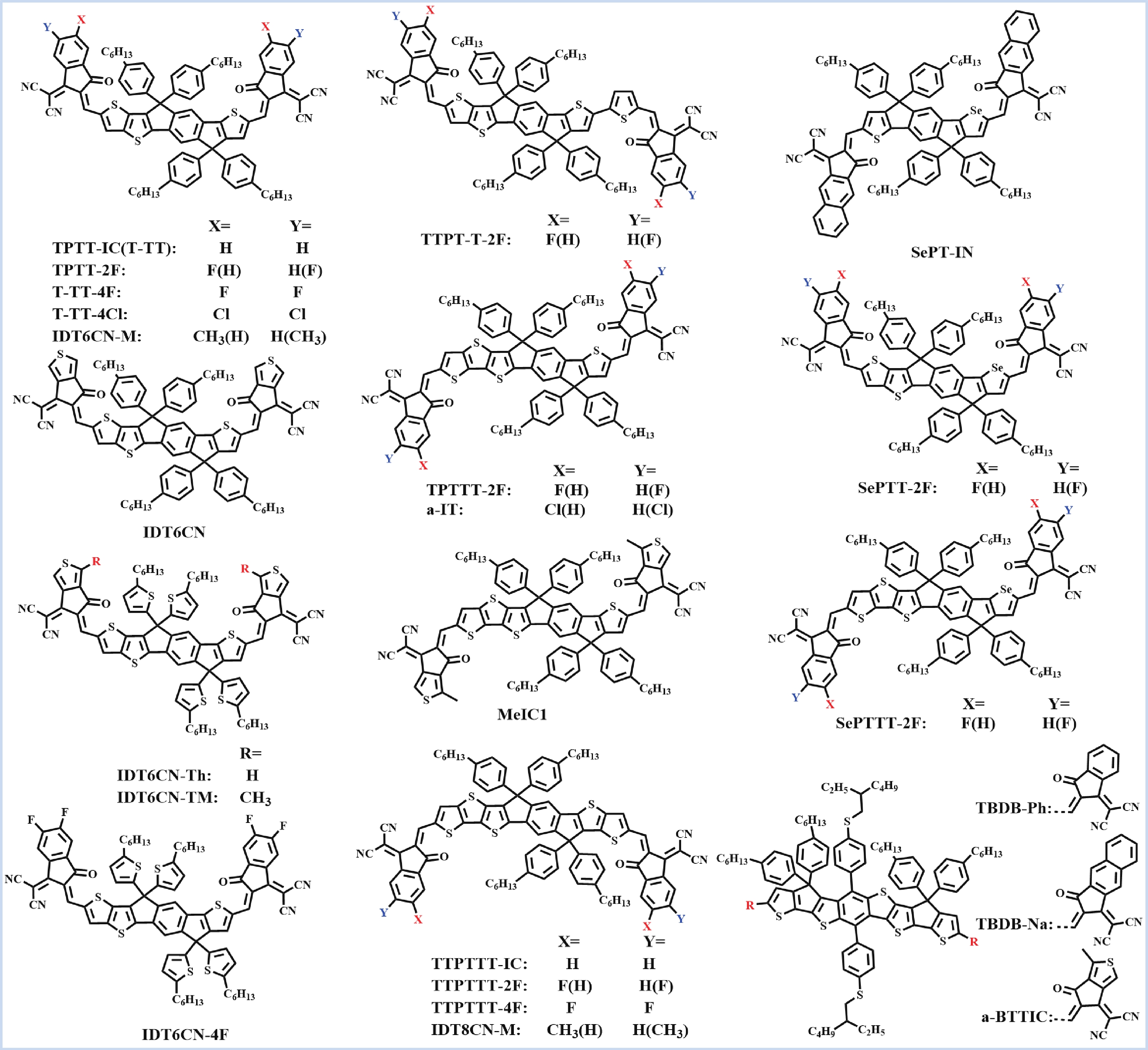
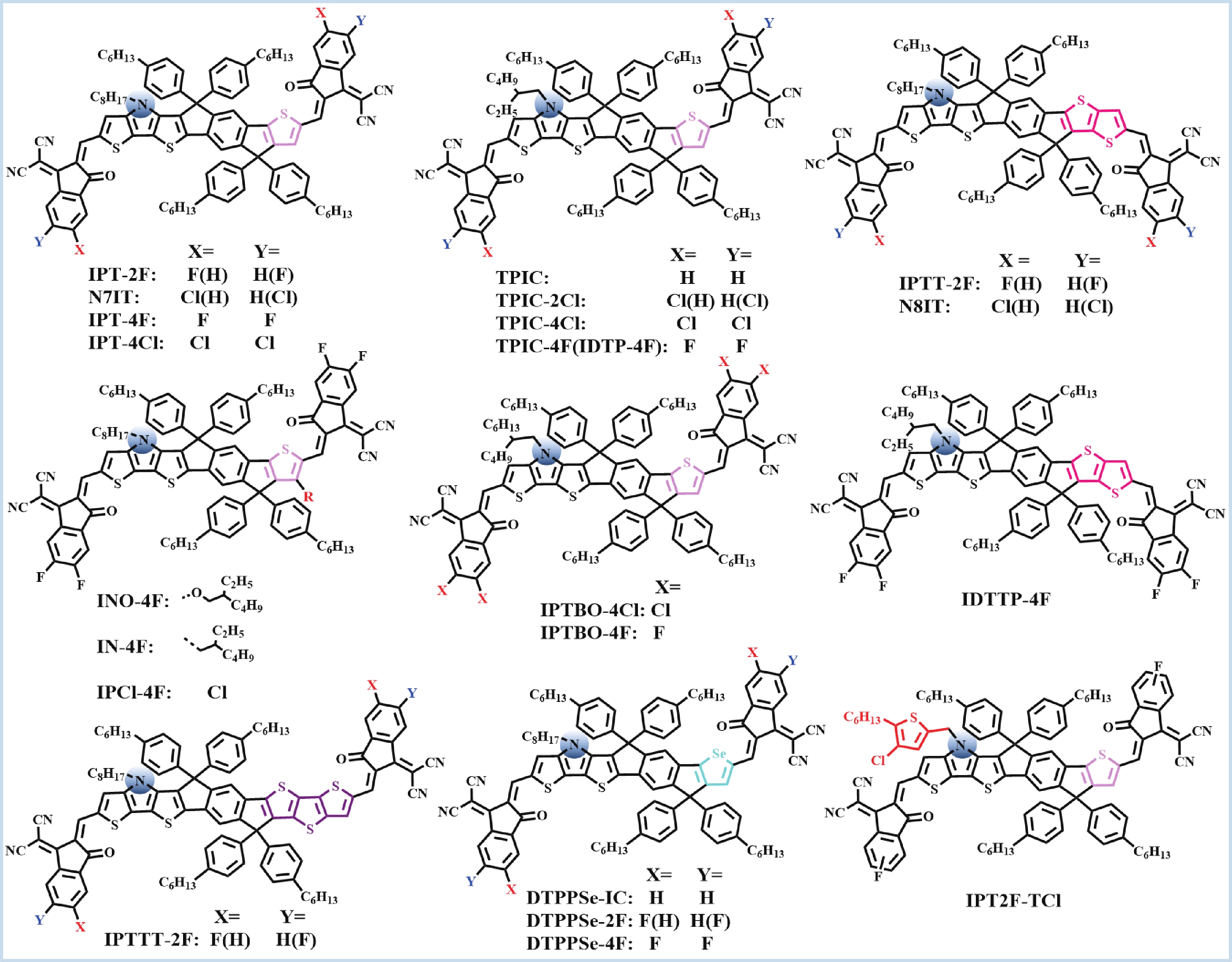
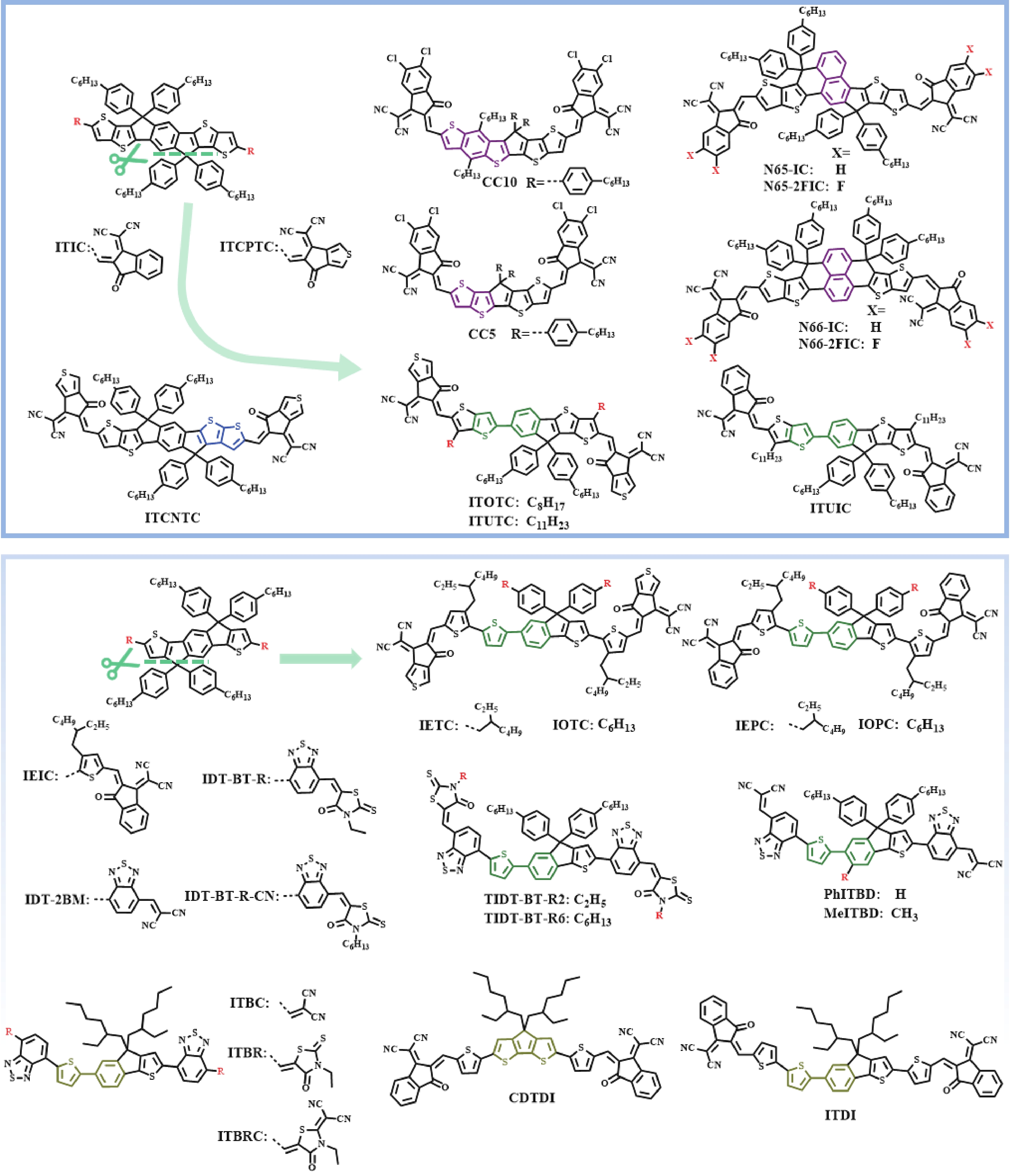
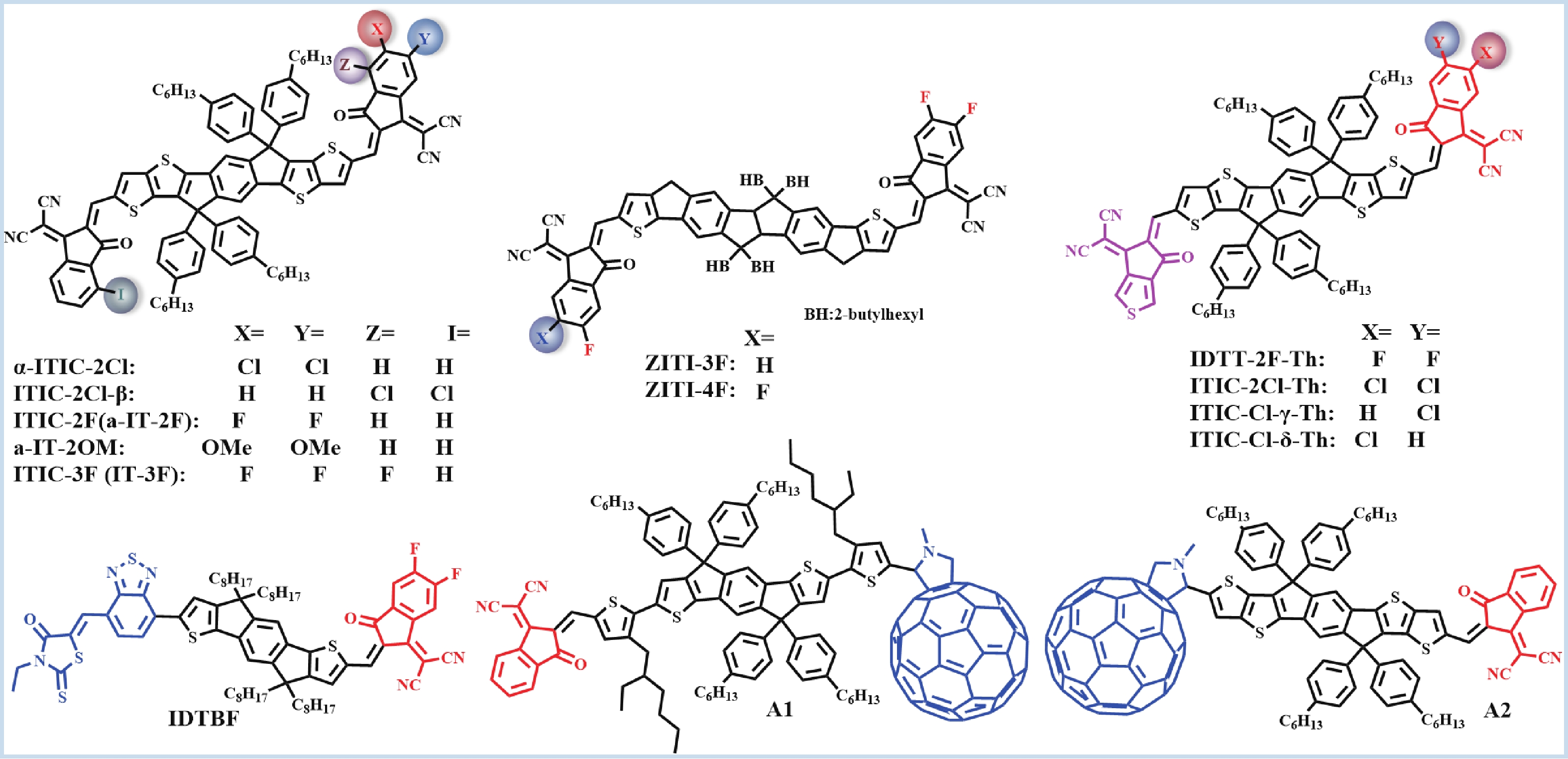
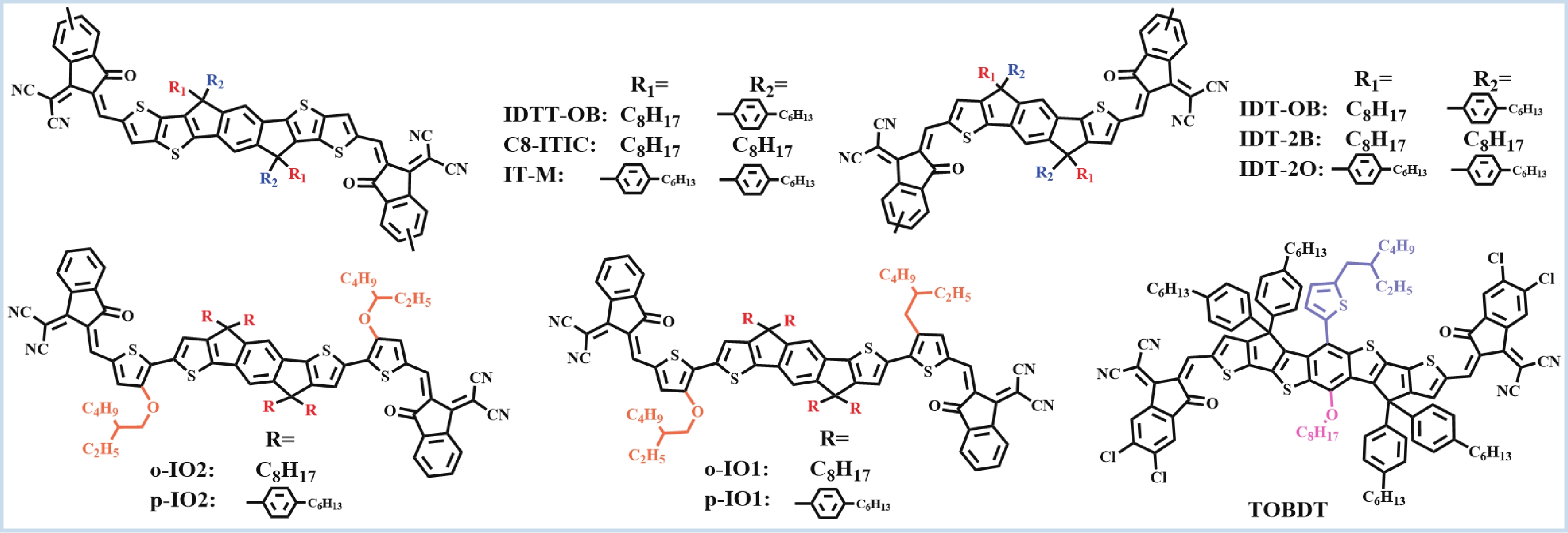
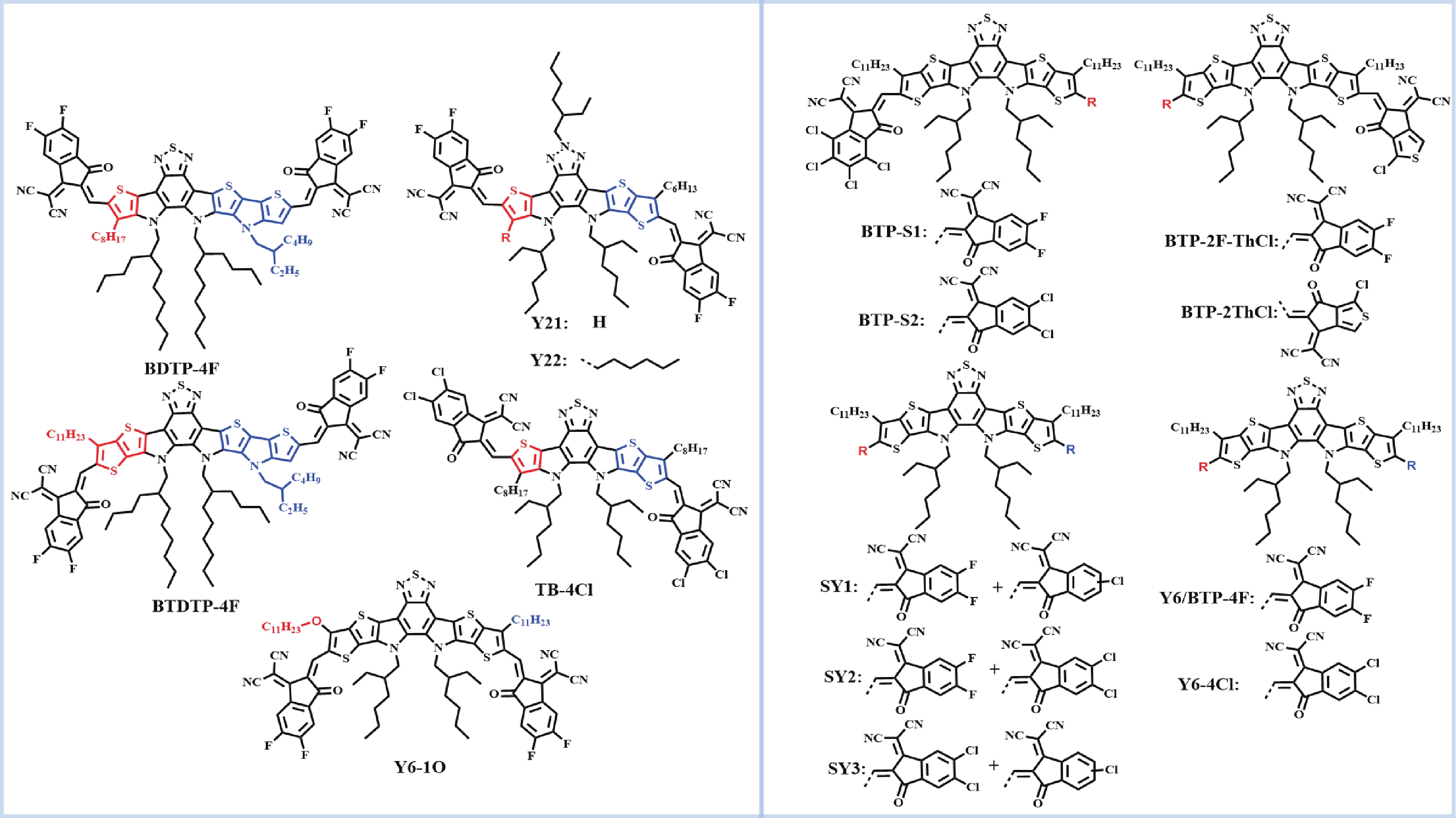
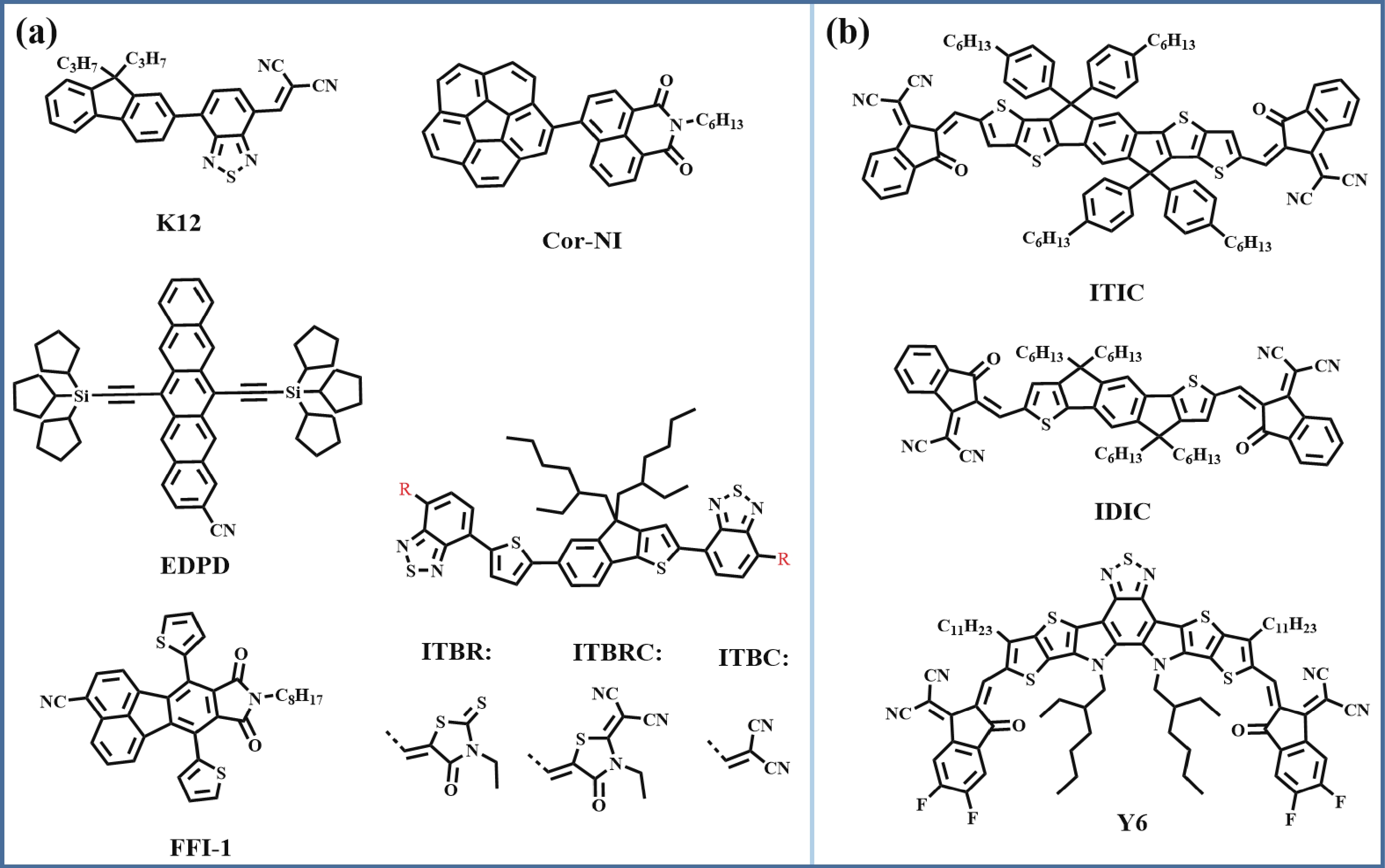
Recently, polymer solar cells developed very fast due to the application of non-fullerence acceptors. Substituting asymmetric small molecules for symmetric small molecule acceptors in the photoactive layer is a strategy to improve the performance of polymer solar cells. The asymmetric design of the molecule is very beneficial for exciton dissociation and charge transport and will also fine-tune the molecular energy level to adjust the open-circuit voltage (Voc) further. The influence on the absorption range and absorption intensity will cause the short-circuit current density (Jsc) to change, resulting in higher device performance. The effect on molecular aggregation and molecular stacking of asymmetric structures can directly change the microscopic morphology, phase separation size, and the active layer's crystallinity. Very recently, thanks to the ingenious design of active layer materials and the optimization of devices, asymmetric non-fullerene polymer solar cells (A-NF-PSCs) have achieved remarkable development. In this review, we have summarized the latest developments in asymmetric small molecule acceptors (A-NF-SMAs) with the acceptor–donor–acceptor (A–D–A) and/or acceptor–donor–acceptor–donor–acceptor (A–D–A–D–A) structures, and the advantages of asymmetric small molecules are explored from the aspects of charge transport, molecular energy level and active layer accumulation morphology. -
References
[1] Winzenberg K N, Kemppinen P, Scholes F H, et al. Indan-1, 3-dione electron-acceptor small molecules for solution-processable solar cells: A structure-property correlation. Chem Commun, 2013, 49, 6307 doi: 10.1039/c3cc42293c[2] Zhou T L, Jia T, Kang B N, et al. Nitrile-substituted QA derivatives: New acceptor materials for solution-processable organic bulk heterojunction solar cells. Adv Energy Mater, 2011, 1, 431 doi: 10.1002/aenm.201100082[3] Fang Y, Pandey A K, Nardes A M, et al. A narrow optical gap small molecule acceptor for organic solar cells. Adv Energy Mater, 2013, 3, 54 doi: 10.1002/aenm.201200372[4] Nielsen C B, Voroshazi E, Holliday S, et al. Efficient truxenone-based acceptors for organic photovoltaics. J Mater Chem A, 2013, 1, 73 doi: 10.1039/C2TA00548D[5] Bloking J T, Han X, Higgs A T, et al. Solution-processed organic solar cells with power conversion efficiencies of 2.5% using benzothiadiazole/imide-based acceptors. Chem Mater, 2011, 23, 5484 doi: 10.1021/cm203111k[6] Lin Y Z, Wang H F, Li Y F, et al. A star-shaped electron acceptor based on 5, 5'-bibenzothiadiazole for solution processed solar cells. J Mater Chem A, 2013, 1, 14627 doi: 10.1039/c3ta13747c[7] Lin Y Z, Cheng P, Li Y F, et al. A 3D star-shaped non-fullerene acceptor for solution-processed organic solar cells with a high open-circuit voltage of 1.18 V. Chem Commun, 2012, 48, 4773 doi: 10.1039/c2cc31511d[8] Li J F, Sun C K, Tang A L, et al. Utilizing an electron-deficient thieno[3, 4-c]pyrrole-4, 6-dione (TPD) unit as a π-bridge to improve the photovoltaic performance of A–π–D–π–A type acceptors. J Mater Chem C, 2020, 8, 15981 doi: 10.1039/D0TC04601A[9] Wang J Y, Zhan X W. Fused-ring electron acceptors for photovoltaics and beyond. Acc Chem Res, 2021, 54, 132 doi: 10.1021/acs.accounts.0c00575[10] Chen W Q, Zhang Q C. Recent progress in non-fullerene small molecule acceptors in organic solar cells (OSCs). J Mater Chem C, 2017, 5, 1275 doi: 10.1039/C6TC05066B[11] Sun H, Song X, Xie J, et al. PDI derivative through fine-tuning the molecular structure for fullerene-free organic solar cells. ACS Appl Mater Interfaces, 2017, 9, 29924 doi: 10.1021/acsami.7b08282[12] Fan X B, Gao J H, Wang W, et al. Ladder-type nonacyclic arene bis(thieno[3, 2-b]thieno)cyclopentafluorene as a promising building block for non-fullerene acceptors. Chem Asian J, 2019, 14, 1814 doi: 10.1002/asia.201801669[13] Liu Q S, Jiang Y F, Jin K, et al. 18% efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[14] Lin Y Z, Wang J Y, Zhang Z G, et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv Mater, 2015, 27, 1170 doi: 10.1002/adma.201404317[15] Lin Y Z, Li Y F, Zhan X W. A solution-processable electron acceptor based on dibenzosilole and diketopyrrolopyrrole for organic solar cells. Adv Energy Mater, 2013, 3, 724 doi: 10.1002/aenm.201200911[16] Li H Y, Earmme T, Ren G Q, et al. Beyond fullerenes: Design of nonfullerene acceptors for efficient organic photovoltaics. J Am Chem Soc, 2014, 136, 14589 doi: 10.1021/ja508472j[17] Sharenko A, Proctor C M, van der Poll T S, et al. A high-performing solution-processed small molecule: Perylene diimide bulk heterojunction solar cell. Adv Mater, 2013, 25, 4403 doi: 10.1002/adma.201301167[18] Lin Y Z, Zhao F W, Wu Y, et al. Mapping polymer donors toward high-efficiency fullerene free organic solar cells. Adv Mater, 2017, 29, 1604155 doi: 10.1002/adma.201604155[19] Chen X, Zheng S H. On the study of influence of molecular arrangements and dipole moment on exciton binding energy in solid state. Int J Quantum Chem, 2021, 121, e26511 doi: 10.1002/qua.26511[20] Benatto L, de Almeida Sousa K R, Koehler M. Driving force for exciton dissociation in organic solar cells: The influence of donor and acceptor relative orientation. J Phys Chem C, 2020, 124, 13580 doi: 10.1021/acs.jpcc.0c03116[21] Cao J R, Qu S Y, Yang L Q, et al. An asymmetric acceptor enabling 77.51% fill factor in organic solar cells. Sci Bull, 2020, 65, 1876 doi: 10.1016/j.scib.2020.08.004[22] Ma R J, Liu T, Luo Z H, et al. Adding a third component with reduced miscibility and higher LUMO level enables efficient ternary organic solar cells. ACS Energy Lett, 2020, 5, 2711 doi: 10.1021/acsenergylett.0c01364[23] Yin L X, Yuan Q Q, Li Y Q. D–A–A'-type asymmetric small molecules based on triphenylamine-diketopyrrolopyrrole/5, 6-difluoro-2, 1, 3-benzothiadiazole backbone for organic photovoltaic materials. New J Chem, 2020, 44, 13319 doi: 10.1039/D0NJ02239J[24] Congreve D N, Lee J, Thompson N J, et al. External quantum efficiency above 100% in a singlet-exciton-fission-based organic photovoltaic cell. Science, 2013, 340, 334 doi: 10.1126/science.1232994[25] Schwenn P E, Gui K, Nardes A M, et al. A small molecule non-fullerene electron acceptor for organic solar cells. Adv Energy Mater, 2011, 1, 73 doi: 10.1002/aenm.201000024[26] Shu Y, Lim Y F, Li Z, et al. A survey of electron-deficient pentacenes as acceptors in polymer bulk heterojunction solar cells. Chem Sci, 2011, 2, 363 doi: 10.1039/C0SC00433B[27] Zhou Y, Ding L, Shi K, et al. A non-fullerene small molecule as efficient electron acceptor in organic bulk heterojunction solar cells. Adv Mater, 2012, 24, 957 doi: 10.1002/adma.201103927[28] Li C, Fu H T, Xia T, et al. Asymmetric nonfullerene small molecule acceptors for organic solar cells. Adv Energy Mater, 2019, 9, 1900999 doi: 10.1002/aenm.201900999[29] Tang C Q, Chen S C, Shang Q, et al. Asymmetric indenothiophene-based non-fullerene acceptors for efficient polymer solar cells. Sci China Mater, 2017, 60, 707 doi: 10.1007/s40843-017-9059-3[30] Yuan J, Zhang Y Q, Zhou L Y, et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule, 2019, 3, 1140 doi: 10.1016/j.joule.2019.01.004[31] Guo X, Fan Q P, Wu J N, et al. Optimized active layer morphologies via ternary copolymerization of polymer donors for 17.6 % efficiency organic solar cells with enhanced fill factor. Angew Chem Int Ed, 2021, 60, 2322 doi: 10.1002/anie.202010596[32] Li S X, Zhan L L, Jin Y Z, et al. Asymmetric electron acceptors for high-efficiency and low-energy-loss organic photovoltaics. Adv Mater, 2020, 32, 2001160 doi: 10.1002/adma.202001160[33] Zhang C X, Liu R G, Mak C H, et al. Photophysics of organic photovoltaic devices: A review. J Photonics Energy, 2018, 8, 021001 doi: 10.1117/1.JPE.8.021001[34] Ilmi R, Haque A, Khan M S. High efficiency small molecule-based donor materials for organic solar cells. Org Electron, 2018, 58, 53 doi: 10.1016/j.orgel.2018.03.048[35] Qi B, Wang J. Fill factor in organic solar cells. Phys Chem Chem Phys, 2013, 15, 8972 doi: 10.1039/c3cp51383a[36] Chen Y Z, Bai F J, Peng Z X, et al. Asymmetric alkoxy and alkyl substitution on nonfullerene acceptors enabling high-performance organic solar cells. Adv Energy Mater, 2021, 11, 2003141 doi: 10.1002/aenm.202003141[37] Li C, Xie Y P, Fan B B, et al. A nonfullerene acceptor utilizing a novel asymmetric multifused-ring core unit for highly efficient organic solar cells. J Mater Chem C, 2018, 6, 4873 doi: 10.1039/C8TC01229F[38] Song J L, Li C, Ye L L, et al. Extension of indacenodithiophene backbone conjugation enables efficient asymmetric A–D–A type non-fullerene acceptors. J Mater Chem A, 2018, 6, 18847 doi: 10.1039/C8TA07334A[39] Liu S N, Zhao B F, Cong Z Y, et al. Influences of the terminal groups on the performances of asymmetric small molecule acceptors-based polymer solar cells. Dyes Pigments, 2020, 178, 108388 doi: 10.1016/j.dyepig.2020.108388[40] Gao W, Liu T, Zhong C, et al. Asymmetrical small molecule acceptor enabling nonfullerene polymer solar cell with fill factor approaching 79%. ACS Energy Lett, 2018, 3, 1760 doi: 10.1021/acsenergylett.8b00825[41] Li X S, Li C, Ye L L, et al. Asymmetric A–D–π–A-type nonfullerene small molecule acceptors for efficient organic solar cells. J Mater Chem A, 2019, 7, 19348 doi: 10.1039/C9TA06476A[42] Gao W, Zhang M, Liu T, et al. Asymmetrical ladder-type donor-induced polar small molecule acceptor to promote fill factors approaching 77% for high-performance nonfullerene polymer solar cells. Adv Mater, 2018, 30, 1800052 doi: 10.1002/adma.201800052[43] Gao W, Wu F, Liu T, et al. Multifunctional asymmetrical molecules for high-performance perovskite and organic solar cells. J Mater Chem A, 2019, 7, 2412 doi: 10.1039/C8TA10975C[44] Gao W, Liu T, Sun R, et al. Dithieno[3, 2-b: 2ʹ, 3ʹ-d]pyrrol-fused asymmetrical electron acceptors: A study into the effects of nitrogen-functionalization on reducing nonradiative recombination loss and dipole moment on morphology. Adv Sci, 2020, 7, 1902657 doi: 10.1002/advs.201902657[45] Gao W, An Q S, Zhong C, et al. Designing an asymmetrical isomer to promote the LUMO energy level and molecular packing of a non-fullerene acceptor for polymer solar cells with 12.6% efficiency. Chem Sci, 2018, 9, 8142 doi: 10.1039/C8SC02018C[46] Li C, Song J L, Ye L L, et al. High-performance eight-membered indacenodithiophene-based asymmetric A-D-A type non-fullerene acceptors. Sol RRL, 2019, 3, 1800246 doi: 10.1002/solr.201800246[47] Li C, Song J L, Cai Y H, et al. Heteroatom substitution-induced asymmetric A-D-A type non-fullerene acceptor for efficient organic solar cells. J Energy Chem, 2020, 40, 144 doi: 10.1016/j.jechem.2019.03.009[48] Li C, Xia T, Song J L, et al. Asymmetric selenophene-based non-fullerene acceptors for high-performance organic solar cells. J Mater Chem A, 2019, 7, 1435 doi: 10.1039/C8TA11197A[49] Wang X C, Han J H, Jiang H X, et al. Regulation of molecular packing and blend morphology by finely tuning molecular conformation for high-performance nonfullerene polymer solar cells. ACS Appl Mater Interfaces, 2019, 11, 44501 doi: 10.1021/acsami.9b14981[50] Gao W, Liu T, Li J W, et al. Simultaneously increasing open-circuit voltage and short-circuit current to minimize the energy loss in organic solar cells via designing asymmetrical non-fullerene acceptor. J Mater Chem A, 2019, 7, 11053 doi: 10.1039/C9TA02283J[51] Zhang X M, Li M M, Wang Q, et al. Near-infrared absorbing non-fullerene acceptors with dithienopyrrole as π spacer for organic solar cells. Chin J Appl Chem, 2019, 36, 1023 doi: 10.11944/j.issn.1000-0518.2019.09.190048[52] Geng Y F, Tang A L, Tajima K, et al. Conjugated materials containing dithieno[3, 2-b: 2', 3'-d]pyrrole and its derivatives for organic and hybrid solar cell applications. J Mater Chem A, 2019, 7, 64 doi: 10.1039/C8TA09383K[53] Yang L Q, Song X, Yu J S, et al. Tuning of the conformation of asymmetric nonfullerene acceptors for efficient organic solar cells. J Mater Chem A, 2019, 7, 22279 doi: 10.1039/C9TA07634D[54] Yang L Q, Hu Z H, Zhang Z H, et al. Molecular engineering of acceptors to control aggregation for optimized nonfullerene solar cells. J Mater Chem A, 2020, 8, 5458 doi: 10.1039/D0TA00651C[55] Ma R J, Li G, Li D D, et al. Understanding the effect of end group halogenation in tuning miscibility and morphology of high-performance small molecular acceptors. Sol RRL, 2020, 4, 2000250 doi: 10.1002/solr.202000250[56] Li G, Li D D, Ma R J, et al. Efficient modulation of end groups for the asymmetric small molecule acceptors enabling organic solar cells with over 15% efficiency. J Mater Chem A, 2020, 8, 5927 doi: 10.1039/D0TA01032D[57] Cao J R, Qu S Y, Yu J S, et al. 13.76% efficiency nonfullerene solar cells enabled by selenophene integrated dithieno[3, 2-b:2', 3'-d]pyrrole asymmetric acceptors. Mater Chem Front, 2020, 4, 924 doi: 10.1039/C9QM00775J[58] Guo Q, Ma R J, Hu J, et al. Over 15% efficiency polymer solar cells enabled by conformation tuning of newly designed asymmetric small-molecule acceptors. Adv Funct Mater, 2020, 30, 2000383 doi: 10.1002/adfm.202000383[59] Luo Z H, Ma R J, Xiao Y Q, et al. Conformation-tuning effect of asymmetric small molecule acceptors on molecular packing, interaction, and photovoltaic performance. Small, 2020, 16, 2001942 doi: 10.1002/smll.202001942[60] Zhang Z H, Yang L Q, Hu Z H, et al. Charge density modulation on asymmetric fused-ring acceptors for high-efficiency photovoltaic solar cells. Mater Chem Front, 2020, 4, 1747 doi: 10.1039/D0QM00123F[61] Luo Z H, Li G H, Wu K L, et al. Asymmetric thieno[2, 3-b]thiophene-based electron acceptor featuring a seven fused-ring electron donor unit as core for nonfullerene organic photovoltaics. Org Electron, 2018, 62, 82 doi: 10.1016/j.orgel.2018.07.018[62] Jiao C C, Guo Z Q, Sun B Q, et al. An acceptor–donor–acceptor type non-fullerene acceptor with an asymmetric backbone for high performance organic solar cells. J Mater Chem C, 2020, 8, 6293 doi: 10.1039/D0TC00981D[63] Hu W, Du X Y, Zhuang W L, et al. Axisymmetric and asymmetric naphthalene-bisthienothiophene based nonfullerene acceptors: On constitutional isomerization and photovoltaic performance. ACS Appl Energy Mater, 2020, 3, 5734 doi: 10.1021/acsaem.0c00689[64] Zhang M Q, Ma Y L, Zheng Q D. Asymmetric indenothienothiophene-based unfused core for A-D-A type nonfullerene acceptors. Dyes Pigments, 2020, 180, 108495 doi: 10.1016/j.dyepig.2020.108495[65] Huang B, Chen L, Jin X F, et al. Alkylsilyl functionalized copolymer donor for annealing-free high performance solar cells with over 11% efficiency: Crystallinity induced small driving force. Adv Funct Mater, 2018, 28, 1800606 doi: 10.1002/adfm.201800606[66] Kang Z J, Ma Y L, Zheng Q D. Asymmetric indenothiophene-based nonfullerene acceptors for binary- and ternary-blend polymer solar cells. Dyes Pigments, 2019, 170, 107555 doi: 10.1016/j.dyepig.2019.107555[67] Hong L, Yao H F, Yu R N, et al. Investigating the trade-off between device performance and energy loss in nonfullerene organic solar cells. ACS Appl Mater Interfaces, 2019, 11, 29124 doi: 10.1021/acsami.9b10243[68] Bai W Y, Xu X P, Li Q Y, et al. Efficient nonfullerene polymer solar cells enabled by small-molecular acceptors with a decreased fused-ring core. Small Methods, 2018, 2, 1700373 doi: 10.1002/smtd.201700373[69] Un Kim Y, Eun Park G, Choi S, et al. A new n-type semiconducting molecule with an asymmetric indenothiophene core for a high-performing non-fullerene type organic solar cell. J Mater Chem C, 2017, 5, 7182 doi: 10.1039/C7TC00706J[70] Li Q Y, Xiao J Y, Tang L M, et al. Thermally stable high performance non-fullerene polymer solar cells with low energy loss by using ladder-type small molecule acceptors. Org Electron, 2017, 44, 217 doi: 10.1016/j.orgel.2017.02.008[71] Xiao J Y, Chen Z M, Zhang G C, et al. Efficient device engineering for inverted non-fullerene organic solar cells with low energy loss. J Mater Chem C, 2018, 6, 4457 doi: 10.1039/C8TC00705E[72] Kang Z J, Chen S C, Ma Y L, et al. Push-pull type non-fullerene acceptors for polymer solar cells: Effect of the donor core. ACS Appl Mater Interfaces, 2017, 9, 24771 doi: 10.1021/acsami.7b05417[73] Zhang Y, Wang Y, Xie Z Y, et al. Preparation of non-fullerene acceptors with a multi-asymmetric configuration in a one-pot reaction for organic solar cells. J Mater Chem C, 2020, 8, 17229 doi: 10.1039/D0TC04749J[74] Hu D Q, Yang Q G, Zheng Y J, et al. 15.3% efficiency all-small-molecule organic solar cells achieved by a locally asymmetric F, Cl disubstitution strategy. Adv Sci, 2021, 8, 2004262 doi: 10.1002/advs.202004262[75] Pan F, Li X J, Bai S, et al. High electron mobility fluorinated indacenodithiophene small molecule acceptors for organic solar cells. Chin Chem Lett, 2021, 32, 1257 doi: 10.1016/j.cclet.2020.08.051[76] Lai H J, Chen H, Zhou J D, et al. 3D interpenetrating network for high-performance nonfullerene acceptors via asymmetric chlorine substitution. J Phys Chem Lett, 2019, 10, 4737 doi: 10.1021/acs.jpclett.9b01931[77] Aldrich T J, Matta M, Zhu W, et al. Fluorination effects on indacenodithienothiophene acceptor packing and electronic structure, end-group redistribution, and solar cell photovoltaic response. J Am Chem Soc, 2019, 141, 3274 doi: 10.1021/jacs.8b13653[78] Li M, Zhou Y Y, Zhang J Q, et al. Tuning the dipole moments of nonfullerene acceptors with an asymmetric terminal strategy for highly efficient organic solar cells. J Mater Chem A, 2019, 7, 8889 doi: 10.1039/C8TA12530A[79] Gao B W, Yao H F, Hou J X, et al. Multi-component non-fullerene acceptors with tunable bandgap structures for efficient organic solar cells. J Mater Chem A, 2018, 6, 23644 doi: 10.1039/C8TA09830A[80] Ye L L, Xie Y P, Xiao Y Q, et al. Asymmetric fused-ring electron acceptor with two distinct terminal groups for efficient organic solar cells. J Mater Chem A, 2019, 7, 8055 doi: 10.1039/C9TA01285K[81] Lai H J, Chen H, Shen Y, et al. Using chlorine atoms to fine-tune the intermolecular packing and energy levels of efficient nonfullerene acceptors. ACS Appl Energy Mater, 2019, 2, 7663 doi: 10.1021/acsaem.9b01667[82] Zhang J Y, Liu W R, Chen S S, et al. One-pot synthesis of electron-acceptor composite enables efficient fullerene-free ternary organic solar cells. J Mater Chem A, 2018, 6, 22519 doi: 10.1039/C8TA08961B[83] Duan T N, Hou L C, Fu J H, et al. An asymmetric end-capping strategy enables a new non-fullerene acceptor for organic solar cells with efficiency over 10%. Chem Commun, 2020, 56, 6531 doi: 10.1039/D0CC01739F[84] Zhao Y, Luo Z H, Li G H, et al. De novo design of small molecule acceptors via fullerene/non-fullerene hybrids for polymer solar cells. Chem Commun, 2018, 54, 9801 doi: 10.1039/C8CC04845B[85] Feng S Y, Zhang C E, Liu Y H, et al. Fused-ring acceptors with asymmetric side chains for high-performance thick-film organic solar cells. Adv Mater, 2017, 29, 1703527 doi: 10.1002/adma.201703527[86] Feng S Y, Zhang C, Bi Z Z, et al. Controlling molecular packing and orientation via constructing a ladder-type electron acceptor with asymmetric substituents for thick-film nonfullerene solar cells. ACS Appl Mater Interfaces, 2019, 11, 3098 doi: 10.1021/acsami.8b19596[87] Lee J, Song S, Huang J F, et al. Bandgap tailored nonfullerene acceptors for low-energy-loss near-infrared organic photovoltaics. ACS Mater Lett, 2020, 2, 395 doi: 10.1021/acsmaterialslett.9b00512[88] Chen X B, Kan B, Kan Y Y, et al. As-cast ternary organic solar cells based on an asymmetric side-chains featured acceptor with reduced voltage loss and 14.0% efficiency. Adv Funct Mater, 2020, 30, 1909535 doi: 10.1002/adfm.201909535[89] Liu X Z, Wei Y N, Zhang X, et al. An A-D-A'-D-A type unfused nonfullerene acceptor for organic solar cells with approaching 14% efficiency. Sci China Chem, 2021, 64, 228 doi: 10.1007/s11426-020-9868-8[90] Liu T, Zhang Y D, Shao Y M, et al. Asymmetric acceptors with fluorine and chlorine substitution for organic solar cells toward 16.83% efficiency. Adv Funct Mater, 2020, 30, 2000456 doi: 10.1002/adfm.202000456[91] Luo Z H, Ma R J, Liu T, et al. Fine-tuning energy levels via asymmetric end groups enables polymer solar cells with efficiencies over 17%. Joule, 2020, 4, 1236 doi: 10.1016/j.joule.2020.03.023[92] Zhang J S, Han Y F, Zhang W X, et al. High-efficiency thermal-annealing-free organic solar cells based on an asymmetric acceptor with improved thermal and air stability. ACS Appl Mater Interfaces, 2020, 12, 57271 doi: 10.1021/acsami.0c17423[93] Cai F F, Zhu C, Yuan J, et al. Efficient organic solar cells based on a new "Y-series" non-fullerene acceptor with an asymmetric electron-deficient-core. Chem Commun, 2020, 56, 4340 doi: 10.1039/C9CC10076H[94] Cai F F, Peng H J, Chen H G, et al. An asymmetric small molecule acceptor for organic solar cells with a short circuit current density over 24 mA cm–2. J Mater Chem A, 2020, 8, 15984 doi: 10.1039/D0TA01636E[95] Zhang M, Gao W, Zhang F J, et al. Efficient ternary non-fullerene polymer solar cells with PCE of 11.92% and FF of 76. 5%. Energy Environ Sci, 2018, 11, 841 doi: 10.1039/C8EE00215K[96] Jeong C H, Kim Y U, Park C G, et al. Improved performance of non-fullerene polymer solar cells by simple structural change of asymmetric acceptor based on indenothiophene. Synth Met, 2018, 246, 164 doi: 10.1016/j.synthmet.2018.10.014 -
Proportional views





 DownLoad:
DownLoad: